User login
New AI-enhanced bandages poised to transform wound treatment
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
Skin has different daytime and nighttime needs, emerging circadian research suggests
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO –
“Paying attention to the circadian rhythm of the skin is every bit as important as moisturizing the skin,” Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “It is paramount to both your morning and evening skin regimen routine,” she added.
Circadian rhythms are physical, mental, and behavioral changes that follow a 24-hour cycle. “These natural processes respond primarily to light and dark and affect most living things, including animals, plants, and microbes,” she said. “The circadian system is composed of peripheral circadian oscillators in many other cells, including the skin.”
The science has been around awhile, but dermatologists didn’t understand its impact until recently, she said.
In 1729, the French astronomer Jean-Jacques d’Ortous de Mairan demonstrated that mimosa leaves, which open at dawn and close at dusk, continued this cycle even when kept in darkness. In the 1970s, Seymour Benzer and Ronald Konopka showed that mutations in an unknown gene disrupted the circadian clock of fruit flies.
And in 2017, the Nobel Prize in Physiology or Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for discovering molecular mechanisms that control circadian rhythm. Using fruit flies as a model, they isolated a gene that controls the normal daily biological rhythm.
“They showed that this gene encodes a protein that accumulates in the cell during the night and is then degraded during the day, and they identified additional protein components, exposing the mechanism governing the self-sustaining clockwork inside the cell,” said Dr. Shamban.
In humans and other mammals, the primary body clock is located in the suprachiasmatic nucleus, a cluster of approximately 10,000 neurons located on either side of the midline above the optic chiasma, about 3 cm behind the eyes. Several clock genes have been identified that regulate and control transcription and translation.
“Expression of these core clock genes inside the cell influences many signaling pathways, which allows the cells to identify the time of day and perform their appropriate function,” Dr. Shamban said. “Furthermore, phosphorylation of core clock proteins leads to degradation to keep the 24-hour cycle in sync.”
Photoreceptive molecules known as opsins also appear to play a role in regulating the skin’s clock. A systematic review of 22 articles published in 2020 found that opsins are present in keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells, and they have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging in human and nonhuman species.
“You may wonder, why does the skin respond so nicely to light?” Dr. Shamban said. “Because it contains opsins, and light exposure through opsin-regulated pathways stimulates melanin production.”
Patients can support their skin’s clock genes by understanding that skin barrier functions such as photoprotection and sebum production are increased during the day, while skin permeability processes such as DNA repair, cell proliferation, and blood flow are enhanced at night.
“Your skin has different daytime and nighttime needs,” Dr. Shamban commented. “Simply put, daytime is defense, and nighttime is offense. I think we’ve known this intuitively, but to know that there is science supporting this idea is important.”
Dr. Shamban wrote the book “Heal Your Skin: The Breakthrough Plan for Renewal” (Wiley, 2011). She disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2023
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2
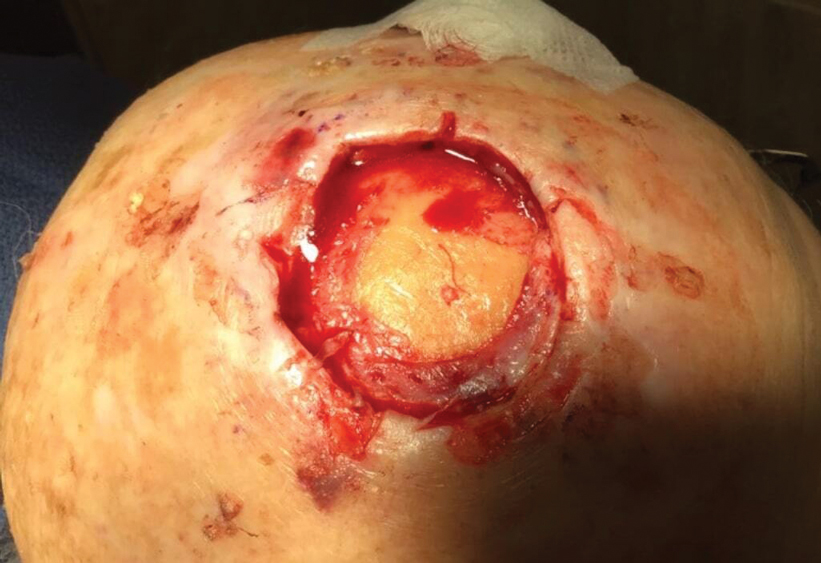
Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).
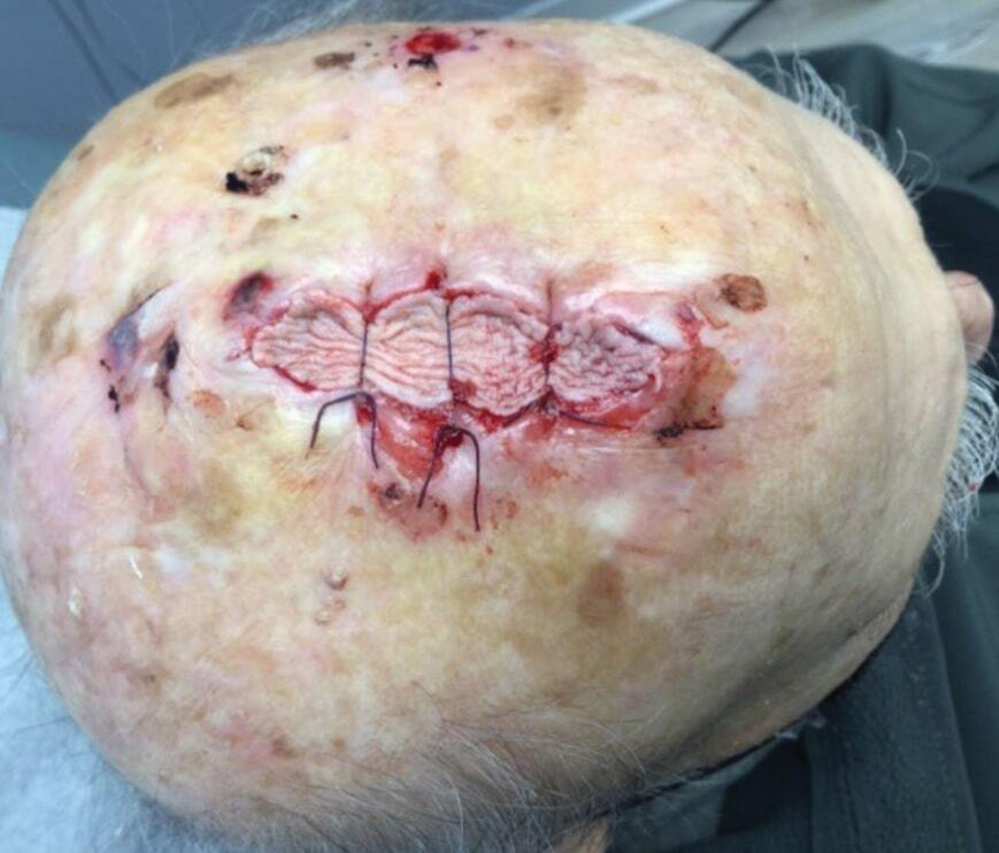
Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.
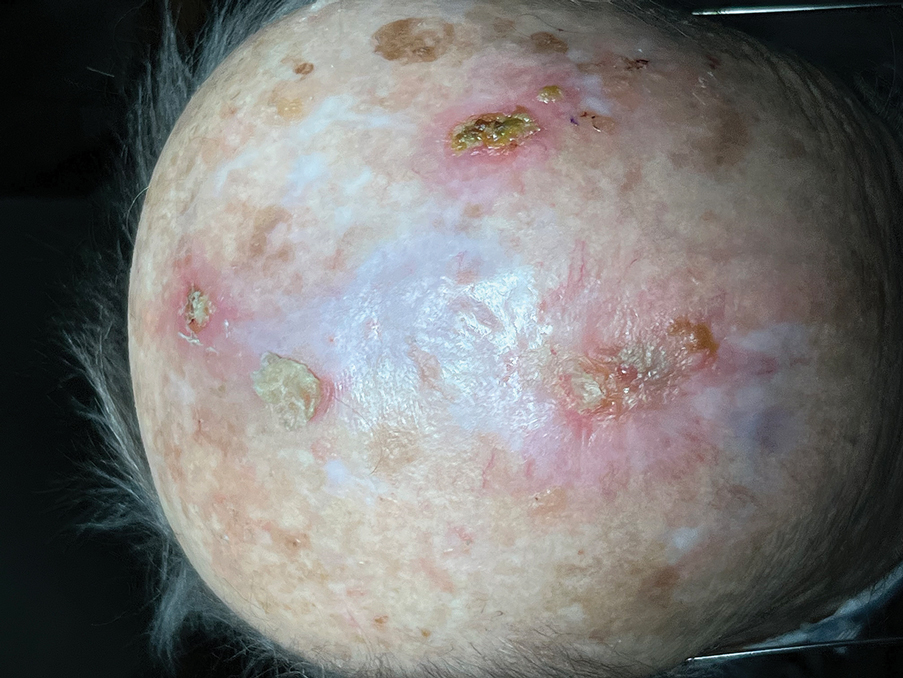
Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
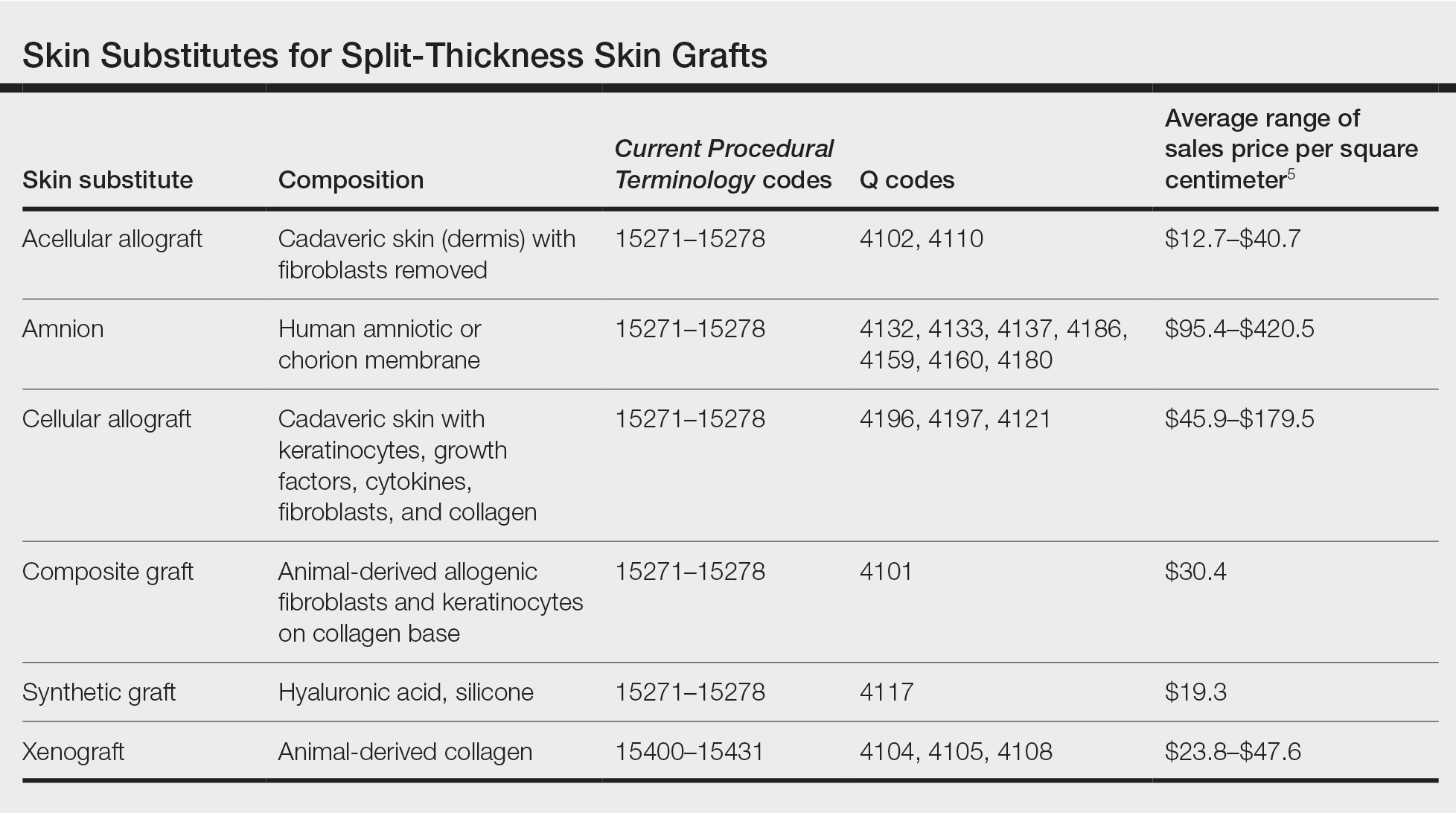
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).
Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
A step forward in diabetic foot disease management
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Palifermin-Associated Cutaneous Papular Rash of the Head and Neck
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.
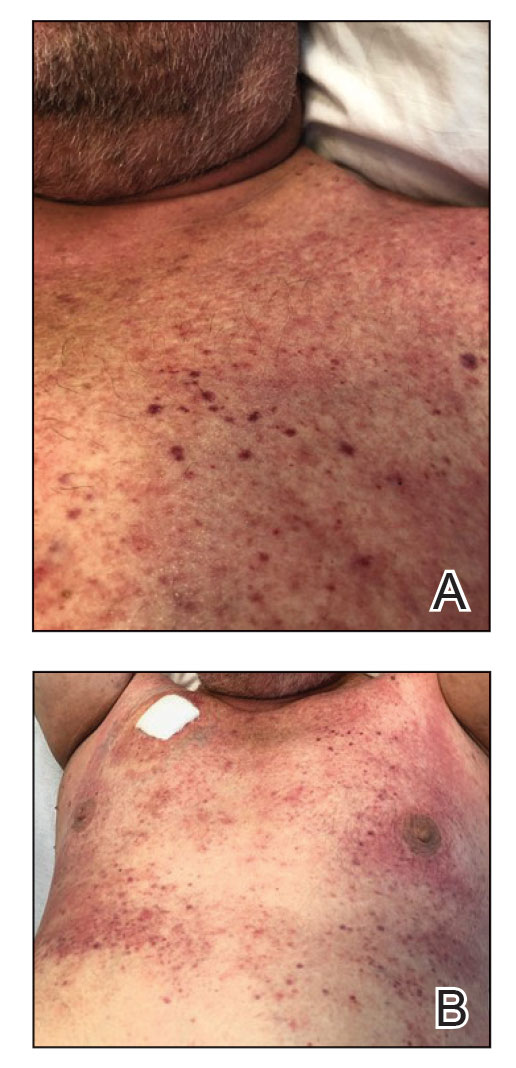
Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
Practice Points
- Palifermin is a recombinant keratinocyte growth factor that is US Food and Drug Administration approved to prevent oral mucositis in patients undergoing chemotherapy or radiation therapy.
- Histologically, the rash can resemble verrucae with evidence of hypergranulosis, hyperorthokeratosis, and papillomatosis.
- Cutaneous reactions have been reported with use of palifermin and generally are benign and self-limited with removal of the offending agent.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
‘New standard of care’ for capecitabine hand-foot syndrome
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers reported in a study that has been hailed by experts as “practice changing.”
Hand-foot syndrome causes painful, bleeding blisters and ulcers on the palms and soles. It often leads to dose reductions and sometimes even discontinuations, both of which limit the effectiveness of capecitabine, a standard oral chemotherapy drug widely used for colorectal and breast cancers.
In a new study presented at the annual meeting of the American Society of Clinical Oncology, Indian researchers reported that a cheap, safe, and widely available over-the-counter nonsteroidal anti-inflammatory gel containing 1% diclofenac reduced the incidence of hand-foot syndrome by 75% among patients with cancer being treated with capecitabine.
Up until now, the oral anti-inflammatory celecoxib (Celebrex) was the only agent proven to prevent the problem, but it’s rarely used because of the risk for strokes, gastric bleeding, and other issues, none of which are a concern with topical diclofenac, which osteoarthritis patients have used safely for years.
The Indian trial, dubbed D-Torch, establishes “1% topical diclofenac gel as the new standard of care to prevent capecitabine-associated hand-foot syndrome,” said investigator and study presenter Atul Batra, MD, a medical oncologist at the All India Institute of Medical Sciences, New Delhi.
Dr. Batra told ASCO Daily News that there is no need for a second trial. “We don’t feel there’s a need to replicate these results” in a larger study “because this was adequately powered, and the results speak for themselves. There’s no confusion about these results. Diclofenac is clearly effective.”
Dr. Batra also commented that his clinic now uses topical diclofenac routinely during capecitabine treatment and that he hopes oncology practices elsewhere will do the same.
Diclofenac gel is sold under the brand name Voltaren and is also available as a generic; in the United States, a 150-gram tube costs about $18 at Walmart.
‘The most practice-changing study’ at ASCO 2023
Audience members at ASCO’s annual meeting immediately saw the importance of the study.
Tarah Ballinger, MD, a breast cancer specialist at Indiana University, Indianapolis, said on Twitter that “this might be the most practice changing study I heard at ASCO23.” Topical diclofenac is “widely available, affordable, [and] addresses [a] major” quality of life issue.
The study discussant at the meeting, gastrointestinal cancer specialist Pallavi Kumar, MD, of the University of Pennsylvania, Philadelphia, concurred: “For me as a GI oncologist, topical diclofenac for prevention of HFS for patients on capecitabine is practice changing,” she said.
The takeaway is “that topical diclofenac significantly reduces the incidence of grade 2 or higher HFS in patients receiving capecitabine.” The results are “very impressive,” Dr. Kumar said.
Study details
The idea for the new study came after Batra and colleagues realized that celecoxib, a COX-2 enzyme inhibitor, helps prevent capecitabine hand-foot syndrome (HFS) by blocking a key process that leads to it, the up-regulation of COX-2 and subsequent release of proinflammatory prostaglandins.
They turned to diclofenac gel hoping to get the same effect but more safely; diclofenac is also a COX-2 blocker, and its topical formulation has a strong safety record.
To test the approach, the team randomly assigned 130 patients to topical diclofenac and 133 to placebo – the gel vehicle without the medication – while they were being treated with capecitabine for 12 weeks; 56% were being treated for breast cancer and the rest for gastrointestinal cancers.
Subjects rubbed one fingertip’s worth of gel – about half a gram – on each palm and the back of each hand twice a day. The dose was about 4 grams/day, which is well below maximal dosages for osteoarthritis (up to 32 g/day over all affected joints). Adherence to treatment was about 95% in both arms.
By the end of 12 weeks, the incidence of grade 2 or higher HFS was 3.8% in the diclofenac arm (5 patients) versus 15% (n = 20) with placebo (P = .003), a 75% risk reduction.
The incidence of any grade HFS was 6.1% in the treatment group versus 18.1% with placebo (P = .003).
Hand-foot syndrome led to dose reductions of capecitabine in 13.5% of placebo but only 3.8% of those in the diclofenac group (P = .002).
The findings held regardless of whether patients were being treated for breast or GI cancer or if they were men or women.
Other capecitabine-induced adverse events, including diarrhea, mucositis, and myelosuppression, were not significantly different between the groups.
The treatment arms were well balanced, with a median age of 47 years in both groups and women making up about 70% of each. About 40% of subjects in each group were on capecitabine monotherapy with the rest on combination treatments. The mean dose of capecitabine was just over 1,880 mg/m2 in both groups.
At the meeting, Dr. Batra was asked if topical diclofenac would also work for another common problem in oncology: hand-food syndrome occurring as a side-effect with VEGF–tyrosine kinase inhibitors. He didn’t think so because it probably has a different cause than capecitabine HFS, one not strongly related to COX-2 up-regulation.
The study was partly funded by the Indian Supportive Care of Cancer Association. The investigators reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ASCO 2023
Extracellular Matrix–Based Collagen Dressings for Scalp Repair Following Mohs Micrographic Surgery
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.
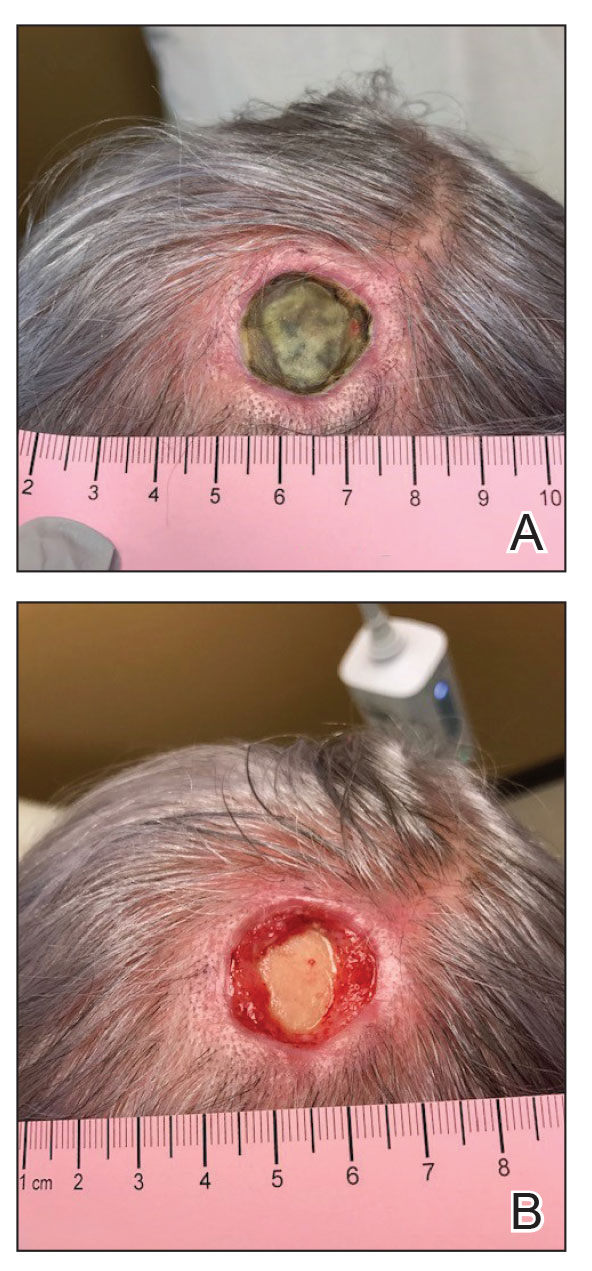
A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
To the Editor:
Squamous cell carcinoma (SCC) is the second most common cancer of the scalp.1 Mohs micrographic surgery is used to treat SCC, and it commonly generates a 2.5×2.5-cm open wound with exposed bone.2 Although Mohs micrographic surgery effectively treats cutaneous lesions, it carries a high risk for complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.3 Recommended therapies to decrease these complications include linear closures, flaps, and peripheral autograft tissue.4 However, these procedures do not come without risks and carry their own complications. Therefore, we suggest a safe, less-invasive initial approach using a synthetic extracellular matrix (ECM)–based collagen dressing for secondary wound closure.

A 76-year-old woman presented to the infectious disease clinic at Monument Health Rapid City Clinic (Rapid City, South Dakota) for evaluation of a dehisced scalp wound 3 months following Mohs micrographic surgery for scalp SCC. The wound underwent primary closure following surgery and dehisced shortly after (Figure 1A). Various oral antimicrobials were used by the dermatologist to assist with wound closure but without success. The patient was referred to the wound clinic for management. At the first appointment, all necrotic tissue was debrided and the cranium was exposed in the wound base (Figure 1B). The wound measured 2.3×2.3×0.2 cm. An ECM-containing collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was used to provide a scaffold for wound closure (Figure 2A). It was dressed with the petroleum-based gauze Xeroform (Cardinal Health) and covered with dry gauze to prevent evaporation and provide moist wound healing. The wound developed some budding tissue islands 3 weeks after weekly ECM-based collagen dressing applications (Figure 3A). The wound continued to decrease in size and formed an isthmus by the second month of therapy (Figure 3B). The wound fully closed within 3 months and showed minimal scarring after 3 years (Figure 2B).
![A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure. A, An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. B, The wound showed minimal scarring 3 years after closure.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig2_AB.jpg)
Chronic wounds usually get trapped in the inflammatory stage of wound healing due to destruction of growth factors and ECM by metalloproteases (MMPs), which creates a vicious cycle and wound stalling. Wound debridement converts a chronic wound back into an acute wound, which is the first step of healing. Following wound debridement, collagen-based dressings can assist with healing by binding the destructive MMPs, and ECM matrix promotes the building of new tissue. The 3 most commonly used ECM-based collagen dressings are Endoform, PuraPly AM (Organogenesis Inc), and Puracol Ultra ECM (Medline Industries, Inc).
![A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application A, Budding tissue islands developed on a scalp wound 3 weeks after application of an extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]). B, An isthmus developed 7 weeks after application](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_Fig3_AB.jpg)
Endoform is ovine-based collagen and provides a natural porous bioscaffold for rapid cell infiltration.5 It contains more than 150 ECM proteins along with residual vascular channels that help re-establish new vasculature. Ovine-based collagen contains collagen types I, III, and IV arranged as native fibers that retain the 3-dimensional architecture present in tissue ECM.5 Although MMPs are essential in normal healing, the elevated presence of MMPs has been linked to stalled wound healing. Clinical observation and assessment may not be sufficient to identify a wound with elevated protease activity that can break down ECM, affect wound fibroblasts, and impair growth factor response. Although collagen ECM itself does not contain any growth factors, it preserves the destruction of native ECM and growth factors by MMPs by functioning as a sacrificial substrate. The addition of 0.3% ionic silver to the ECM has been shown to decrease bacterial growth and prevent biofilm formation.6
PuraPly AM is a native, type I porcine collagen matrix embedded with the
Puracol Ultra ECM is made of porcine mesothelium and is comprised of types I, III, and IV collagens; elastin; fibronectin; laminin; and proteoglycans. It also contains fibroblast growth factors, contributing to angiogenesis in the wound.9
Application of ECM-based collagen dressings on debrided wounds requires moisture for absorption. Because cranium wounds lack sufficient exudate production, dermal templates need to be hydrated with sterile normal saline before application and covered with a moisture-retaining dressing. Extracellular matrix–based dressings are biodegradable and can be reapplied every 5 to 7 days. For chronic wounds, application of collagen dressings, such as Endoform, is essential and could be considered as the first step prior to switching to more advanced wound care modalities.6,10 Additional studies investigating ECM-containing may determine their comparative efficacy.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491-508. doi:10.1007/s40257-016-0207-3
- Kimyai-Asadi A, Goldberg LH, Peterson SR, et al. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628-634. doi:10.1016/j.jaad.2005.03.023
- Merritt BG, Lee NY, Brodland DG, et al. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol. 2012;67:1302-1309. doi:10.1016/j.jaad.2012.05.041
- Yu WY, Salmon P, Thuener J, et al. Mohs surgery for advanced tumors of the scalp. Dermatol Surg. 2019;45(suppl 2):S110-S117.
- Endoform. Aroa Biosurgery Limited website. Accessed May 22, 2023. https://aroa.com/product/endoform/
- Liden BA, May BC. Clinical outcomes following the use of ovine forestomach matrix (endoform dermal template) to treat chronic wounds. Adv Skin Wound Care. 2013;26:164-167. doi:10.1097/01.ASW.0000428862.34294.d4
- PuraPly AM. Organogenesis website. Accessed May 22, 2023. https://organogenesis.com/surgical-sports-medicine/puraplyam/
- Bain MA, Koullias GJ, Morse K, et al. Type I collagen matrix plus polyhexamethylene biguanide antimicrobial for the treatment of cutaneous wounds. J Comp Eff Res. 2020;9:691-703. doi:10.2217/cer-2020-0058
- Puracol Ultra ECM Collagen Wound Dressings. Medical Industries, LP website. May 22, 2023. https://punchout.medline.com/product/Puracol-Ultra-Extracellular-Matrix-ECM-Collagen-Wound-Dressing/Collagen-Dressings/Z05-PF188619?question=&index=P4&indexCount=4
- Raizman R, Hill R, Woo K. Prospective multicenter evaluation of an advanced extracellular matrix for wound management. Adv Skin Wound Care. 2020;33:437-444. doi:10.1097/01.ASW.0000667052.74087.d6
Practice Points
- Patients who undergo Mohs micrographic surgery on the scalp are prone to developing complications such as infection, wound dehiscence, and partial or full-thickness skin graft necrosis.
- Use of extracellular matrix–based dressings may assist with deep wound healing on the scalp.
Chondrodermatitis Nodularis Helicis After Mohs Micrographic Surgery and Radiation Therapy
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.
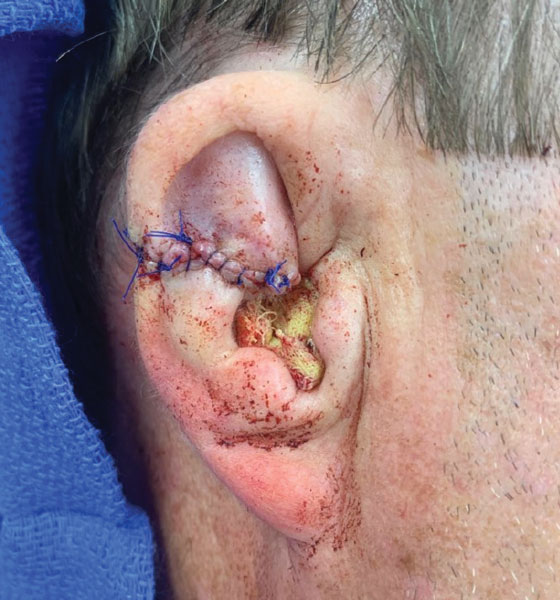
A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.
Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).
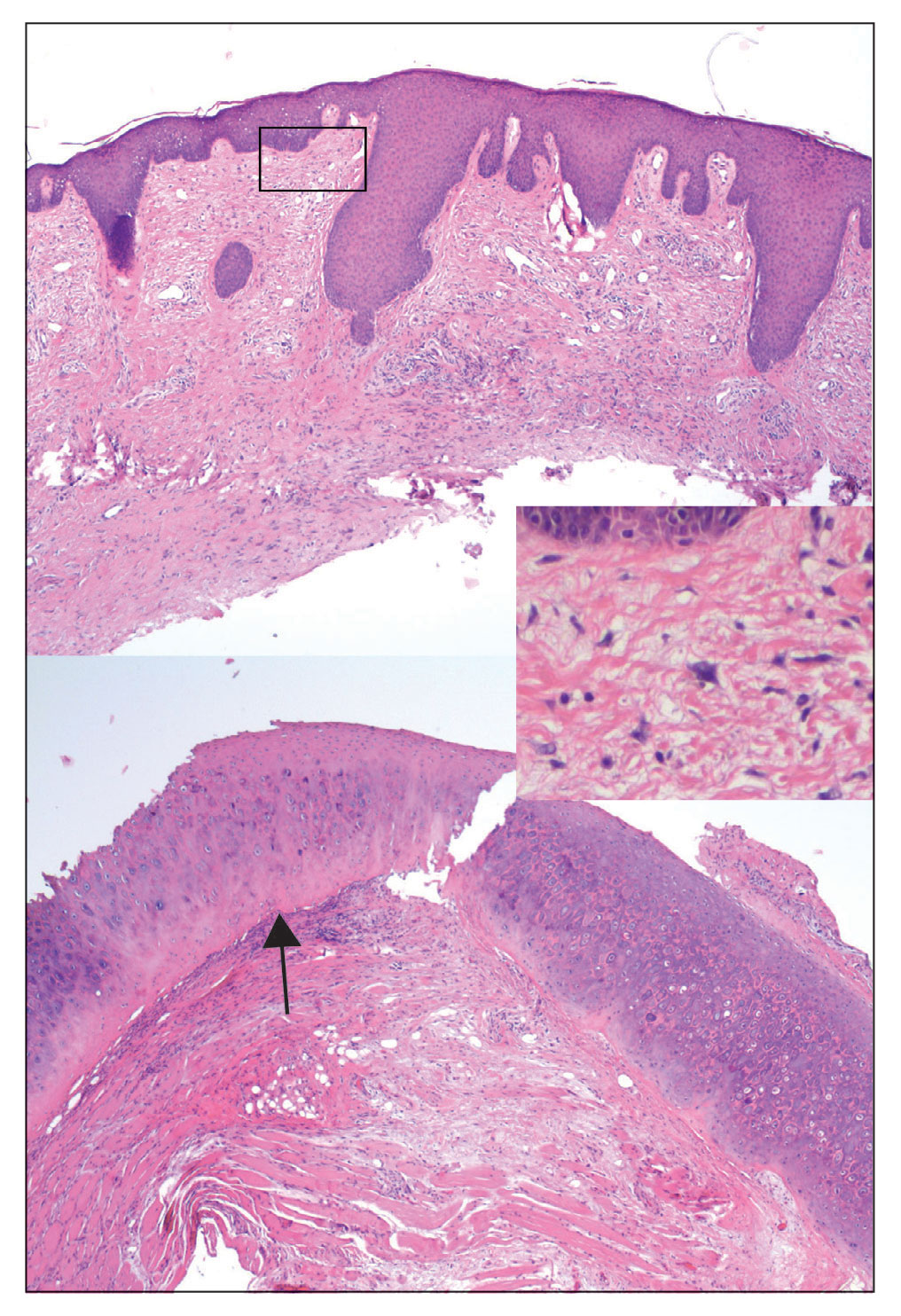
Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
Practice Points
- Although chondrodermatitis nodularis helicis (CNH) is benign by nature, it can mimic tumor recurrence when it presents close to the site of prior Mohs micrographic surgery (MMS). Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Skin lesions in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis.
Beta-blocker gel shows promise for diabetic foot ulcers
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN


![An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound. An extracellular matrix–based collagen dressing (Endoform Natural Restorative Bioscaffold [Aroa Biosurgery Inc]) was applied to the wound.](https://cdn.mdedge.com/files/s3fs-public/CT111005033_e_300x300.jpg)
