User login
Is Telomere Length Linked to Migraine Risk in Younger Adults?
Key clinical point: In American adults aged 20-50 years, a shorter telomere length was associated with an increased risk for migraine; however, no such association was found in adults older than 50 years.
Major findings: In adults aged 20-50 years, a decrease in the ratio of telomeric repeats to single-copy genes was associated with an increased risk for migraine (odds ratio [OR], 1.48; P = .047). Those with the shortest telomeres were at a greater risk for migraine than those with the longest telomeres (OR, 1.35; P = .043). Such an association was not observed in adults older than 50 years.
Study details: This cross-sectional study analyzed data from the US National Health and Nutrition Examination Survey (1999-2002) on migraine and leukocyte telomere length in 6169 adults with or without migraine.
Disclosure: The study was supported by the Natural Science Foundation of Hebei Province and the Central Government Guides Local Funds for Science and Technology Development. The authors declared no conflicts of interest.
Source: Geng D, Liu H, Wang H, et al. Telomere length exhibits inverse association with migraine among Americans aged 20–50 years, without implications beyond age 50: A cross-sectional study. Sci Rep. 2024;14:22597. Source
Key clinical point: In American adults aged 20-50 years, a shorter telomere length was associated with an increased risk for migraine; however, no such association was found in adults older than 50 years.
Major findings: In adults aged 20-50 years, a decrease in the ratio of telomeric repeats to single-copy genes was associated with an increased risk for migraine (odds ratio [OR], 1.48; P = .047). Those with the shortest telomeres were at a greater risk for migraine than those with the longest telomeres (OR, 1.35; P = .043). Such an association was not observed in adults older than 50 years.
Study details: This cross-sectional study analyzed data from the US National Health and Nutrition Examination Survey (1999-2002) on migraine and leukocyte telomere length in 6169 adults with or without migraine.
Disclosure: The study was supported by the Natural Science Foundation of Hebei Province and the Central Government Guides Local Funds for Science and Technology Development. The authors declared no conflicts of interest.
Source: Geng D, Liu H, Wang H, et al. Telomere length exhibits inverse association with migraine among Americans aged 20–50 years, without implications beyond age 50: A cross-sectional study. Sci Rep. 2024;14:22597. Source
Key clinical point: In American adults aged 20-50 years, a shorter telomere length was associated with an increased risk for migraine; however, no such association was found in adults older than 50 years.
Major findings: In adults aged 20-50 years, a decrease in the ratio of telomeric repeats to single-copy genes was associated with an increased risk for migraine (odds ratio [OR], 1.48; P = .047). Those with the shortest telomeres were at a greater risk for migraine than those with the longest telomeres (OR, 1.35; P = .043). Such an association was not observed in adults older than 50 years.
Study details: This cross-sectional study analyzed data from the US National Health and Nutrition Examination Survey (1999-2002) on migraine and leukocyte telomere length in 6169 adults with or without migraine.
Disclosure: The study was supported by the Natural Science Foundation of Hebei Province and the Central Government Guides Local Funds for Science and Technology Development. The authors declared no conflicts of interest.
Source: Geng D, Liu H, Wang H, et al. Telomere length exhibits inverse association with migraine among Americans aged 20–50 years, without implications beyond age 50: A cross-sectional study. Sci Rep. 2024;14:22597. Source
Sustained Remission of Nonopioid Medication Overuse Headache with Erenumab in Chronic Migraine
Key clinical point: Erenumab was effective in achieving and sustaining the remission of medication overuse headache (MOH) in adults with chronic migraine (CM) and nonopioid MOH, with adverse events reflecting the known safety profile of erenumab.
Major findings: At 6 months, 140 mg erenumab was significantly more effective than placebo in achieving increased MOH remission (odds ratio [OR], 2.01; P < .001) and sustained MOH remission (OR, 2.63; P < .001). The most common treatment-emergent adverse events in both erunumab groups were constipation (15.2%) and COVID-19 (13.9%); no new adverse events were reported.
Study details: This phase 4 randomized controlled trial included 584 adults with CM and MOH in the nonopioid-treated cohort who did not respond to one or more preventive treatments. Participants were randomly assigned to receive monthly injections of erenumab (70 mg or 140 mg) or placebo for 24 weeks.
Disclosures: This study was funded by Amgen. Some authors declared being employees or stockholders of Amgen, and others declared having ties with various sources, including Amgen.
Source: Tepper SJ, Dodick DW, Lanteri-Minet M, et al. Efficacy and safety of erenumab for nonopioid medication overuse headache in chronic migraine: A phase 4, randomized, placebo-controlled trial. JAMA Neurol. Published online September 16, 2024. Source
Key clinical point: Erenumab was effective in achieving and sustaining the remission of medication overuse headache (MOH) in adults with chronic migraine (CM) and nonopioid MOH, with adverse events reflecting the known safety profile of erenumab.
Major findings: At 6 months, 140 mg erenumab was significantly more effective than placebo in achieving increased MOH remission (odds ratio [OR], 2.01; P < .001) and sustained MOH remission (OR, 2.63; P < .001). The most common treatment-emergent adverse events in both erunumab groups were constipation (15.2%) and COVID-19 (13.9%); no new adverse events were reported.
Study details: This phase 4 randomized controlled trial included 584 adults with CM and MOH in the nonopioid-treated cohort who did not respond to one or more preventive treatments. Participants were randomly assigned to receive monthly injections of erenumab (70 mg or 140 mg) or placebo for 24 weeks.
Disclosures: This study was funded by Amgen. Some authors declared being employees or stockholders of Amgen, and others declared having ties with various sources, including Amgen.
Source: Tepper SJ, Dodick DW, Lanteri-Minet M, et al. Efficacy and safety of erenumab for nonopioid medication overuse headache in chronic migraine: A phase 4, randomized, placebo-controlled trial. JAMA Neurol. Published online September 16, 2024. Source
Key clinical point: Erenumab was effective in achieving and sustaining the remission of medication overuse headache (MOH) in adults with chronic migraine (CM) and nonopioid MOH, with adverse events reflecting the known safety profile of erenumab.
Major findings: At 6 months, 140 mg erenumab was significantly more effective than placebo in achieving increased MOH remission (odds ratio [OR], 2.01; P < .001) and sustained MOH remission (OR, 2.63; P < .001). The most common treatment-emergent adverse events in both erunumab groups were constipation (15.2%) and COVID-19 (13.9%); no new adverse events were reported.
Study details: This phase 4 randomized controlled trial included 584 adults with CM and MOH in the nonopioid-treated cohort who did not respond to one or more preventive treatments. Participants were randomly assigned to receive monthly injections of erenumab (70 mg or 140 mg) or placebo for 24 weeks.
Disclosures: This study was funded by Amgen. Some authors declared being employees or stockholders of Amgen, and others declared having ties with various sources, including Amgen.
Source: Tepper SJ, Dodick DW, Lanteri-Minet M, et al. Efficacy and safety of erenumab for nonopioid medication overuse headache in chronic migraine: A phase 4, randomized, placebo-controlled trial. JAMA Neurol. Published online September 16, 2024. Source
Triptans Outperform Newer Drugs in Acute Treatment of Migraine
Key clinical point: Triptans, including eletriptan, rizatriptan, sumatriptan, and zolmitriptan, were more efficacious than newer and more expensive medications, such as lasmiditan and rimegepant, for the acute treatment of migraine.
Major findings: All active interventions were superior to placebo in achieving freedom from pain at 2 hours (odds ratio [OR], 1.73) with naratriptan and (OR, 5.19) for eletriptan. Eletriptan was the most effective for pain relief at two hours (OR, 1.46-3.01), followed by rizatriptan (OR, 1.59-2.44), sumatriptan (OR, 1.35-2.04), and zolmitriptan (OR, 1.47-1.96). For sustained pain freedom, eletriptan and ibuprofen were the most effective.
Study details: This network meta-analysis of 137 randomized controlled trials included 89,445 adults with migraine who received one of 17 drugs, including antipyretics, ditans, gepants, nonsteroidal anti-inflammatory drugs, and triptans, or placebo.
Disclosures: This study was funded by the National Institute for Health and Care Research Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Several authors reported having ties with various sources.
Source: Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
Key clinical point: Triptans, including eletriptan, rizatriptan, sumatriptan, and zolmitriptan, were more efficacious than newer and more expensive medications, such as lasmiditan and rimegepant, for the acute treatment of migraine.
Major findings: All active interventions were superior to placebo in achieving freedom from pain at 2 hours (odds ratio [OR], 1.73) with naratriptan and (OR, 5.19) for eletriptan. Eletriptan was the most effective for pain relief at two hours (OR, 1.46-3.01), followed by rizatriptan (OR, 1.59-2.44), sumatriptan (OR, 1.35-2.04), and zolmitriptan (OR, 1.47-1.96). For sustained pain freedom, eletriptan and ibuprofen were the most effective.
Study details: This network meta-analysis of 137 randomized controlled trials included 89,445 adults with migraine who received one of 17 drugs, including antipyretics, ditans, gepants, nonsteroidal anti-inflammatory drugs, and triptans, or placebo.
Disclosures: This study was funded by the National Institute for Health and Care Research Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Several authors reported having ties with various sources.
Source: Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
Key clinical point: Triptans, including eletriptan, rizatriptan, sumatriptan, and zolmitriptan, were more efficacious than newer and more expensive medications, such as lasmiditan and rimegepant, for the acute treatment of migraine.
Major findings: All active interventions were superior to placebo in achieving freedom from pain at 2 hours (odds ratio [OR], 1.73) with naratriptan and (OR, 5.19) for eletriptan. Eletriptan was the most effective for pain relief at two hours (OR, 1.46-3.01), followed by rizatriptan (OR, 1.59-2.44), sumatriptan (OR, 1.35-2.04), and zolmitriptan (OR, 1.47-1.96). For sustained pain freedom, eletriptan and ibuprofen were the most effective.
Study details: This network meta-analysis of 137 randomized controlled trials included 89,445 adults with migraine who received one of 17 drugs, including antipyretics, ditans, gepants, nonsteroidal anti-inflammatory drugs, and triptans, or placebo.
Disclosures: This study was funded by the National Institute for Health and Care Research Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Several authors reported having ties with various sources.
Source: Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
Obesity Etiology
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Persistent headaches and nightmares
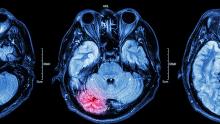
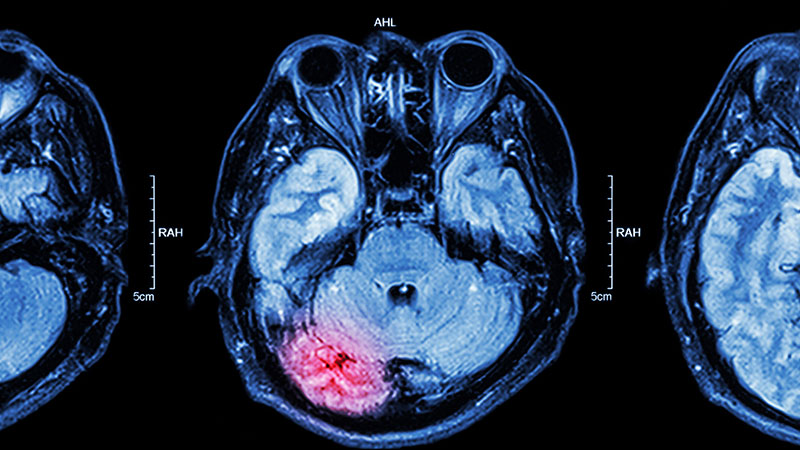
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 15-year-old girl presented to the emergency department with complaints of persistent headaches, nightmares, and difficulty concentrating in school over the past 3 months. The patient had recently experienced a traumatic event, a severe car accident in which a close friend was critically injured. Since the incident, the patient has been exhibiting increased irritability, avoidance of activities that she previously enjoyed, and a noticeable withdrawal from social interactions. Additionally, she reported recurrent flashbacks to the accident, often triggered by sounds resembling car engines. On physical examination, the patient appeared anxious and exhibited hypervigilance. An MRI of the brain was performed to rule out any organic causes of her symptoms, revealing an area of increased signal intensity in the left cerebellar hemisphere (as highlighted in the image).
Utilization, Cost, and Prescription Trends of Antipsychotics Prescribed by Dermatologists for Medicare Patients
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
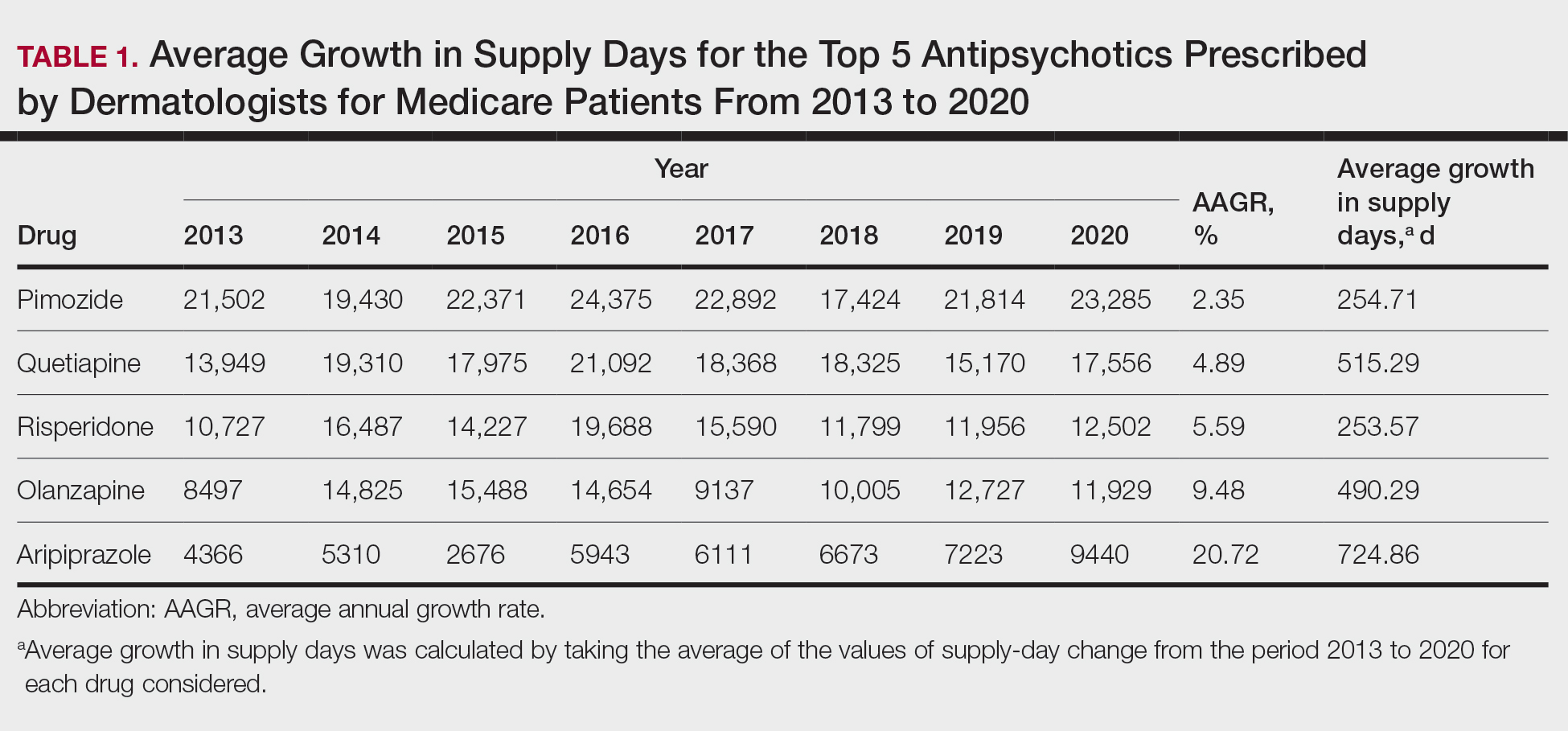
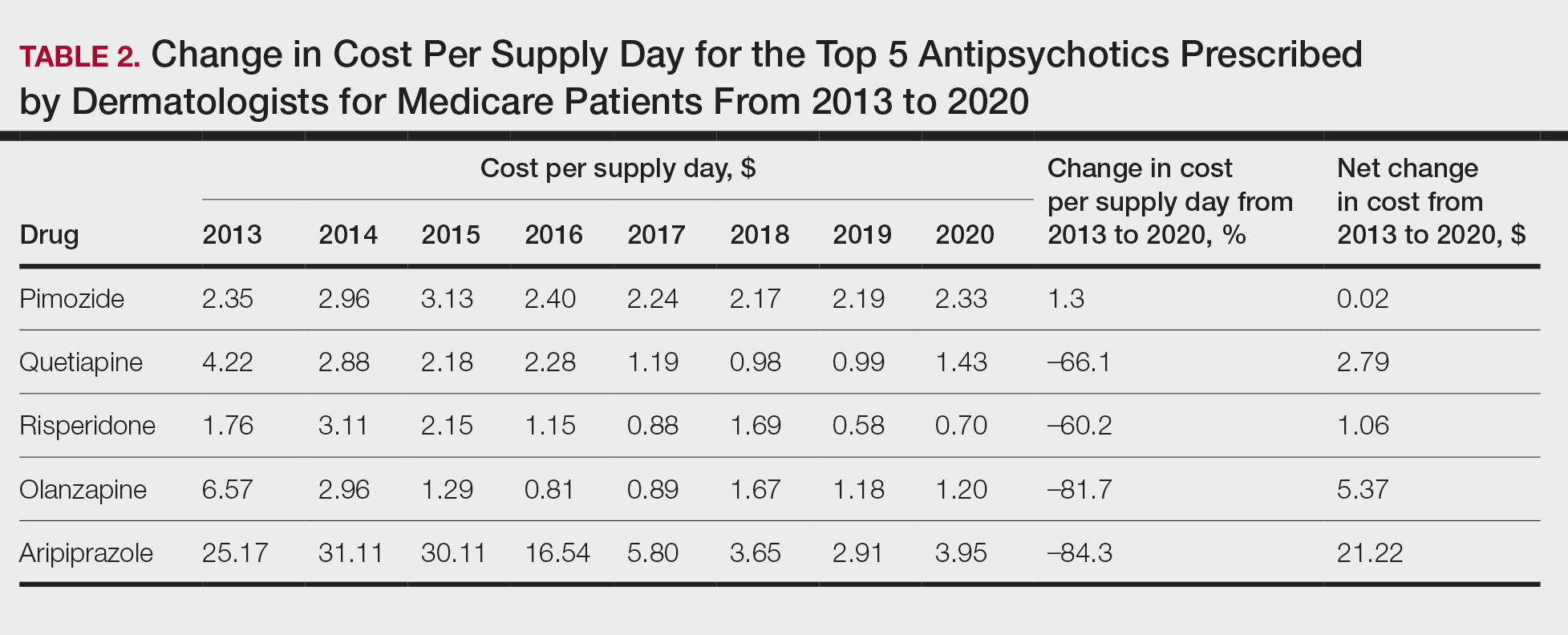
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).
There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).


There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).


There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
Practice Points
- Dermatologists are frontline medical providers who can be useful in screening for primary psychiatric disorders in patients with dermatologic manifestations.
- Second-generation antipsychotics are effective for treating many psychiatric disorders.
Swollen elbow and knee joints
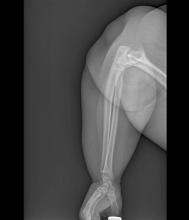
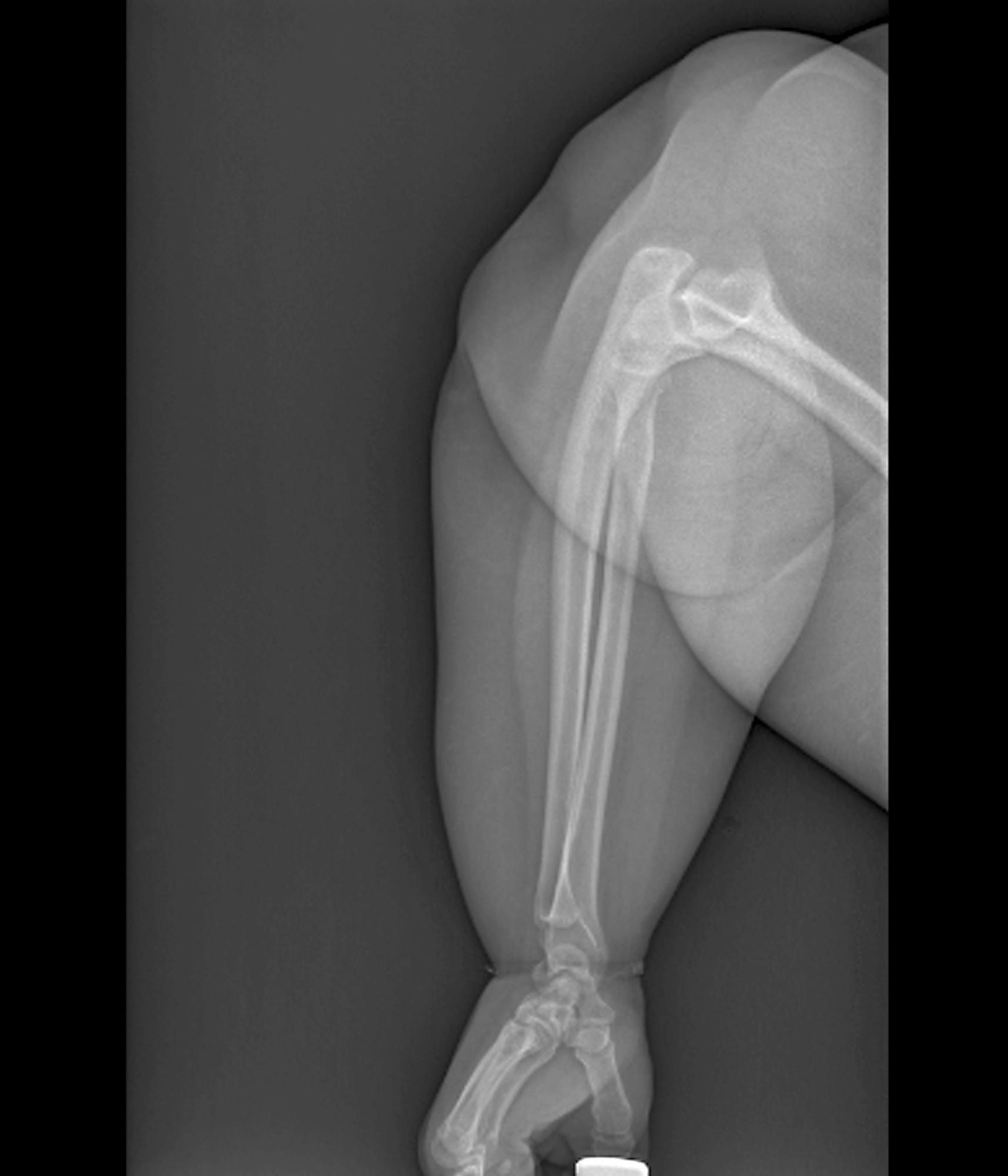
Obesity is a chronic disease affecting more than 20% of adults in the United States. In 2022, prevalence was 20.5% among those aged 18 to 24 and 39.9% among those aged 45 to 54 years. This patient meets criteria for obesity (BMI ≥ 30), and it is likely that her obesity contributed to development of T2D, hypertension, osteoarthritis, and joint edema (as shown in the image).
Patients with obesity are at high risk of developing cardiometabolic disease and osteoarthritis. Obesity is a key driver of T2D and cardiovascular disease development through its influence on insulin and lipid metabolism and proinflammatory changes. Factors associated with obesity that foster arthritis development include the erosive effects of adiponectin and leptin on cartilage and direct inflammation in joint tissues.
It is important for patients with obesity and comorbid T2D and hypertension to receive multidisciplinary care designed to address all aspects of their health and minimize their risk for progression. The primary goal for this patient should be to promote weight loss safely while also improving her glycemic control and blood pressure. For patients with obesity and comorbid T2D, the American Diabetes Association recommends glucagon-like peptide-1 receptor agonists (GLP-1 RAs; semaglutide or liraglutide) or the dual gastric inhibitory polypeptide (GIP)/GLP-1 RA tirzepatide. The GLP-1 RA drugs reduce the risk for major cardiovascular events for patients with T2D while also providing substantial reductions in glucose levels without increasing hypoglycemia risk. They are available in higher doses (semaglutide 2.4 mg weekly, liraglutide 3.0 mg daily) for patients with obesity. These drugs also have salubrious effects on cardiorenal health and reduce progression of kidney disease. Tirzepatide produced greater reductions in A1c vs semaglutide in patients with T2D in the SURPASS-2 trial. It also has been shown to reduce atherosclerotic cardiovascular events in patients with overweight or obesity (without diabetes) in a post hoc analysis of the SURMOUNT-1 trial. Its effect on a broad set of cardiac, renal, and metabolic outcomes is being studied in the ongoing SURMOUNT-MMO trial. The American Gastroenterological Association and other organizations recommend treatment with antiobesity medications along with lifestyle modifications for patients with obesity (BMI ≥ 30) and weight-related complications (BMI > 27).
Pharmacologic interventions for osteoarthritis include nonsteroidal anti-inflammatory drugs, including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib. These may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical nonsteroidal anti-inflammatory drugs also may be useful. For more intractable joint pain, options include injecting corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
In addition, comprehensive care includes lifestyle modifications designed to promote weight loss, reduce sodium, and increase exercise and intake of healthy foods. While maintaining intensive lifestyle modifications can be challenging, achieving weight loss of ≥ 5% can improve cardiometabolic risk factors in patients with obesity and T2D. Greater benefit is seen with greater reductions in body weight. Other interventions include behavioral modification and encouragement of increased physical activity to the extent of the patient's ability. Achieving substantial weight loss also could help relieve stress on the patient's joints, improve physical function, and mitigate osteoarthritis-related pain. The patient also may benefit from nonpharmacologic approaches to joint pain, including hot or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Obesity is a chronic disease affecting more than 20% of adults in the United States. In 2022, prevalence was 20.5% among those aged 18 to 24 and 39.9% among those aged 45 to 54 years. This patient meets criteria for obesity (BMI ≥ 30), and it is likely that her obesity contributed to development of T2D, hypertension, osteoarthritis, and joint edema (as shown in the image).
Patients with obesity are at high risk of developing cardiometabolic disease and osteoarthritis. Obesity is a key driver of T2D and cardiovascular disease development through its influence on insulin and lipid metabolism and proinflammatory changes. Factors associated with obesity that foster arthritis development include the erosive effects of adiponectin and leptin on cartilage and direct inflammation in joint tissues.
It is important for patients with obesity and comorbid T2D and hypertension to receive multidisciplinary care designed to address all aspects of their health and minimize their risk for progression. The primary goal for this patient should be to promote weight loss safely while also improving her glycemic control and blood pressure. For patients with obesity and comorbid T2D, the American Diabetes Association recommends glucagon-like peptide-1 receptor agonists (GLP-1 RAs; semaglutide or liraglutide) or the dual gastric inhibitory polypeptide (GIP)/GLP-1 RA tirzepatide. The GLP-1 RA drugs reduce the risk for major cardiovascular events for patients with T2D while also providing substantial reductions in glucose levels without increasing hypoglycemia risk. They are available in higher doses (semaglutide 2.4 mg weekly, liraglutide 3.0 mg daily) for patients with obesity. These drugs also have salubrious effects on cardiorenal health and reduce progression of kidney disease. Tirzepatide produced greater reductions in A1c vs semaglutide in patients with T2D in the SURPASS-2 trial. It also has been shown to reduce atherosclerotic cardiovascular events in patients with overweight or obesity (without diabetes) in a post hoc analysis of the SURMOUNT-1 trial. Its effect on a broad set of cardiac, renal, and metabolic outcomes is being studied in the ongoing SURMOUNT-MMO trial. The American Gastroenterological Association and other organizations recommend treatment with antiobesity medications along with lifestyle modifications for patients with obesity (BMI ≥ 30) and weight-related complications (BMI > 27).
Pharmacologic interventions for osteoarthritis include nonsteroidal anti-inflammatory drugs, including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib. These may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical nonsteroidal anti-inflammatory drugs also may be useful. For more intractable joint pain, options include injecting corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
In addition, comprehensive care includes lifestyle modifications designed to promote weight loss, reduce sodium, and increase exercise and intake of healthy foods. While maintaining intensive lifestyle modifications can be challenging, achieving weight loss of ≥ 5% can improve cardiometabolic risk factors in patients with obesity and T2D. Greater benefit is seen with greater reductions in body weight. Other interventions include behavioral modification and encouragement of increased physical activity to the extent of the patient's ability. Achieving substantial weight loss also could help relieve stress on the patient's joints, improve physical function, and mitigate osteoarthritis-related pain. The patient also may benefit from nonpharmacologic approaches to joint pain, including hot or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Obesity is a chronic disease affecting more than 20% of adults in the United States. In 2022, prevalence was 20.5% among those aged 18 to 24 and 39.9% among those aged 45 to 54 years. This patient meets criteria for obesity (BMI ≥ 30), and it is likely that her obesity contributed to development of T2D, hypertension, osteoarthritis, and joint edema (as shown in the image).
Patients with obesity are at high risk of developing cardiometabolic disease and osteoarthritis. Obesity is a key driver of T2D and cardiovascular disease development through its influence on insulin and lipid metabolism and proinflammatory changes. Factors associated with obesity that foster arthritis development include the erosive effects of adiponectin and leptin on cartilage and direct inflammation in joint tissues.
It is important for patients with obesity and comorbid T2D and hypertension to receive multidisciplinary care designed to address all aspects of their health and minimize their risk for progression. The primary goal for this patient should be to promote weight loss safely while also improving her glycemic control and blood pressure. For patients with obesity and comorbid T2D, the American Diabetes Association recommends glucagon-like peptide-1 receptor agonists (GLP-1 RAs; semaglutide or liraglutide) or the dual gastric inhibitory polypeptide (GIP)/GLP-1 RA tirzepatide. The GLP-1 RA drugs reduce the risk for major cardiovascular events for patients with T2D while also providing substantial reductions in glucose levels without increasing hypoglycemia risk. They are available in higher doses (semaglutide 2.4 mg weekly, liraglutide 3.0 mg daily) for patients with obesity. These drugs also have salubrious effects on cardiorenal health and reduce progression of kidney disease. Tirzepatide produced greater reductions in A1c vs semaglutide in patients with T2D in the SURPASS-2 trial. It also has been shown to reduce atherosclerotic cardiovascular events in patients with overweight or obesity (without diabetes) in a post hoc analysis of the SURMOUNT-1 trial. Its effect on a broad set of cardiac, renal, and metabolic outcomes is being studied in the ongoing SURMOUNT-MMO trial. The American Gastroenterological Association and other organizations recommend treatment with antiobesity medications along with lifestyle modifications for patients with obesity (BMI ≥ 30) and weight-related complications (BMI > 27).
Pharmacologic interventions for osteoarthritis include nonsteroidal anti-inflammatory drugs, including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib. These may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical nonsteroidal anti-inflammatory drugs also may be useful. For more intractable joint pain, options include injecting corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
In addition, comprehensive care includes lifestyle modifications designed to promote weight loss, reduce sodium, and increase exercise and intake of healthy foods. While maintaining intensive lifestyle modifications can be challenging, achieving weight loss of ≥ 5% can improve cardiometabolic risk factors in patients with obesity and T2D. Greater benefit is seen with greater reductions in body weight. Other interventions include behavioral modification and encouragement of increased physical activity to the extent of the patient's ability. Achieving substantial weight loss also could help relieve stress on the patient's joints, improve physical function, and mitigate osteoarthritis-related pain. The patient also may benefit from nonpharmacologic approaches to joint pain, including hot or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 24-year-old woman presents for swollen and painful elbow and knee joints. The patient is 5 ft 7 in tall and weighs 235 lb (BMI 36.8). The patient says she has been overweight since her preteen years and has never been involved in sports or exercise activities. She has gained a significant amount of weight in the past 2 years since beginning work in an insurance office. She has lived at home with her parents since graduating from college.
Her elbows are tender to the touch; further examination reveals tender joints at her wrists, knees, and hips as well. Extremities are thick because of obesity.
Medical history includes diagnosis of type 2 diabetes (T2D) at age 22. In the office, her blood pressure is elevated (150/85 mm Hg), heart rate is 110 beats/min, and respiratory rate is 18 breaths/min. Lab results indicate A1c = 8.5%, low-density lipoprotein cholesterol = 145 mg/dL, and estimated glomerular filtration rate = 90 mL/min/1.73 m2; all other results are within normal range. Her only current medication is metformin 1000 mg daily.
In-office radiography reveals no obvious bone or joint damage.
Multiple Sclerosis Highlights From ECTRIMS 2024
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (MS) presented at the European Committee for Treatment and Research in Multiple Sclerosis 2024 Congress is reported by Dr Patricia Coyle from Stony Brook University Hospital, in Stony Brook, New York.
Dr Coyle first discusses a registry study looking at initiation of monoclonal antibody therapy for patients with pediatric-onset MS. Results showed a significant reduction in disability at age 23 and beyond when therapy was initiated in childhood.
Next, Dr Coyle discusses a trial examining the safety and efficacy of frexalimab, a second-generation anti-CD40L antibody. In an open-label extension trial through 72 weeks, frexalimab provided a sustained reduction of disease activity, as measured by MRI, and was well tolerated.
She then details a study looking at the effects of disease-modifying therapies (DMTs) on pregnancy outcomes in patients with MS. Using a German MS registry, researchers looked at 3722 pregnancies, 2885 with DMT exposure, and concluded that most pregnancy outcomes are unaffected by DMT exposure; however, the data showed the potential risk for reduced birth rates.
Finally, Dr Coyle examines the efficacy of the Bruton tyrosine kinase (BTK) inhibitor tolebrutinib, as evidenced by the HERCULES trial and the two GEMINI trials. In HERCULES, the BTK inhibitor reduced 6-month disability progression by a significant 31% compared with placebo.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; National Institute of Neurological Disorders and Stroke; Sanofi Genzyme

Facial Angioedema, Rash, and “Mastitis” in a 31-Year-Old Female
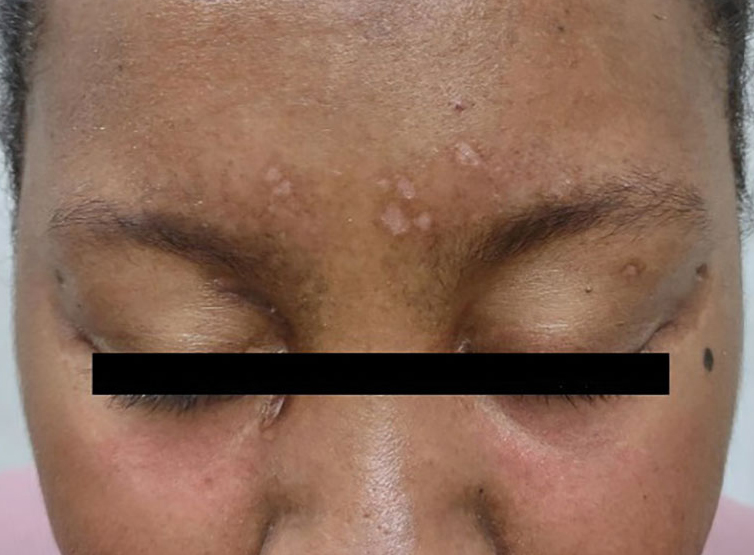
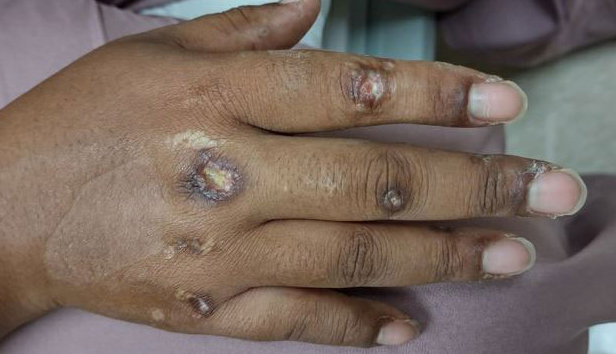
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.

DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
Vancomycin AUC-Dosing Initiative at a Regional Antibiotic Stewardship Collaborative
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.
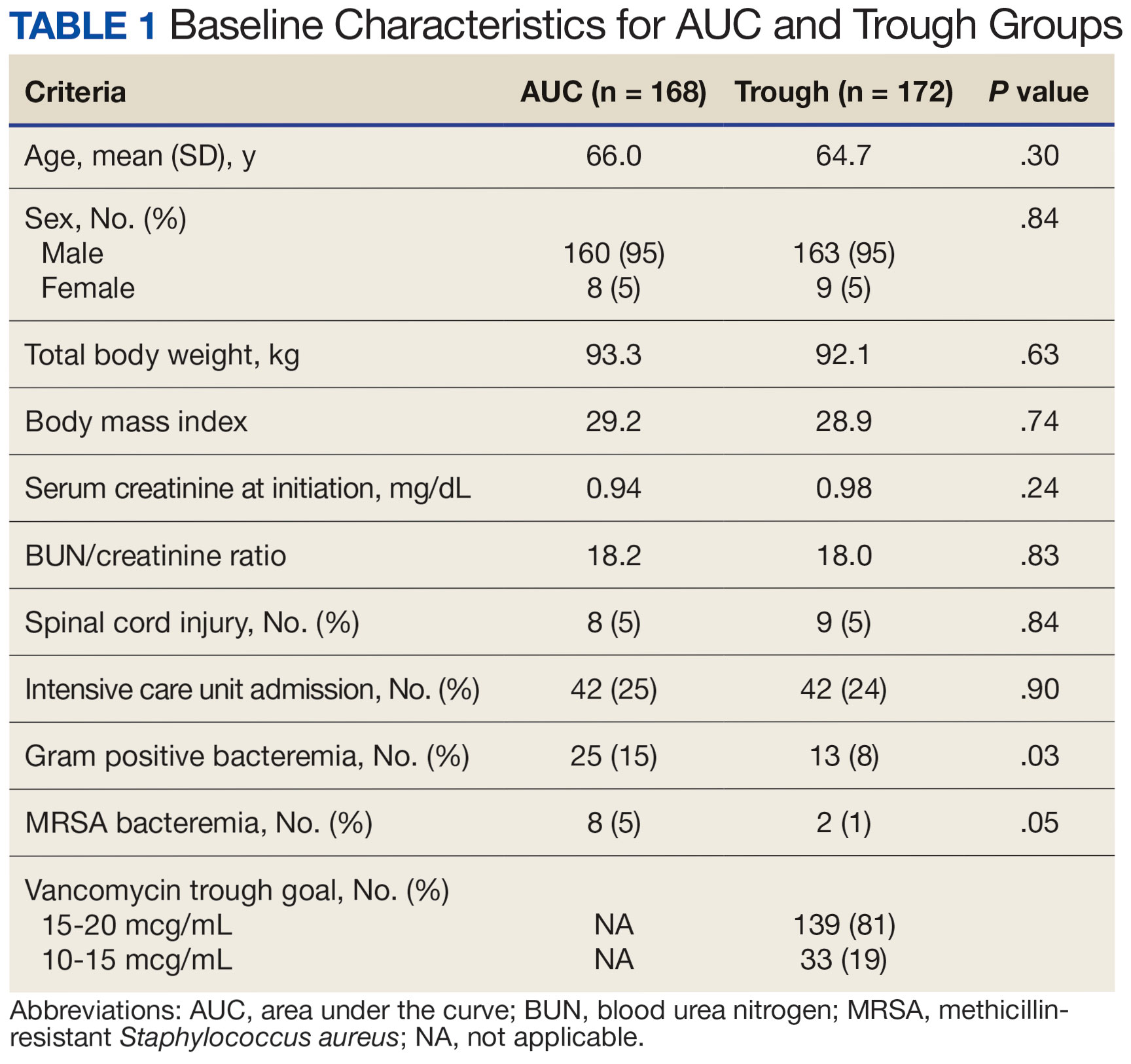

We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
Antimicrobial resistance is a global threat and burden to health care, with > 2.8 million antibiotic-resistant infections occurring annually in the United States.1 To combat this issue and improve patient care, the US Department of Veterans Affairs (VA) has implemented antimicrobial stewardship programs (ASPs) across its health care systems. ASPs are multidisciplinary teams that promote evidence-based use of antimicrobials through activities supporting appropriate selection, dosing, route, and duration of antimicrobial therapy. ASP best practices are also included in the Joint Commission and Centers for Medicare and Medicaid Services accreditation standards.2
The foundational charge for VA facilities to develop and maintain ASPs was outlined in 2014 and updated in 2023 in the Veterans Health Administration (VHA) Directive 1031 on antimicrobial stewardship programs.2 This directive outlines specific requirements for all VA ASPs, including personnel, staffing levels, and the roles and responsibilities of all team members. VHA now requires that Veterans Integrated Services Networks (VISNs) establish robust ASP collaboratives. A VISN ASP collaborative consists of stewardship champions from each VA medical center in the VISN and is designed to support, develop, and enhance ASP programs across all facilities within that VISN.2 Some VISNs may lack an ASP collaborative altogether, and others with existing groups may seek ways to expand their collaboratives in line with the updated directive. Prior to VHA Directive 1031, the VA Sunshine Healthcare Network (VISN 8) established an ASP collaborative. This article describes the structure and activities of the VISN 8 ASP collaborative and highlights a recent VISN 8 quality assurance initiative related to vancomycin area under the curve (AUC) dosing that illustrates how ASP collaboratives can enhance stewardship and clinical care across broad geographic areas.
VISN 8 ASP
The VHA, the largest integrated US health care system, is divided into 18 VISNs that provide regional systems of care to enhance access and meet the local health care needs of veterans.3 VISN 8 serves > 1.5 million veterans across 165,759 km2 in Florida, South Georgia, Puerto Rico, and the US Virgin Islands.4 The network is composed of 7 health systems with 8 medical centers and > 60 outpatient clinics. These facilities provide comprehensive acute, primary, and specialty care, as well as mental health and extended care services in inpatient, outpatient, nursing home, and home care settings.4
The 2023 VHA Directive 1031 update recognizes the importance of VISN-level coordination of ASP activities to enhance the standardization of care and build partnerships in stewardship across all levels of care. The VISN 8 ASP collaborative workgroup (ASPWG) was established in 2015. Consistent with Directive 1031, the ASPWG is guided by clinician and pharmacist VISN leads. These leads serve as subject matter experts, facilitate access to resources, establish VISN-level consensus, and enhance communication among local ASP champions at medical centers within the VISN. All 7 health systems include = 1 ASP champion (clinician or pharmacist) in the ASPWG. Ad hoc members, whose routine duties are not solely focused on antimicrobial stewardship, contribute to specific stewardship projects as needed. For example, the ASPWG has included internal medicine, emergency department, community living center pharmacists, representatives from pharmacy administration, and trainees (pharmacy students and residents, and infectious diseases fellows) in antimicrobial stewardship initiatives. The inclusion of non-ASP champions is not discussed in VHA Directive 1031. However, these members have made valuable contributions to the ASPWG.
The ASPWG meets monthly. Agendas and priorities are developed by the VISN pharmacist and health care practitioner (HCP) leads. Monthly discussions may include but are not limited to a review of national formulary decisions, VISN goals and metrics, infectious diseases hot topics, pharmacoeconomic initiatives, strong practice presentations, regulatory and accreditation preparation, preparation of tracking reports, as well as the development of both patient-level and HCPlevel tools, resources, and education materials. This forum facilitates collaborative learning: members process and synthesize information, share and reframe ideas, and listen to other viewpoints to gain a complete understanding as a group.5 For example, ASPWG members have leaned on each other to prepare for Joint Commission accreditation surveys and strengthen the VISN 8 COVID-19 program through the rollout of vaccines and treatments. Other collaborative projects completed over the past few years included a penicillin allergy testing initiative and anti-methicillin-resistant Staphylococcus aureus (MRSA) and pseudomonal medication use evaluations. This team-centric problem-solving approach is highly effective while also fostering professional and social relationships. However, collaboratives could be perceived to have drawbacks. There may be opportunity costs if ASP time is allocated for issues that have already been addressed locally or concerns that standardization might hinder rapid adoption of practices at individual sites. Therefore, participation in each distinct group initiative is optional. This allows sites to choose projects related to their high priority areas and maintain bandwidth to implement practices not yet adopted by the larger group.
The ASPWG tracks metrics related to antimicrobial use with quarterly data presented by the VISN pharmacist lead. Both inpatient and outpatient metrics are evaluated, such as days of therapy per 1000 days and outpatient antibiotic prescriptions per 1000 unique patients. Facilities are benchmarked against their own historical data and other VISN sites, as well as other VISNs across the country. When outliers are identified, facilities are encouraged to conduct local projects to identify reasons for different antimicrobial use patterns and subsequent initiatives to optimize antimicrobial use. Benchmarking against VISN facilities can be useful since VISN facilities may be more similar than facilities in different geographic regions. Each year, the ASPWG reviews the current metrics, makes adjustments to address VISN priorities, and votes for approval of the metrics that will be tracked in the coming year.
Participation in an ASP collaborative streamlines the rollout of ASP and quality improvement initiatives across multiple sites, allowing ASPs to impact a greater number of veterans and evaluate initiatives on a larger scale. In 2019, with the anticipation of revised vancomycin dosing and monitoring guidelines, our ASPWG began to strategize the transition to AUC-based vancomycin monitoring.6 This multisite initiative showcases the strengths of implementing and evaluating practice changes as part of an ASP collaborative.
Vancomycin Dosing
The antibiotic vancomycin is used primarily for the treatment of MRSA infections.6 The 2020 consensus guidelines for vancomycin therapeutic monitoring recommend using the AUC to minimum inhibitory concentration (MIC) ratio as the pharmacodynamic target for serious MRSA infections, with an AUC/MIC goal of 400 to 600 mcg*h/mL.6 Prior guidelines recommended using vancomycin trough concentrations of 15 to 20 mcg/mL as a surrogate for this AUC target. However, subsequent studies have shown that trough-based dosing is associated with higher vancomycin exposures, supratherapeutic AUCs, and increased risk of vancomycin-associated acute kidney injury (AKI).7,8 Therefore, more direct AUC estimation is now recommended.6 The preferred approach for AUC calculations is through Bayesian modeling. Due to limited resources and software availability, many facilities use an alternative method involving 2 postdistributive serum vancomycin concentrations and first-order pharmacokinetic equations. This approach can optimize vancomycin dosing but is more mathematically and logistically challenging. Transitioning from troughto AUC-based vancomycin monitoring requires careful planning and comprehensive staff education.
In 2019, the VISN 8 ASPWG created a comprehensive vancomycin AUC toolkit to facilitate implementation. Components included a pharmacokinetic management policy and procedure, a vancomycin dosing guide, a progress note template, educational materials specific to pharmacy, nursing, laboratory, and medical services, a pharmacist competency examination, and a vancomycin AUC calculator (eAppendix). Each component was developed by a subgroup with the understanding that sites could incorporate variations based on local practices and needs.

The vancomycin AUC calculator was developed to be user-friendly and included safety validation protocols to prevent the entry of erroneous data (eg, unrealistic patient weight or laboratory values). The calculator allowed users to copy data into the electronic health record to avoid manual transcription errors and improve operational efficiency. It offered suggested volume of distribution estimates and 2 methods to estimate elimination constant (Ke ) depending on the patient’s weight.9,10 Creatinine clearance could be estimated using serum creatinine or cystatin C and considered amputation history. The default AUC goal in the calculator was 400 to 550 mcg*h/mL. This range was chosen based on consensus guidelines, data suggesting increased risk of AKI with AUCs > 515 mcg*h/mL, and the preference for conservative empiric dosing in the generally older VA population.11 The calculator suggested loading doses of about 25 mg/kg with a 2500 mg limit. VHA facilities could make limited modifications to the calculator based on local policies and procedures (eg, adjusting default infusion times or a dosing intervals).
The VISN 8 Pharmacy Pharmacokinetic Dosing Manual was developed as a comprehensive document to guide pharmacy staff with dosing vancomycin across diverse patient populations. This document included recommendations for renal function assessment, patient-specific considerations when choosing an empiric vancomycin dose, methods of ordering vancomycin peak, trough, and surveillance levels, dose determination based on 2 levels, and other clinical insights or frequently asked questions.
ASPWG members presented an accredited continuing education webinar for pharmacists, which reviewed the rationale for AUC-targeted dosing, changes to the current pharmacokinetic dosing program, case-based scenarios across various patient populations, and potential challenges associated with vancomycin AUC-based dosing. A recording of the live training was also made available. A vancomycin AUC dosing competency test was developed with 11 basic pharmacokinetic and case-based questions and comprehensive explanations provided for each answer.
VHA facilities implemented AUC dosing in a staggered manner, allowing for lessons learned at earlier adopters to be addressed proactively at later sites. The dosing calculator and education documents were updated iteratively as opportunities for improvement were discovered. ASPWG members held local office hours to address questions or concerns from staff at their facilities. Sharing standardized materials across the VISN reduced individual site workload and complications in rolling out this complex new process.
VISN-WIDE QUALITY ASSURANCE
At the time of project conception, 4 of 7 VISN 8 health systems had transitioned to AUC-based dosing. A quality assurance protocol to compare patient outcomes before and after changing to AUC dosing was developed. Each site followed local protocols for project approval and data were deidentified, collected, and aggregated for analysis.
The primary objectives were to compare the incidence of AKI and persistent bacteremia and assess rates of AUC target attainment (400-600 mcg*h/mL) in the AUC-based and trough-based dosing groups.6 Data for both groups included anthropomorphic measurements, serum creatinine, amputation status, vancomycin dosing, and infection characteristics. The X2 test was used for categorical data and the t test was used for continuous data. A 2-tailed α of 0.05 was used to determine significance. Each site sequentially reviewed all patients receiving ≥ 48 hours of intravenous vancomycin over a 3-month period and contributed up to 50 patients for each group. Due to staggered implementation, the study periods for sites spanned 2018 to 2023. A minimum 6-month washout period was observed between the trough and AUC groups at each site. Patients were excluded if pregnant, receiving renal replacement therapy, or presenting with AKI at the time of vancomycin initiation.
There were 168 patients in the AUC group and 172 patients in the trough group (Table 1). The rate of AUC target attainment with the initial dosing regimen varied across sites from 18% to 69% (mean, 48%). Total daily vancomycin exposure was lower in the AUC group compared with the trough group (2402 mg vs 2605 mg, respectively), with AUC-dosed patients being less likely to experience troughs level ≥ 15 or 20 mcg/mL (Table 2). There was a statistically significant lower rate of AKI in the AUC group: 2.4% in the AUC group (range, 2%-3%) vs 10.4% (range 7%-12%) in the trough group (P = .002). Rates of AKI were comparable to those observed in previous interventions.6 There was no statistical difference in length of stay, time to blood culture clearance, or rate of persistent bacteremia in the 2 groups, but these assessments were limited by sample size.


We did not anticipate such variability in initial target attainment across sites. The multisite quality assurance design allowed for qualitative evaluation of variability in dosing practices, which likely arose from sites and individual pharmacists having some flexibility in adjusting dosing tool parameters. Further analysis revealed that the facility with low initial target attainment was not routinely utilizing vancomycin loading doses. Sites routinely use robust loading doses achieved earlier and more consistent target attainment. Some sites used a narrower AUC target range in certain clinical scenarios (eg, > 500 mcg*h/mL for septic patients and < 500 mcg*h/mL for patients with less severe infections) rather than the 400 to 550 mcg*h/mL range for all patients. Sites targeting broader AUC ranges for all patients had higher rates of target attainment. Reviewing differences among sites allowed the ASPWG to identify best practices to optimize future care.
CONCLUSIONS
VHA ASPs must meet the standards outlined in VHA Directive 1031, including the new requirement for each VISN to develop an ASP collaborative. The VISN 8 ASPWG demonstrates how ASP champions can collaborate to solve common issues, complete tasks, explore new infectious diseases concepts, and impact large veteran populations. Furthermore, ASP collaboratives can harness their collective size to complete robust quality assurance evaluations that might otherwise be underpowered if completed at a single center. A limitation of the collaborative model is that a site with a robust ASP may already have specific practices in place. Expanding the ASP collaborative model further highlights the VHA role as a nationwide leader in ASP best practices.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Updated December 2019. Accessed September 10, 2024. https:// www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- US Department of Veterans Affairs. Antimicrobial stewardship programs. Updated September 22, 2023. Accessed September 13, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=11458
- US Department of Veterans Affairs, Veteran Health Administration. Veterans Integrated Service Networks (VISNs). Accessed September 13, 2024. https://www.va.gov/HEALTH/visns.asp
- US Department of Veterans Affairs. Veterans Health Administration, Veterans Integrated Service Networks, VISN 08. Updated September 10, 2024. Accessed September 13, 2024. https://department.va.gov/integrated-service-networks/visn-08/
- Andreev I. What is collaborative learning? Theory, examples of activities. Valamis. Updated July 10, 2024. Accessed September 10, 2024. https://www.valamis.com/hub/collaborative-learning
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
- Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycinassociated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi:10.1128/AAC.01293-17
- Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62(1):e01684-17. doi:10.1128/AAC.01684-17
- Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin-clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311-315.
- Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081-3086. doi:10.1093/jac/dky310
- Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycIn exposure and failure rates among adult hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis. 2020;70(8):1536-1545. doi:10.1093/cid/ciz460