User login
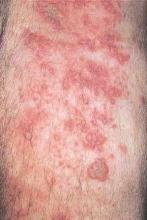
Biologic treatment in pregnancy requires balancing risks
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at obnews@frontlinemedcom.com.
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at obnews@frontlinemedcom.com.
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at obnews@frontlinemedcom.com.
ACR: Push back when insurance decisions force TNFi change
SAN FRANCISCO – Stopping a tumor necrosis factor inhibitor for nonmedical reasons is associated with significantly worse clinical outcomes and increased health care resource use, according to a review of 166 rheumatoid arthritis patients from the Physicians Consulting Network.
That’s probably not a surprise; the main value of the findings is that they provide ammunition to push back against insurance decisions that lead to stopping or switching tumor necrosis factor inhibitors (TNFi) that are helping patients.
After being stable on their TNFi for at least 6 months, 25 patients in the study were forced to stop their medication for a range of nonmedical economic reasons such as increased copays, change of insurance, or loss of job health insurance. Another 58 switched to a new TNFi for similar reasons. Those 83 patients were matched to 83 others who were also stable on their TNFi for at least 6 months but continued the therapy. Data came from chart reviews by rheumatologists, and all the patients were under the care of physicians participating in the Physicians Consulting Network, which provides feedback to GfK, a market research firm.
Over the next year, 48% of switchers/discontinuers were deemed to have well controlled RA by their rheumatologists, versus 84% of continuers. Switchers and discontinuers were almost 4 times as likely to flare, and more than 10 times as likely to have severe RA at the end of the year.
Switchers/discontinuers also had more inpatient days and emergency department and urgent care visits. Switchers and discontinuers had more than six times greater odds of visiting emergency departments and urgent care clinics at least once, compared with continuers. The differences were statistically significant. In short, payers may have saved on the front end, but probably lost on the back end.
“These real world data demonstrate that patients are significantly affected by switching or discontinuing their stable and effective [TNFi] therapy for rheumatoid arthritis. I don’t think there is any patient who asks to have their [TNFi] switched when they are doing well, and it would be uncommon for a rheumatologist to switch or discontinue [a TNFi] when patients are doing well. These are insurance-driven things that are impacting negatively on patient care, and this should stop. The [American College of Rheumatology] and other groups need to work together to get insurance companies to stop intervening in this way,” said investigator Dr. Douglas Wolf, medical director at Atlanta Gastroenterology Associates.
Similar problems have been reported when TNFi drugs are switched or stopped in Crohn’s disease, psoriasis, and other immunologic conditions, he said at the annual meeting of the American College of Rheumatology.
Switchers and discontinuers were more likely than were continuers to be Hispanic (27.7% vs. 15.7%; P = .041), but otherwise there were no significant differences between cohorts in baseline sociodemographic and disease characteristics, comorbidities, medication use, or resource utilization.
The investigators plan to continue the project to see if TNFi switchers fair better than discontinuers.
Dr. Wolf and other investigators disclosed relationships with AbbVie, including some who received payments from the company to participate in the research. Three authors are employees of AbbVie, maker of adalimumab (Humira).
SAN FRANCISCO – Stopping a tumor necrosis factor inhibitor for nonmedical reasons is associated with significantly worse clinical outcomes and increased health care resource use, according to a review of 166 rheumatoid arthritis patients from the Physicians Consulting Network.
That’s probably not a surprise; the main value of the findings is that they provide ammunition to push back against insurance decisions that lead to stopping or switching tumor necrosis factor inhibitors (TNFi) that are helping patients.
After being stable on their TNFi for at least 6 months, 25 patients in the study were forced to stop their medication for a range of nonmedical economic reasons such as increased copays, change of insurance, or loss of job health insurance. Another 58 switched to a new TNFi for similar reasons. Those 83 patients were matched to 83 others who were also stable on their TNFi for at least 6 months but continued the therapy. Data came from chart reviews by rheumatologists, and all the patients were under the care of physicians participating in the Physicians Consulting Network, which provides feedback to GfK, a market research firm.
Over the next year, 48% of switchers/discontinuers were deemed to have well controlled RA by their rheumatologists, versus 84% of continuers. Switchers and discontinuers were almost 4 times as likely to flare, and more than 10 times as likely to have severe RA at the end of the year.
Switchers/discontinuers also had more inpatient days and emergency department and urgent care visits. Switchers and discontinuers had more than six times greater odds of visiting emergency departments and urgent care clinics at least once, compared with continuers. The differences were statistically significant. In short, payers may have saved on the front end, but probably lost on the back end.
“These real world data demonstrate that patients are significantly affected by switching or discontinuing their stable and effective [TNFi] therapy for rheumatoid arthritis. I don’t think there is any patient who asks to have their [TNFi] switched when they are doing well, and it would be uncommon for a rheumatologist to switch or discontinue [a TNFi] when patients are doing well. These are insurance-driven things that are impacting negatively on patient care, and this should stop. The [American College of Rheumatology] and other groups need to work together to get insurance companies to stop intervening in this way,” said investigator Dr. Douglas Wolf, medical director at Atlanta Gastroenterology Associates.
Similar problems have been reported when TNFi drugs are switched or stopped in Crohn’s disease, psoriasis, and other immunologic conditions, he said at the annual meeting of the American College of Rheumatology.
Switchers and discontinuers were more likely than were continuers to be Hispanic (27.7% vs. 15.7%; P = .041), but otherwise there were no significant differences between cohorts in baseline sociodemographic and disease characteristics, comorbidities, medication use, or resource utilization.
The investigators plan to continue the project to see if TNFi switchers fair better than discontinuers.
Dr. Wolf and other investigators disclosed relationships with AbbVie, including some who received payments from the company to participate in the research. Three authors are employees of AbbVie, maker of adalimumab (Humira).
SAN FRANCISCO – Stopping a tumor necrosis factor inhibitor for nonmedical reasons is associated with significantly worse clinical outcomes and increased health care resource use, according to a review of 166 rheumatoid arthritis patients from the Physicians Consulting Network.
That’s probably not a surprise; the main value of the findings is that they provide ammunition to push back against insurance decisions that lead to stopping or switching tumor necrosis factor inhibitors (TNFi) that are helping patients.
After being stable on their TNFi for at least 6 months, 25 patients in the study were forced to stop their medication for a range of nonmedical economic reasons such as increased copays, change of insurance, or loss of job health insurance. Another 58 switched to a new TNFi for similar reasons. Those 83 patients were matched to 83 others who were also stable on their TNFi for at least 6 months but continued the therapy. Data came from chart reviews by rheumatologists, and all the patients were under the care of physicians participating in the Physicians Consulting Network, which provides feedback to GfK, a market research firm.
Over the next year, 48% of switchers/discontinuers were deemed to have well controlled RA by their rheumatologists, versus 84% of continuers. Switchers and discontinuers were almost 4 times as likely to flare, and more than 10 times as likely to have severe RA at the end of the year.
Switchers/discontinuers also had more inpatient days and emergency department and urgent care visits. Switchers and discontinuers had more than six times greater odds of visiting emergency departments and urgent care clinics at least once, compared with continuers. The differences were statistically significant. In short, payers may have saved on the front end, but probably lost on the back end.
“These real world data demonstrate that patients are significantly affected by switching or discontinuing their stable and effective [TNFi] therapy for rheumatoid arthritis. I don’t think there is any patient who asks to have their [TNFi] switched when they are doing well, and it would be uncommon for a rheumatologist to switch or discontinue [a TNFi] when patients are doing well. These are insurance-driven things that are impacting negatively on patient care, and this should stop. The [American College of Rheumatology] and other groups need to work together to get insurance companies to stop intervening in this way,” said investigator Dr. Douglas Wolf, medical director at Atlanta Gastroenterology Associates.
Similar problems have been reported when TNFi drugs are switched or stopped in Crohn’s disease, psoriasis, and other immunologic conditions, he said at the annual meeting of the American College of Rheumatology.
Switchers and discontinuers were more likely than were continuers to be Hispanic (27.7% vs. 15.7%; P = .041), but otherwise there were no significant differences between cohorts in baseline sociodemographic and disease characteristics, comorbidities, medication use, or resource utilization.
The investigators plan to continue the project to see if TNFi switchers fair better than discontinuers.
Dr. Wolf and other investigators disclosed relationships with AbbVie, including some who received payments from the company to participate in the research. Three authors are employees of AbbVie, maker of adalimumab (Humira).
AT THE ACR ANNUAL MEETING
Key clinical point: The findings provide ammunition to push back against insurance decisions that lead to stopping or switching tumor necrosis factor inhibitors (TNFi) that are helping patients.
Major finding: After a year of follow-up, 48% of switchers/discontinuers were deemed to have well controlled RA by their rheumatologists, versus 84% of continuers. Switchers and discontinuers were almost 4 times as likely to flare, and more than 10 times as likely to have severe RA at the end of the year.
Data source: Chart review of 166 RA patients.
Disclosures: The investigators disclosed relationships with AbbVie, including payments from the company to participate in the research. Three authors are employed by AbbVie, maker of adalimumab (Humira).
New case of MS possibly related to tocilizumab
Canadian researchers have reported the first case of a patient developing multiple sclerosis (MS) while taking tocilizumab for rheumatoid arthritis.
A 48-year-old woman with a 20-year history of rheumatoid arthritis was seen for right hemisensory symptoms that began as numbness and pain on her right foot that spread to her trunk, arm, and face over a week, Dr. Philippe Beauchemin and Dr. Robert Carruthers of the University of British Columbia, Vancouver, noted in Multiple Sclerosis Journal. She had received many treatments for rheumatoid arthritis, including hydroxychloroquine, etanercept, and adalimumab. She also was on methotrexate, vitamin D, naproxen, and dexlansoprazole. MRI showed 20 lesions consistent with the diagnosis of MS. Doctors immediately discontinued tocilizumab and methotrexate. Three months later, another MRI showed two new lesions confirming MS diagnosis.
“There is absolutely no proof of a causal relationship between tocilizumab and MS in this patient, but it is also not excluded that tocilizumab might have caused secondary autoimmunity in CNS,” the authors wrote. The drug is in efficacy trials for patients with neuromyelitis optica spectrum disorder.
Two scenarios could explain this potential relationship, according to an accompanying commentary by Dr. Manuel Comabella of the Multiple Sclerosis Center of Catalonia, Vall d’Hebron University Hospital, Barcelona. The patient may have developed demyelinating lesions as a consequence of previous exposure to anti-TNF agents, and MS was later precipitated by treatment with tocilizumab; or that the drug itself can trigger a demyelinating disorder. “IL-6 may have immunosuppressive properties … that when absent by the effect of anti-IL6 agents, predisposes the individual to demyelinating conditions.”
Read the article in Multiple Sclerosis Journal (doi: 10:1177/1352458515623862).
Canadian researchers have reported the first case of a patient developing multiple sclerosis (MS) while taking tocilizumab for rheumatoid arthritis.
A 48-year-old woman with a 20-year history of rheumatoid arthritis was seen for right hemisensory symptoms that began as numbness and pain on her right foot that spread to her trunk, arm, and face over a week, Dr. Philippe Beauchemin and Dr. Robert Carruthers of the University of British Columbia, Vancouver, noted in Multiple Sclerosis Journal. She had received many treatments for rheumatoid arthritis, including hydroxychloroquine, etanercept, and adalimumab. She also was on methotrexate, vitamin D, naproxen, and dexlansoprazole. MRI showed 20 lesions consistent with the diagnosis of MS. Doctors immediately discontinued tocilizumab and methotrexate. Three months later, another MRI showed two new lesions confirming MS diagnosis.
“There is absolutely no proof of a causal relationship between tocilizumab and MS in this patient, but it is also not excluded that tocilizumab might have caused secondary autoimmunity in CNS,” the authors wrote. The drug is in efficacy trials for patients with neuromyelitis optica spectrum disorder.
Two scenarios could explain this potential relationship, according to an accompanying commentary by Dr. Manuel Comabella of the Multiple Sclerosis Center of Catalonia, Vall d’Hebron University Hospital, Barcelona. The patient may have developed demyelinating lesions as a consequence of previous exposure to anti-TNF agents, and MS was later precipitated by treatment with tocilizumab; or that the drug itself can trigger a demyelinating disorder. “IL-6 may have immunosuppressive properties … that when absent by the effect of anti-IL6 agents, predisposes the individual to demyelinating conditions.”
Read the article in Multiple Sclerosis Journal (doi: 10:1177/1352458515623862).
Canadian researchers have reported the first case of a patient developing multiple sclerosis (MS) while taking tocilizumab for rheumatoid arthritis.
A 48-year-old woman with a 20-year history of rheumatoid arthritis was seen for right hemisensory symptoms that began as numbness and pain on her right foot that spread to her trunk, arm, and face over a week, Dr. Philippe Beauchemin and Dr. Robert Carruthers of the University of British Columbia, Vancouver, noted in Multiple Sclerosis Journal. She had received many treatments for rheumatoid arthritis, including hydroxychloroquine, etanercept, and adalimumab. She also was on methotrexate, vitamin D, naproxen, and dexlansoprazole. MRI showed 20 lesions consistent with the diagnosis of MS. Doctors immediately discontinued tocilizumab and methotrexate. Three months later, another MRI showed two new lesions confirming MS diagnosis.
“There is absolutely no proof of a causal relationship between tocilizumab and MS in this patient, but it is also not excluded that tocilizumab might have caused secondary autoimmunity in CNS,” the authors wrote. The drug is in efficacy trials for patients with neuromyelitis optica spectrum disorder.
Two scenarios could explain this potential relationship, according to an accompanying commentary by Dr. Manuel Comabella of the Multiple Sclerosis Center of Catalonia, Vall d’Hebron University Hospital, Barcelona. The patient may have developed demyelinating lesions as a consequence of previous exposure to anti-TNF agents, and MS was later precipitated by treatment with tocilizumab; or that the drug itself can trigger a demyelinating disorder. “IL-6 may have immunosuppressive properties … that when absent by the effect of anti-IL6 agents, predisposes the individual to demyelinating conditions.”
Read the article in Multiple Sclerosis Journal (doi: 10:1177/1352458515623862).
FROM MULTIPLE SCLEROSIS JOURNAL
ACR: The pain of inflammatory disease goes beyond the physical
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
AT THE ACR ANNUAL MEETING
Key clinical point: Ask patients what they are worried about; you might put them at ease by addressing their misconceptions.
Major finding: Overall, 60% of RA patients and 71% of axSpA patients were fearful that their future suffering would be as bad as their past suffering.
Data source: A French survey of 474 patients.
Disclosures: Foundation Arthritis Jacques Courtin and UCB Pharma funded the study. The senior investigator has no relevant disclosures. Two investigators are UCB employees.
ACR: Etanercept during pregnancy doubles the odds of major malformations
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
AT THE ACR ANNUAL MEETING
Key clinical point: Although etanercept exposure was associated with more than twofold higher odds of major structural defects, there was no pattern to the defects and no biological plausibility to etanercept causing the defects.
Major finding: There were 33 major structural defects in children born to etanercept women versus 7 in a disease comparison group, translating to a more than doubling of risk with etanercept (OR, 2.37; 95% CI, 1.02-5.52).
Data source: Prospective investigation of 830 pregnant women.
Disclosures: The presenting investigator disclosed funding from 14 companies, including Amgen, the maker of etanercept, and AbbVie, the maker of adalimumab.
ACR: Fewer GCA relapses with add-on abatacept
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
AT THE ACR ANNUAL MEETING
Key clinical point: Abatacept appears to be a useful adjunct for giant cell arteritis.
Major finding: Relapse-free survival at 12 months was 48% in the abatacept group and 31% in the placebo group (P = .049).
Data source: Randomized, placebo-controlled study of 49 patients with giant cell arteritis.
Disclosures: The National Institutes of Health funded the study. Nearly all of the investigators disclosed relationships with Bristol-Myers Squibb, which provided the abatacept.
‘Hot’ joints may predict RA joint damage
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: A raised skin temperature over an inflamed joint may identify RA patients at risk of joint damage.
Major finding: Patients with hot joints had a nearly fourfold higher risk of x-ray damage at follow-up, compared with those with cool joints.
Data source: An observational pilot study involving 208 RA patients.
Disclosures: No conflicts of interest were declared.
Oral steroid dose, duration affect diabetes risk for people with RA
People with rheumatoid arthritis who take oral glucocorticoids have an increased risk of diabetes, the magnitude of which is dependent on dose and treatment duration, based on an analysis of two databases.
Dr. Mohammad Movahedi of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) led researchers conducting the observational study using the U.K. primary care database (CPRD) of 21,962 RA patients and the U.S. National Data Bank for Rheumatic Diseases (NDB) involving 12,657 RA patients (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39537).
The hazard ratio (HR) for developing diabetes was 1.30 (95% confidence interval, 1.17-1.45) for patients taking oral glucocorticoids in the CPRD cohort and 1.61 (95% CI, 1.37-1.89) for people in the NDB cohort.
While doses taken more than 6 months ago did not influence current risk, each 5-mg increase of current oral glucocorticoid dose was associated with a 25% and 30% increased risk of diabetes in the CPRD and NDB cohorts, respectively.
According to the researchers, identifying the risk of diabetes with various doses and durations of therapy allows clinicians and patients to make informed decisions about treatment as well as balance the benefits and harms.
The research team plans to examine the threshold for cost-effective diabetes screening in patients receiving steroids for RA.
Individual investigators reported support for their work from the Medical Research Council, the Canadian Institute for Health Research, the Rheumatology Research Foundation, and the NIHR Manchester Musculoskeletal Biomedical Research Unit.
People with rheumatoid arthritis who take oral glucocorticoids have an increased risk of diabetes, the magnitude of which is dependent on dose and treatment duration, based on an analysis of two databases.
Dr. Mohammad Movahedi of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) led researchers conducting the observational study using the U.K. primary care database (CPRD) of 21,962 RA patients and the U.S. National Data Bank for Rheumatic Diseases (NDB) involving 12,657 RA patients (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39537).
The hazard ratio (HR) for developing diabetes was 1.30 (95% confidence interval, 1.17-1.45) for patients taking oral glucocorticoids in the CPRD cohort and 1.61 (95% CI, 1.37-1.89) for people in the NDB cohort.
While doses taken more than 6 months ago did not influence current risk, each 5-mg increase of current oral glucocorticoid dose was associated with a 25% and 30% increased risk of diabetes in the CPRD and NDB cohorts, respectively.
According to the researchers, identifying the risk of diabetes with various doses and durations of therapy allows clinicians and patients to make informed decisions about treatment as well as balance the benefits and harms.
The research team plans to examine the threshold for cost-effective diabetes screening in patients receiving steroids for RA.
Individual investigators reported support for their work from the Medical Research Council, the Canadian Institute for Health Research, the Rheumatology Research Foundation, and the NIHR Manchester Musculoskeletal Biomedical Research Unit.
People with rheumatoid arthritis who take oral glucocorticoids have an increased risk of diabetes, the magnitude of which is dependent on dose and treatment duration, based on an analysis of two databases.
Dr. Mohammad Movahedi of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) led researchers conducting the observational study using the U.K. primary care database (CPRD) of 21,962 RA patients and the U.S. National Data Bank for Rheumatic Diseases (NDB) involving 12,657 RA patients (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39537).
The hazard ratio (HR) for developing diabetes was 1.30 (95% confidence interval, 1.17-1.45) for patients taking oral glucocorticoids in the CPRD cohort and 1.61 (95% CI, 1.37-1.89) for people in the NDB cohort.
While doses taken more than 6 months ago did not influence current risk, each 5-mg increase of current oral glucocorticoid dose was associated with a 25% and 30% increased risk of diabetes in the CPRD and NDB cohorts, respectively.
According to the researchers, identifying the risk of diabetes with various doses and durations of therapy allows clinicians and patients to make informed decisions about treatment as well as balance the benefits and harms.
The research team plans to examine the threshold for cost-effective diabetes screening in patients receiving steroids for RA.
Individual investigators reported support for their work from the Medical Research Council, the Canadian Institute for Health Research, the Rheumatology Research Foundation, and the NIHR Manchester Musculoskeletal Biomedical Research Unit.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The magnitude of increased diabetes risk associated with oral glucocorticoids is dependent on duration of treatment and dose.
Major finding: People with RA who were taking oral corticosteroids had up to 60% higher risk of developing diabetes, compared with nonusers.
Data source: An observational study using two databases: the U.K. primary care database of 21,962 RA patients and the U.S. National Data Bank for Rheumatic Diseases involving 12,657 RA patients.
Disclosures: Individual investigators reported support for their work from the Medical Research Council, the Canadian Institute for Health Research, the Rheumatology Research Foundation, and the NIHR Manchester Musculoskeletal Biomedical Research Unit.
Second dose of herpes zoster vaccine beneficial to seniors
The herpes zoster vaccine should be administered earlier rather than later in order to achieve optimal immune response, but an additional booster shot for individuals 70 years or older is also advisable.
This is according to a recent study published in the Journal of Infectious Diseases, which looked at four distinct cohorts – 200 subjects at least 70 years old who had already received the herpes zoster vaccine (ZV) 10 years or more prior (Group 1), 200 subjects at least 70 years old who had never received ZV (Group 2), 100 subjects ages 60-69 years old who had never received ZV (Group 3), and 100 subjects 50-59 years old who had never received ZV (Group 4) – to determine the efficacy of relatively late ZV administration on inducing an adequate immune response (J Infect Dis. 2016 Jan 1;213[1]:14-22. doi: 10.1093/infdis/jiv480).
“During aging there is a progressive decline in immune responsiveness to vaccination and a shortening of the duration of vaccine-induced immunity,” wrote Dr. Myron J. Levin of the University of Colorado, Denver, and his associates, who added that “as an initial step in investigating the potential for reversing this decline in efficacy, we determined that a booster dose of ZV administered to adults [at least] 70 years of age elicits a varicella-zoster virus (VZV) antibody response that is noninferior to that of ZV administered as a first dose.”
Dr. Levin and his coinvestigators separated subjects into one of the four aforementioned cohorts, enrolling individuals who had a history of varicella and had been U.S. residents for at least 30 years, but had no history of herpes zoster prior to enrollment. All individuals received a single, subcutaneous, deltoid region ZV (Zostavax) injection of 0.65 mL on the first day of the study, with subsequent blood samples collected and analyzed at 1, 6, and 52 weeks after receiving ZV. Subjects in Group 2 were matched with subjects in Group 1 based on age to compare results.
Baseline levels of both interferon gamma (IFN-gamma) and interleukin 2 (IL-2) were significantly higher in Group 1 than in Group 2. The geometric mean count of VZV-specific effector memory cells per million peripheral blood mononuclear cells in Group 1 was 47 at baseline, 88 at week 1, 90 at week 6, and 65 at week 52, vs. 36 at baseline, 65 at week 1, 73 at week 6, and 37 at week 52 in Group 2 (P less than .05). Similar disparities were seen between Groups 3 and 4, too, with Group 4 having consistently and significantly higher geometric mean counts at each collection of blood samples throughout the study.
“All age groups developed an increase in GMT [geometric mean titer] at week 1 after ZV receipt that peaked at week 6,” the authors explained. However, “the booster dose of ZV administered to adults [at least] 70 years old after [at least] 10 years elicited a GMT and geometric mean fold-rise in VZV antibody titer that was noninferior to that of ZV administered as a first dose to subjects [at least] 70 years old.”
The indication of that, the authors conclude, is that cell-mediated immunity is affected by ZV and enhanced by a stronger, or more recent, dose. This lends credence to the theory that although it is better to get a ZV shot earlier in life, those who are approaching age 70 years can – and, in many cases, should – get a booster shot to strengthen and maintain that immunity.
“Although the practical implications of the current findings are not fully understood, the similarity of [enzyme-linked immunosorbent spot] responses to those observed in the successful efficacy trial of ZV in vaccinees [at least] 60 years of age supports further investigation of administration of ZV at an early age vs. at a later age and further investigation of a booster dose for elderly individuals at an appropriate interval after initial immunization against HZ,” Dr. Levin and his coauthors concluded.
The study was funded by Merck, Sharp & Dohme. Dr. Levin reported having received grants, personal fees, and royalty payments from Merck. Several other coauthors disclosed individual potential conflicts of interest as well.
The herpes zoster vaccine should be administered earlier rather than later in order to achieve optimal immune response, but an additional booster shot for individuals 70 years or older is also advisable.
This is according to a recent study published in the Journal of Infectious Diseases, which looked at four distinct cohorts – 200 subjects at least 70 years old who had already received the herpes zoster vaccine (ZV) 10 years or more prior (Group 1), 200 subjects at least 70 years old who had never received ZV (Group 2), 100 subjects ages 60-69 years old who had never received ZV (Group 3), and 100 subjects 50-59 years old who had never received ZV (Group 4) – to determine the efficacy of relatively late ZV administration on inducing an adequate immune response (J Infect Dis. 2016 Jan 1;213[1]:14-22. doi: 10.1093/infdis/jiv480).
“During aging there is a progressive decline in immune responsiveness to vaccination and a shortening of the duration of vaccine-induced immunity,” wrote Dr. Myron J. Levin of the University of Colorado, Denver, and his associates, who added that “as an initial step in investigating the potential for reversing this decline in efficacy, we determined that a booster dose of ZV administered to adults [at least] 70 years of age elicits a varicella-zoster virus (VZV) antibody response that is noninferior to that of ZV administered as a first dose.”
Dr. Levin and his coinvestigators separated subjects into one of the four aforementioned cohorts, enrolling individuals who had a history of varicella and had been U.S. residents for at least 30 years, but had no history of herpes zoster prior to enrollment. All individuals received a single, subcutaneous, deltoid region ZV (Zostavax) injection of 0.65 mL on the first day of the study, with subsequent blood samples collected and analyzed at 1, 6, and 52 weeks after receiving ZV. Subjects in Group 2 were matched with subjects in Group 1 based on age to compare results.
Baseline levels of both interferon gamma (IFN-gamma) and interleukin 2 (IL-2) were significantly higher in Group 1 than in Group 2. The geometric mean count of VZV-specific effector memory cells per million peripheral blood mononuclear cells in Group 1 was 47 at baseline, 88 at week 1, 90 at week 6, and 65 at week 52, vs. 36 at baseline, 65 at week 1, 73 at week 6, and 37 at week 52 in Group 2 (P less than .05). Similar disparities were seen between Groups 3 and 4, too, with Group 4 having consistently and significantly higher geometric mean counts at each collection of blood samples throughout the study.
“All age groups developed an increase in GMT [geometric mean titer] at week 1 after ZV receipt that peaked at week 6,” the authors explained. However, “the booster dose of ZV administered to adults [at least] 70 years old after [at least] 10 years elicited a GMT and geometric mean fold-rise in VZV antibody titer that was noninferior to that of ZV administered as a first dose to subjects [at least] 70 years old.”
The indication of that, the authors conclude, is that cell-mediated immunity is affected by ZV and enhanced by a stronger, or more recent, dose. This lends credence to the theory that although it is better to get a ZV shot earlier in life, those who are approaching age 70 years can – and, in many cases, should – get a booster shot to strengthen and maintain that immunity.
“Although the practical implications of the current findings are not fully understood, the similarity of [enzyme-linked immunosorbent spot] responses to those observed in the successful efficacy trial of ZV in vaccinees [at least] 60 years of age supports further investigation of administration of ZV at an early age vs. at a later age and further investigation of a booster dose for elderly individuals at an appropriate interval after initial immunization against HZ,” Dr. Levin and his coauthors concluded.
The study was funded by Merck, Sharp & Dohme. Dr. Levin reported having received grants, personal fees, and royalty payments from Merck. Several other coauthors disclosed individual potential conflicts of interest as well.
The herpes zoster vaccine should be administered earlier rather than later in order to achieve optimal immune response, but an additional booster shot for individuals 70 years or older is also advisable.
This is according to a recent study published in the Journal of Infectious Diseases, which looked at four distinct cohorts – 200 subjects at least 70 years old who had already received the herpes zoster vaccine (ZV) 10 years or more prior (Group 1), 200 subjects at least 70 years old who had never received ZV (Group 2), 100 subjects ages 60-69 years old who had never received ZV (Group 3), and 100 subjects 50-59 years old who had never received ZV (Group 4) – to determine the efficacy of relatively late ZV administration on inducing an adequate immune response (J Infect Dis. 2016 Jan 1;213[1]:14-22. doi: 10.1093/infdis/jiv480).
“During aging there is a progressive decline in immune responsiveness to vaccination and a shortening of the duration of vaccine-induced immunity,” wrote Dr. Myron J. Levin of the University of Colorado, Denver, and his associates, who added that “as an initial step in investigating the potential for reversing this decline in efficacy, we determined that a booster dose of ZV administered to adults [at least] 70 years of age elicits a varicella-zoster virus (VZV) antibody response that is noninferior to that of ZV administered as a first dose.”
Dr. Levin and his coinvestigators separated subjects into one of the four aforementioned cohorts, enrolling individuals who had a history of varicella and had been U.S. residents for at least 30 years, but had no history of herpes zoster prior to enrollment. All individuals received a single, subcutaneous, deltoid region ZV (Zostavax) injection of 0.65 mL on the first day of the study, with subsequent blood samples collected and analyzed at 1, 6, and 52 weeks after receiving ZV. Subjects in Group 2 were matched with subjects in Group 1 based on age to compare results.
Baseline levels of both interferon gamma (IFN-gamma) and interleukin 2 (IL-2) were significantly higher in Group 1 than in Group 2. The geometric mean count of VZV-specific effector memory cells per million peripheral blood mononuclear cells in Group 1 was 47 at baseline, 88 at week 1, 90 at week 6, and 65 at week 52, vs. 36 at baseline, 65 at week 1, 73 at week 6, and 37 at week 52 in Group 2 (P less than .05). Similar disparities were seen between Groups 3 and 4, too, with Group 4 having consistently and significantly higher geometric mean counts at each collection of blood samples throughout the study.
“All age groups developed an increase in GMT [geometric mean titer] at week 1 after ZV receipt that peaked at week 6,” the authors explained. However, “the booster dose of ZV administered to adults [at least] 70 years old after [at least] 10 years elicited a GMT and geometric mean fold-rise in VZV antibody titer that was noninferior to that of ZV administered as a first dose to subjects [at least] 70 years old.”
The indication of that, the authors conclude, is that cell-mediated immunity is affected by ZV and enhanced by a stronger, or more recent, dose. This lends credence to the theory that although it is better to get a ZV shot earlier in life, those who are approaching age 70 years can – and, in many cases, should – get a booster shot to strengthen and maintain that immunity.
“Although the practical implications of the current findings are not fully understood, the similarity of [enzyme-linked immunosorbent spot] responses to those observed in the successful efficacy trial of ZV in vaccinees [at least] 60 years of age supports further investigation of administration of ZV at an early age vs. at a later age and further investigation of a booster dose for elderly individuals at an appropriate interval after initial immunization against HZ,” Dr. Levin and his coauthors concluded.
The study was funded by Merck, Sharp & Dohme. Dr. Levin reported having received grants, personal fees, and royalty payments from Merck. Several other coauthors disclosed individual potential conflicts of interest as well.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Booster doses of the herpes zoster vaccine should be given earlier rather than later to induce effective immune responses in patients aged 70 years and older.
Major finding: Interferon gamma and interleukin 2 levels were significantly higher in cohorts of subjects at least 70 years of age who received booster doses, both at baseline and after vaccination, than other subjects.
Data source: Cohort study of 600 U.S. residents aged 50 years and older with a history of varicella but no history of herpes zoster.
Disclosures: Study funded by Merck, Sharp & Dohme; Dr. Levin disclosed receiving grants and fees from Merck; several other coauthors revealed individual potential conflicts of interest.
Few teen females prescribed teratogenic meds get contraceptive advice, Rx
Fewer than one-third of adolescent females prescribed a teratogenic medication were counseled about, prescribed, or referred for contraception, according to a retrospective review of data from a single academic pediatric medical center.
The records from 1,694 female patients aged 14-25 years, who received 4,506 medications of Food and Drug Administration pregnancy risk category D or X – mostly commonly topiramate, methotrexate, diazepam, isotretinoin, or enalapril – showed that contraceptive counseling, prescription, or referral occurred in 29% of visits, according to a paper published online Dec. 16 in Pediatrics.
White females were 61% more likely to receive contraceptive provision than were nonwhites, and girls aged 16 years or older were 20% more likely to receive it than were girls aged 14-15 years (Pediatrics 2016, Jan. doi: 10.1542/peds.2015-1454).
Teratogens with a federal surveillance system, such as iPLEDGE or REMS, were associated with twofold increase in the rate of contraceptive provision, but a much lower likelihood of documentation of menstrual and sexual histories.
“This finding was unexpected given that the focus of these systems is to proactively reduce the risk of unplanned pregnancy during drug treatment,” wrote Stephani L. Stancil of Children’s Mercy Hospital, Kansas City, Mo., and coauthors.
“Opportunity exists in these adolescents to increase rates of contraceptive counsel, the prescription of contraception if appropriate, or referral for such care when it becomes necessary to use a medication with known teratogenic potential,” they said.
Children’s Mercy Hospital supported the study. No conflicts of interest were declared.
“Even if a provider does not think it is within his or her scope of care to provide a contraceptive method, asking the questions to assess whether a contraceptive method might be needed from another provider is essential.
“In addition, all providers should be aware of the newer recommendation that all adolescents should be offered the option of a long-acting reversible contraceptive method to prevent pregnancy.
The fact that the Affordable Care Act allows us to provide 18 different contraceptive methods without any cost sharing to the patient should also make adolescent pregnancy prevention easier than ever.”
Erica J. Gibson, M.D., is an expert in adolescent medicine in the department of pediatrics at the University of Vermont Children’s Hospital, Burlington. Her remarks are excerpted from an article published in Pediatrics (2016, Jan. doi:10.1542/peds.2015-3826). She disclosed no conflicts of interest.
“Even if a provider does not think it is within his or her scope of care to provide a contraceptive method, asking the questions to assess whether a contraceptive method might be needed from another provider is essential.
“In addition, all providers should be aware of the newer recommendation that all adolescents should be offered the option of a long-acting reversible contraceptive method to prevent pregnancy.
The fact that the Affordable Care Act allows us to provide 18 different contraceptive methods without any cost sharing to the patient should also make adolescent pregnancy prevention easier than ever.”
Erica J. Gibson, M.D., is an expert in adolescent medicine in the department of pediatrics at the University of Vermont Children’s Hospital, Burlington. Her remarks are excerpted from an article published in Pediatrics (2016, Jan. doi:10.1542/peds.2015-3826). She disclosed no conflicts of interest.
“Even if a provider does not think it is within his or her scope of care to provide a contraceptive method, asking the questions to assess whether a contraceptive method might be needed from another provider is essential.
“In addition, all providers should be aware of the newer recommendation that all adolescents should be offered the option of a long-acting reversible contraceptive method to prevent pregnancy.
The fact that the Affordable Care Act allows us to provide 18 different contraceptive methods without any cost sharing to the patient should also make adolescent pregnancy prevention easier than ever.”
Erica J. Gibson, M.D., is an expert in adolescent medicine in the department of pediatrics at the University of Vermont Children’s Hospital, Burlington. Her remarks are excerpted from an article published in Pediatrics (2016, Jan. doi:10.1542/peds.2015-3826). She disclosed no conflicts of interest.
Fewer than one-third of adolescent females prescribed a teratogenic medication were counseled about, prescribed, or referred for contraception, according to a retrospective review of data from a single academic pediatric medical center.
The records from 1,694 female patients aged 14-25 years, who received 4,506 medications of Food and Drug Administration pregnancy risk category D or X – mostly commonly topiramate, methotrexate, diazepam, isotretinoin, or enalapril – showed that contraceptive counseling, prescription, or referral occurred in 29% of visits, according to a paper published online Dec. 16 in Pediatrics.
White females were 61% more likely to receive contraceptive provision than were nonwhites, and girls aged 16 years or older were 20% more likely to receive it than were girls aged 14-15 years (Pediatrics 2016, Jan. doi: 10.1542/peds.2015-1454).
Teratogens with a federal surveillance system, such as iPLEDGE or REMS, were associated with twofold increase in the rate of contraceptive provision, but a much lower likelihood of documentation of menstrual and sexual histories.
“This finding was unexpected given that the focus of these systems is to proactively reduce the risk of unplanned pregnancy during drug treatment,” wrote Stephani L. Stancil of Children’s Mercy Hospital, Kansas City, Mo., and coauthors.
“Opportunity exists in these adolescents to increase rates of contraceptive counsel, the prescription of contraception if appropriate, or referral for such care when it becomes necessary to use a medication with known teratogenic potential,” they said.
Children’s Mercy Hospital supported the study. No conflicts of interest were declared.
Fewer than one-third of adolescent females prescribed a teratogenic medication were counseled about, prescribed, or referred for contraception, according to a retrospective review of data from a single academic pediatric medical center.
The records from 1,694 female patients aged 14-25 years, who received 4,506 medications of Food and Drug Administration pregnancy risk category D or X – mostly commonly topiramate, methotrexate, diazepam, isotretinoin, or enalapril – showed that contraceptive counseling, prescription, or referral occurred in 29% of visits, according to a paper published online Dec. 16 in Pediatrics.
White females were 61% more likely to receive contraceptive provision than were nonwhites, and girls aged 16 years or older were 20% more likely to receive it than were girls aged 14-15 years (Pediatrics 2016, Jan. doi: 10.1542/peds.2015-1454).
Teratogens with a federal surveillance system, such as iPLEDGE or REMS, were associated with twofold increase in the rate of contraceptive provision, but a much lower likelihood of documentation of menstrual and sexual histories.
“This finding was unexpected given that the focus of these systems is to proactively reduce the risk of unplanned pregnancy during drug treatment,” wrote Stephani L. Stancil of Children’s Mercy Hospital, Kansas City, Mo., and coauthors.
“Opportunity exists in these adolescents to increase rates of contraceptive counsel, the prescription of contraception if appropriate, or referral for such care when it becomes necessary to use a medication with known teratogenic potential,” they said.
Children’s Mercy Hospital supported the study. No conflicts of interest were declared.
FROM PEDIATRICS
Key clinical point: Fewer than one-third of adolescent females prescribed a teratogenic medication also receive contraception provision.
Major finding: Among adolescent females prescribed a known teratogen, contraception provision occurred in 29% of visits.
Data source: A single-center, retrospective study of 1,694 female patients aged 14-25 years, prescribed a medication of FDA pregnancy risk category D or X.
Disclosures: Children’s Mercy Hospital, Kansas City, supported the study. No conflicts of interest were declared.