User login
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.
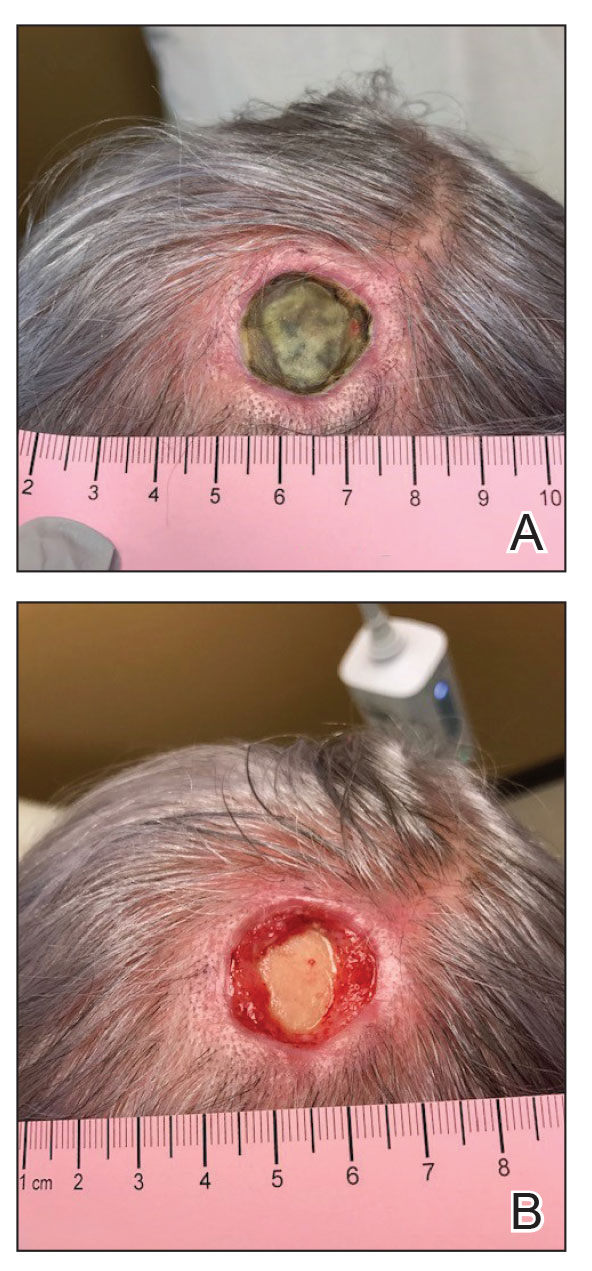
A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
Practice Points
- Patients who undergo Mohs micrographic surgery on the scalp are prone to developing complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.
- Use of extracellular matrix–based dressings may assist with deep wound healing on the scalp.
![An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_300x300.jpg)