User login
New consensus guide on rare drug hypersensitivity reaction
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
TOPLINE:
).
METHODOLOGY:
Data on the evaluation, assessment, and treatment of the rare but potentially life-threatening drug hypersensitivity reaction are lacking.
To support clinicians in diagnosing and managing DRESS, a steering committee conducted a literature review to examine current research, identify evidence, and develop consensus statements. They invited experts from 21 countries across four continents to participate in a Delphi consensus process.
An international panel of 54 experts (including 45 dermatologists) initially assessed 100 statements related to baseline workup, severity of the condition, and treatment. Two more statements were added in the second round.
After revisions and the second round, the group reached consensus for 93 statements overall.
TAKEAWAY:
The statements generating the most disagreement involved diagnosis. The group ultimately supported the value of measuring the viral load of Epstein-Barr virus, cytomegalovirus, and human herpesvirus 6 in all patients with suspected DRESS. The group also agreed on screening for hepatitis A, B, and C in cases of liver involvement and screening for hepatitis B and C before starting systemic therapy.
The group agreed with previous severity criteria that differentiate between mild, moderate, and severe DRESS based on the extent of liver, kidney, and blood involvement and the damage of other organs.
Consensus on treatment was reached for all 12 relevant statements in the first Delphi round. Recommendations included the use of corticosteroids and immediate discontinuation of the drugs causing the reaction.
IN PRACTICE:
“This Delphi exercise aimed to provide a common ground of consensus,” the authors noted. However, “each of the addressed categories needs more in-depth follow-up studies to improve the clinical management of patients.”
SOURCE:
The DRESS Delphi consensus group conducted its exercise under the leadership of Marie-Charlotte Brüggen, MD, of the University Hospital of Zürich. The consensus was published online in the JAMA Dermatology.
LIMITATIONS:
Published evidence was limited because of the low prevalence of DRESS. The consensus statements should therefore be considered with caution and in the context of a clinician’s expertise and available resources. Research gaps also persist in how DRESS may vary with region and ethnicity. The severity thresholds need validation in a revised multicenter statement.
DISCLOSURES:
The consensus review received no outside funding. Dr. Brüggen disclosed relationships with the Swiss National Science Foundation, Christine Kühne – Center for Allergy Research and Education, FreeNovation, LEO Foundation, Olga Mayenfisch Foundation, University of Zürich, LEO Pharma, Pierre Fabre Eczema Foundation, Eli Lilly, AbbVie, GSK, and AstraZeneca. Coauthors disclosed relationships with multiple pharmaceutical companies, foundations, and medical publishing companies.
A version of this article appeared on Medscape.com.
More phase 3 data support use of nemolizumab for prurigo nodularis
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Birch bark–derived treatment reduces daily dressings in patients with epidermolysis bullosa
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
FROM THE EADV CONGRESS
Novel hydrogel holds promise for skin regeneration
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
AT CALDERM 2023
Hospital Dermatology: Review of Research in 2022-2023
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
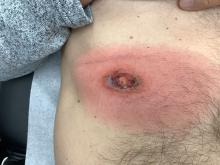
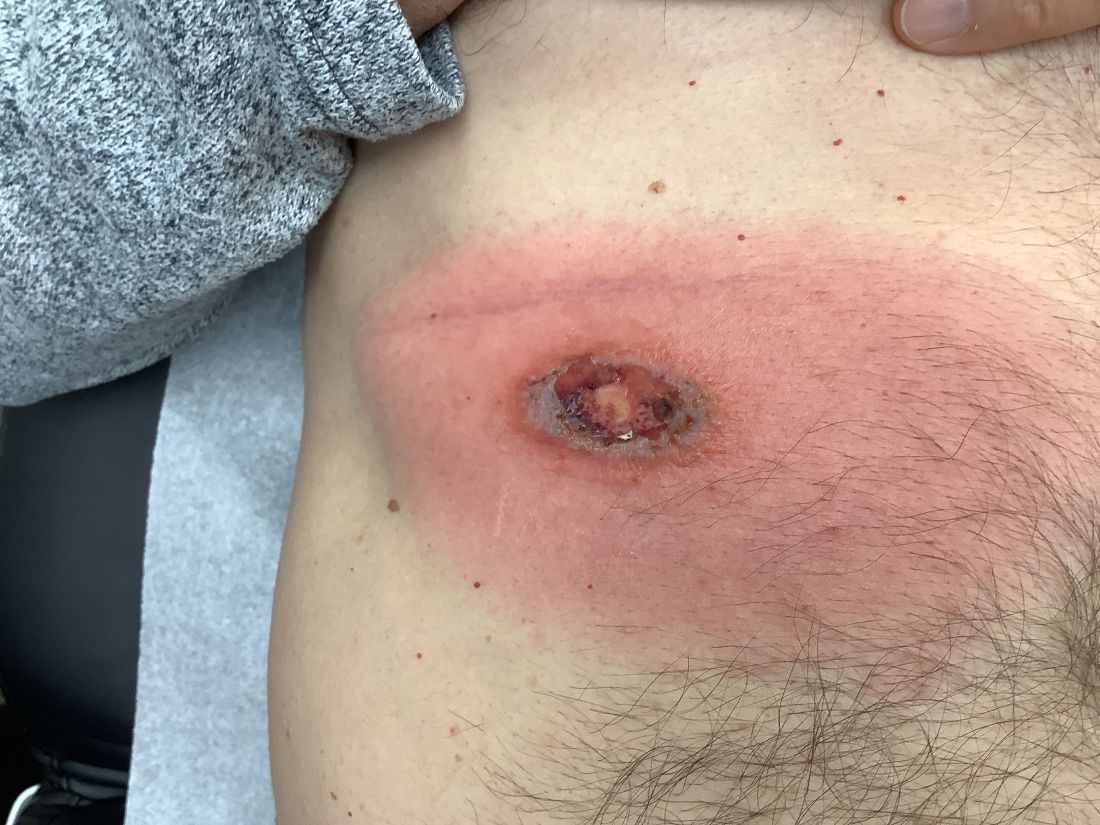
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Practice Points
- A severe hypersensitivity reaction to trimethoprim-sulfamethoxazole—sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes (SCoRCH)—has been described.
- Patients experiencing cutaneous reactions to immune checkpoint inhibitors have improved progression-free and overall survival rates if evaluated by a dermatologist who can optimize skin-directed and targeted therapies.
- Interventions, including shorter time to dermatology outpatient follow-up, are needed to reduce emergency department utilization by patients with hidradenitis suppurativa.
- Asynchronous store-and-forward dermatology e-consultation is effective for immunobullous diseases, vasculitis, herpes zoster, and cellulitis, demonstrating the utility of teledermatology in the inpatient setting, particularly when standardized data capture tools are used.
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.
What’s Eating You? Noble False Widow Spider (Steatoda nobilis)
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
PRACTICE POINTS
- With evidence of a recent population boom of noble false widow spiders in Europe and spread to California, dermatologists should be aware of these spiders and their bites.
- Symptoms of Steatoda bites (steatodism) include immediate pain followed by intense pruritus, swelling, erythema, and possibly systemic symptoms such as fever. Secondary infections such as cellulitis and septicemia are risks.
- The envenomation site should be kept clean to prevent secondary infection, and medical care should be sought when there is evidence of ulceration or cellulitis.
Can zoo poo help manage diabetic foot ulcers?
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Hyperbaric oxygen therapy beneficial for calciphylaxis?
, report Daniela Kroshinsky, MD, MPH, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues.
Although intravenous sodium thiosulfate (IV STS) is considered standard care in the treatment of calciphylaxis, HBOT has been reported to have beneficial effects, they noted.
In their study, the researchers retrospectively reviewed records of 93 patients newly diagnosed with calciphylaxis, seen at Massachusetts General Hospital, between January 2006 and December 2021. They compared mortality and wound healing outcomes for 57 patients treated with IV STS only (control group) with those of 36 patients treated with HBOT plus IV STS (treatment group). Traditional survival analyses and Cox proportional hazard modeling were used to examine mortality data, and mixed effects modeling was used to analyze longitudinal wound outcomes. The study was published in the Journal of the American Academy of Dermatology.
Univariate survival analyses showed that HBOT plus IV STS was associated with significantly longer survival time than IV STS alone (P = .016), particularly for those with nonnephrogenic calciphylaxis (P < .0001), they report. An increased number of HBOT sessions conferred improved mortality outcomes, with 1, 5, 10, and 20 sessions yielding decreasing hazard ratios.
There was also a significant positive association between an increasing number of HBOT sessions and increased wound score (P = .042). Increases were seen with each session.
Anxiety/claustrophobia was the most common side effect reported among those in the HBOT group (22%).
“Given the proposed benefits and seemingly low side effect profile, it is the authors’ recommendation that HBOT be offered as an additional intervention to patients with calciphylaxis, especially if they have open wounds, to improve outcomes and expedite wound healing,” the researchers concluded.
Limitations, they noted, included the small sample size, retrospective design, and the potential for not adequately capturing patients who received external care. They were also unable to match patients by disease or wound severity. Large prospective trials would help clarify the role of HBOT for calciphylaxis, they added.
The researchers reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
, report Daniela Kroshinsky, MD, MPH, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues.
Although intravenous sodium thiosulfate (IV STS) is considered standard care in the treatment of calciphylaxis, HBOT has been reported to have beneficial effects, they noted.
In their study, the researchers retrospectively reviewed records of 93 patients newly diagnosed with calciphylaxis, seen at Massachusetts General Hospital, between January 2006 and December 2021. They compared mortality and wound healing outcomes for 57 patients treated with IV STS only (control group) with those of 36 patients treated with HBOT plus IV STS (treatment group). Traditional survival analyses and Cox proportional hazard modeling were used to examine mortality data, and mixed effects modeling was used to analyze longitudinal wound outcomes. The study was published in the Journal of the American Academy of Dermatology.
Univariate survival analyses showed that HBOT plus IV STS was associated with significantly longer survival time than IV STS alone (P = .016), particularly for those with nonnephrogenic calciphylaxis (P < .0001), they report. An increased number of HBOT sessions conferred improved mortality outcomes, with 1, 5, 10, and 20 sessions yielding decreasing hazard ratios.
There was also a significant positive association between an increasing number of HBOT sessions and increased wound score (P = .042). Increases were seen with each session.
Anxiety/claustrophobia was the most common side effect reported among those in the HBOT group (22%).
“Given the proposed benefits and seemingly low side effect profile, it is the authors’ recommendation that HBOT be offered as an additional intervention to patients with calciphylaxis, especially if they have open wounds, to improve outcomes and expedite wound healing,” the researchers concluded.
Limitations, they noted, included the small sample size, retrospective design, and the potential for not adequately capturing patients who received external care. They were also unable to match patients by disease or wound severity. Large prospective trials would help clarify the role of HBOT for calciphylaxis, they added.
The researchers reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
, report Daniela Kroshinsky, MD, MPH, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues.
Although intravenous sodium thiosulfate (IV STS) is considered standard care in the treatment of calciphylaxis, HBOT has been reported to have beneficial effects, they noted.
In their study, the researchers retrospectively reviewed records of 93 patients newly diagnosed with calciphylaxis, seen at Massachusetts General Hospital, between January 2006 and December 2021. They compared mortality and wound healing outcomes for 57 patients treated with IV STS only (control group) with those of 36 patients treated with HBOT plus IV STS (treatment group). Traditional survival analyses and Cox proportional hazard modeling were used to examine mortality data, and mixed effects modeling was used to analyze longitudinal wound outcomes. The study was published in the Journal of the American Academy of Dermatology.
Univariate survival analyses showed that HBOT plus IV STS was associated with significantly longer survival time than IV STS alone (P = .016), particularly for those with nonnephrogenic calciphylaxis (P < .0001), they report. An increased number of HBOT sessions conferred improved mortality outcomes, with 1, 5, 10, and 20 sessions yielding decreasing hazard ratios.
There was also a significant positive association between an increasing number of HBOT sessions and increased wound score (P = .042). Increases were seen with each session.
Anxiety/claustrophobia was the most common side effect reported among those in the HBOT group (22%).
“Given the proposed benefits and seemingly low side effect profile, it is the authors’ recommendation that HBOT be offered as an additional intervention to patients with calciphylaxis, especially if they have open wounds, to improve outcomes and expedite wound healing,” the researchers concluded.
Limitations, they noted, included the small sample size, retrospective design, and the potential for not adequately capturing patients who received external care. They were also unable to match patients by disease or wound severity. Large prospective trials would help clarify the role of HBOT for calciphylaxis, they added.
The researchers reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.