User login
Early Treatment of Lyme Disease Prompted by Histopathologic Analysis of the Abdomen of an Engorged Tick
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
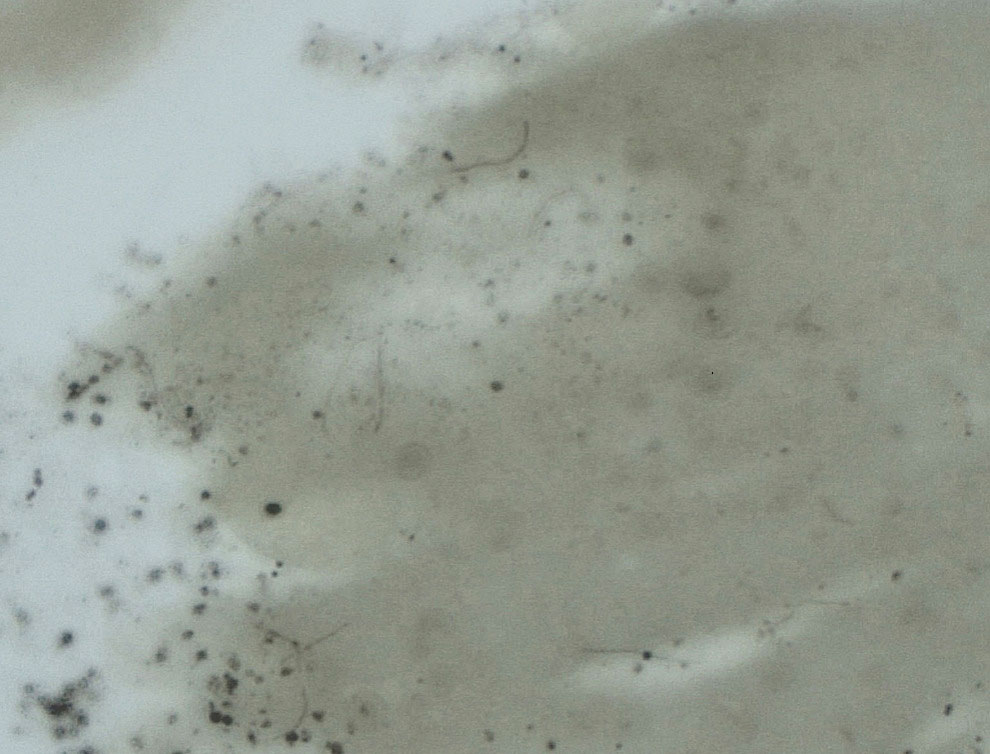
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.
Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
PRACTICE POINTS
- Lyme disease is increasingly common in the United States.
- Lyme disease can cause debilitating sequelae if left untreated, including arthritis, neurologic deficits, and heart block.
- Diagnostic methods for identifying early Lyme disease have limited sensitivity and specificity, necessitating alternative strategies for making an accurate diagnosis and initiating treatment.
Palifermin-Associated Cutaneous Papular Rash of the Head and Neck
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
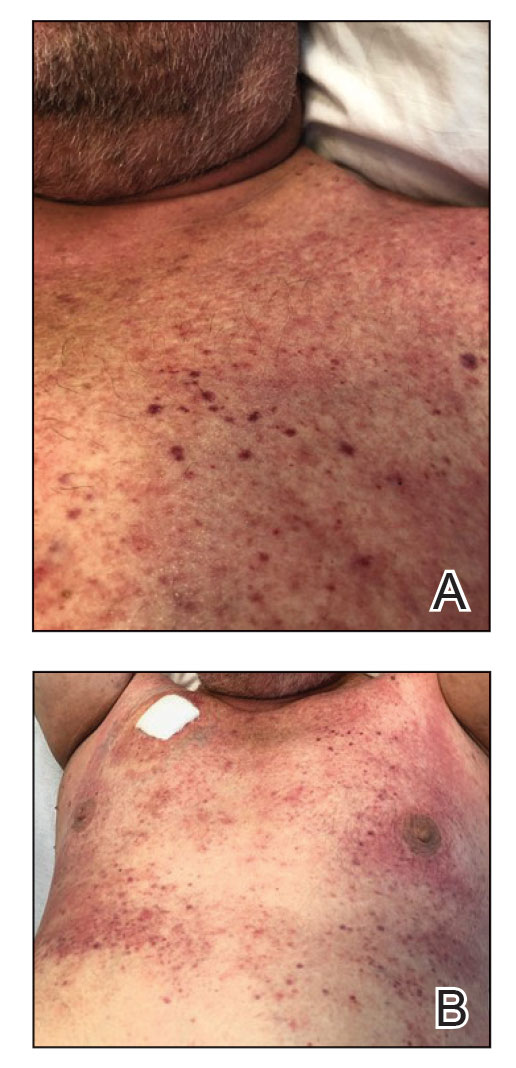
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.
Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
Practice Points
- Palifermin is a recombinant keratinocyte growth factor that is US Food and Drug Administration approved to prevent oral mucositis in patients undergoing chemotherapy or radiation therapy.
- Histologically, the rash can resemble verrucae with evidence of hypergranulosis, hyperorthokeratosis, and papillomatosis.
- Cutaneous reactions have been reported with use of palifermin and generally are benign and self-limited with removal of the offending agent.

