User login
U.S. vs. French guidelines for osteoporosis treatment
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
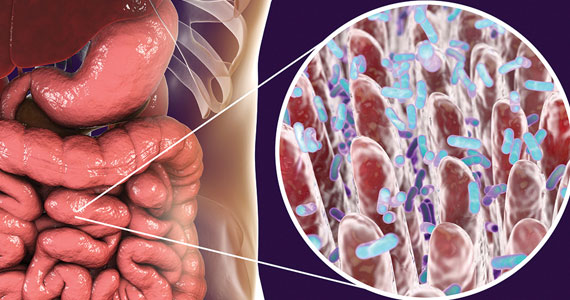
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
High HDL-C levels linked to increased fracture risk
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Regular vitamin D supplements may lower melanoma risk
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
. They also found a trend for benefit with occasional use.
The study, published in Melanoma Research, involved almost 500 individuals attending a dermatology clinic who reported on their use of vitamin D supplements.
Regular users had a significant 55% reduction in the odds of having a past or present melanoma diagnosis, while occasional use was associated with a nonsignificant 46% reduction. The reduction was similar for all skin cancer types.
However, senior author Ilkka T. Harvima, MD, PhD, department of dermatology, University of Eastern Finland and Kuopio (Finland) University Hospital, warned there are limitations to the study.
Despite adjustment for several possible confounding factors, “it is still possible that some other, yet unidentified or untested, factors can still confound the present result,” he said.
Consequently, “the causal link between vitamin D and melanoma cannot be confirmed by the present results,” Dr. Harvima said in a statement.
Even if the link were to be proven, “the question about the optimal dose of oral vitamin D in order to for it to have beneficial effects remains to be answered,” he said.
“Until we know more, national intake recommendations should be followed.”
The incidence of cutaneous malignant melanoma and other skin cancers has been increasing steadily in Western populations, particularly in immunosuppressed individuals, the authors pointed out, and they attributed the rise to an increased exposure to ultraviolet radiation.
While ultraviolet radiation exposure is a well-known risk factor, “the other side of the coin is that public sun protection campaigns have led to alerts that insufficient sun exposure is a significant public health problem, resulting in insufficient vitamin D status.”
For their study, the team reviewed the records of 498 patients aged 21-79 years at a dermatology outpatient clinic who were deemed by an experienced dermatologist to be at risk of any type of skin cancer.
Among these patients, 295 individuals had a history of past or present cutaneous malignancy, with 100 diagnosed with melanoma, 213 with basal cell carcinoma, and 41 with squamous cell carcinoma. A further 70 subjects had cancer elsewhere, including breast, prostate, kidney, bladder, intestine, and blood cancers.
A subgroup of 96 patients were immunocompromised and were considered separately.
The 402 remaining patients were categorized, based on their self-reported use of oral vitamin D preparations, as nonusers (n = 99), occasional users (n = 126), and regular users (n = 177).
Regular use of vitamin D was associated with being more educated (P = .032), less frequent outdoor working (P = .003), lower tobacco pack years (P = .001), and more frequent solarium exposure (P = .002).
There was no significant association between vitamin D use and photoaging, actinic keratoses, nevi, basal or squamous cell carcinoma, body mass index, or self-estimated lifetime exposure to sunlight or sunburns.
However, there were significant associations between regular use of vitamin D and a lower incidence of melanoma and other cancer types.
There were significantly fewer individuals in the regular vitamin D use group with a past or present history of melanoma when compared with the nonuse group, at 18.1% vs. 32.3% (P = .021), or any type of skin cancer, at 62.1% vs. 74.7% (P = .027).
Multivariate logistic regression analysis revealed that regular vitamin D use was significantly associated with a reduced melanoma risk, at an odds ratio vs. nonuse of 0.447 (P = .016).
Occasional use was associated with a reduced, albeit nonsignificant, risk, with an odds ratio versus nonuse of 0.540 (P = .08).
For any type of skin cancers, regular vitamin D use was associated with an odds ratio vs. nonuse of 0.478 (P = .032), while that for occasional vitamin D use was 0.543 (P = .061).
“Somewhat similar” results were obtained when the investigators looked at the subgroup of immunocompromised individuals, although they note that “the number of subjects was low.”
The study was supported by the Cancer Center of Eastern Finland of the University of Eastern Finland, the Finnish Cancer Research Foundation, and the VTR-funding of Kuopio University Hospital. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MELANOMA RESEARCH
New osteoporosis guideline says start with a bisphosphonate
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
FROM THE ANNALS OF INTERNAL MEDICINE
Mind the geriatrician gap
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
Transgender patients on hormone therapy require monitoring
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
PAU, France – Transgender patients on hormone therapy have an increased mortality risk and so must be closely monitored, especially in terms of cardiovascular health and oncology, reported Marie D’Assigny, MD, of the department of endocrinology, diabetes, and dietetics at Poitiers (France) University Hospital, at the Infogyn 2022 conference. Because transgender women (those assigned male at birth who have assumed a female gender identity) are at risk of breast cancer, they should also be recommended for breast cancer screening.
Transgender men and women, especially transgender women, “should be deemed high-risk cardiovascular patients, or even very high risk in some cases,” said Dr. D’Assigny. This means that they should be considered candidates for cholesterol-lowering medication earlier than their cisgender counterparts, and a target LDL cholesterol of less than 0.70 g/L (70 mg/dL) should be sought. Likewise, blood pressure must be strictly monitored, especially because it tends to rise when on hormone therapy.
Feminizing hormone therapy requires chemical castration with the use of anti-androgen drugs to achieve a blood testosterone level less than 0.5 ng/mL (1.73 nmol/L). Low-dose cyproterone acetate (< 25 to 50 mg/day) is usually used. Treatment is stopped if a patient undergoes an orchidectomy. For feminizing hormone therapy, administration of 17beta-estradiol transcutaneously (patch or gel) is recommended, because it is associated with a lower risk of thromboembolism than oral administration.
Masculinizing hormone therapy is based on administration of progestogens, then testosterone in the form of an injection (mostly testosterone enanthate via intramuscular injection every 10 days) or percutaneously (gel or patch). There are few contraindications, and treatment is generally well tolerated.
High mortality rate
A recent retrospective study highlighted the mortality and risk factors for death in transgender men and women receiving hormone therapy. More than 4,500 people, mostly male to female transgender women, were enrolled in this study, which was conducted over a 47-year period (1972-2018) at a specialist clinic at Amsterdam UMC.
Over the course of the study, the mortality rate in transgender men and women was twice that of the general population. The death rate was 10.8% in transgender women vs. 2.7% in transgender men, after a follow-up of 40,232 person-years and 17,285 person-years, respectively. In transgender women, mortality was nearly three times that of cisgender women in the general population.
Over the nearly 5 decades of study, there was no improvement in the mortality rate, even over the last 10 years when transgender issues started to be more recognized. The mortality trends are markedly distinct over the years from those observed in the cisgender population, and this is especially true for transgender women compared to transgender men. “Much is still to be done,” said Dr. D’Assigny.
According to the study, cause-specific mortality in transgender women was high for cardiovascular disease and lung cancer, possibly because of a higher smoking rate in this population. HIV-related disease and suicide remained very high in both transgender men and women.
People with gender dysphoria who do not receive treatment for gender reassignment have a suicide rate of 40%, reported François-Xavier Madec, MD, of Foch Hospital in Suresnes, France, at a previous presentation. For transgender men and women who receive care, this rate is lowered to 15%, which is still significantly higher than the rate of 1.6% observed in the general population.
“These causes of death don’t give any indication as to a specific effect of hormone treatment but show that monitoring and, if necessary, treatment of comorbidities and lifestyle-related factors are important in managing transgender patients,” said the study authors.
“Strengthening social acceptance and treating cardiovascular risk factors could also help to reduce mortality in transgender men and women,” they added.
Screening for osteoporosis
In addition to receiving cardiovascular risk factor assessment and monitoring, transgender men and women on hormone therapy should also undergo bone density testing “when risk factors for osteoporosis are present, especially in patients stopping hormone therapy after a gonadectomy,” said Dr. D’Assigny.
Calcium and vitamin D supplements are also recommended for all patients after a gonadectomy, especially in transgender men on testosterone. Osteoporosis screening is recommended for transgender men 10 years after starting treatment with testosterone, then every 10 years.
There is also the risk for breast cancer in transgender women, although the risk is lower than in cisgender women. This risk was highlighted in another study of more than 2,260 transgender women that was carried out by a team at Amsterdam UMC.
A total of 18 cases of breast cancer (15 invasive) were diagnosed after a median 18 years of hormone treatment. This represents an incidence of breast cancer that is 46 times higher than that expected in cisgender men of the same age but 3 times lower than in cisgender women.
The authors noted that “the risk of breast cancer in transgender women increases during a relatively short duration of hormone treatment,” going on to say that “these results suggest that breast cancer screening recommendations are relevant for transgender men and women on hormone therapy.”
Poorly attended screening
All of this means that transgender women older than age 50 years, as well as transgender men who have not had a mastectomy, should be offered a mammogram screening, taking into account the possible presence of implants in the former. Transgender women are also at risk for prostate cancer. Monitoring is personalized according to the individual risk of prostate disease, as it is for cisgender men.
There is no consensus on the monitoring of transgender men on hormone therapy for uterine cancer. Yet there is a risk. “Testosterone causes thinning of the endometrium, which may lead to dysplasia,” said Dr. D’Assigny. A physical examination once a year or a pelvic ultrasound scan every 2 years should form the basis of endometrial and ovarian appearance monitoring.
Transgender women are also at risk for prostate cancer. However, they are less likely to attend a prostate cancer screening test, said Dr. D’Assigny, which means “we need to raise awareness of their benefit in advance.” Vaginal swabs for transgender men and mammograms in transgender women “are resented, on both a physical and emotional level.” As a result, delays in diagnosis are common in transgender men and women.
Globally, access to care is still difficult for transgender patients because they don’t always receive appropriate gynecological monitoring, through fear of judgment or discrimination. Many transgender men and women are reluctant to see a gynecologist, even though they are at risk of gynecological cancers, as well as unwanted pregnancies in transgender men who have not undergone a hysterectomy.
In a demonstration of the collective desire to improve patient care for the transgender community, a literature review was recently published by a French team that analyzed gynecological monitoring methods in transgender patients. In September, the French National Authority for Health also issued a guidance memorandum on the transgender transition pathway, pending new recommendations scheduled for 2023.
A version of this article first appeared on Medscape.com.
This article was translated from the Medscape French edition.
FDA alert: ‘Substantial’ hypocalcemia risk with denosumab use in dialysis patients
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration issued an alert on Nov. 22 that cited preliminary evidence for a “substantial risk” for severe and symptomatic hypocalcemia and serious outcomes related to abnormally low calcium levels in people being treated with dialysis and receiving the osteoporosis medication denosumab (Prolia), including hospitalization and death.
In its alert, the FDA advised clinicians to make sure that people on dialysis who receive Prolia ingest adequate calcium and vitamin D supplementation and undergo frequent blood calcium monitoring, “possibly more often than is already being conducted,” which “may help decrease the likelihood or severity of these risks.”
The agency also called on clinicians to “advise patients on dialysis to immediately seek help if they experience symptoms of hypocalcemia,” such as unusual tingling or numbness in the hands, arms, legs, or feet; painful muscle spasms or cramps; voice box or lung spasms causing difficulty breathing; vomiting; seizures; or irregular heart rhythm.
The FDA had a similar message for people being treated with dialysis who are also receiving Prolia. The alert advised patients to watch for these symptoms and to tell their health care provider if they occur. The agency also advised patients who are undergoing dialysis and receiving Prolia to not stop the agent on their own, without first discussing this step with their care provider.
The FDA also advised providers and patients to contact the agency about episodes of side effects from Prolia (or other medications) via the FDA’s MedWatch program.
Frequent and serious
The FDA explained it issued the alert because of “the frequency and seriousness” of the risk for hypocalcemia and resulting complications. The agency noted that the risk seems most acute for people on dialysis who also receive Prolia, but the risk may also extend to people with advanced kidney disease who are not being treated with hemodialysis.
The alert stemmed from “interim results” in an ongoing safety study of Prolia that the FDA required the agent’s manufacturer, Amgen, to run when the agency first approved denosumab for U.S. marketing in 2010. The FDA said its review of these interim results suggested an increased risk of hypocalcemia with Prolia in patients with advanced kidney disease.
In addition, adverse event reports submitted to the FDA suggested in a separate, internal study that patients on dialysis treated with Prolia are at “substantial risk for severe and symptomatic hypocalcemia, including hospitalization and death.”
The alert explained that “because of the frequency and seriousness of these risks, we are alerting healthcare professionals and patients about them and that we are continuing to evaluate this potential safety issue with Prolia use in patients with advanced kidney disease, particularly those on dialysis.” The FDA added that “we will communicate our final conclusions and recommendations when we have completed our review or have more information to share.”
A version of this article first appeared on Medscape.com.
Does subclinical hyperthyroidism raise fracture risk?
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN