User login
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
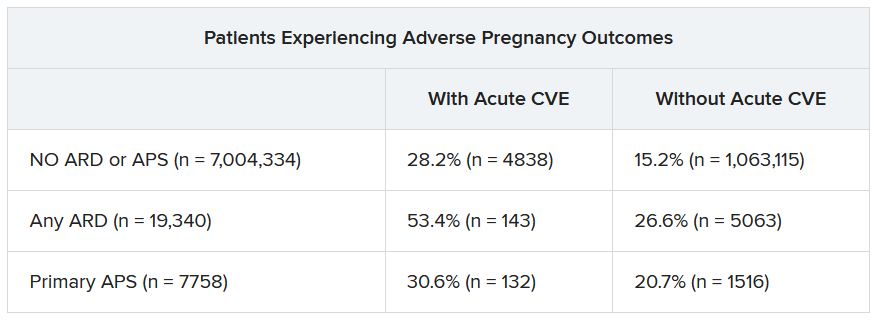
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
News & Perspectives from Ob.Gyn. News
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
CONFERENCE COVERAGE
MS, DMTs, and pregnancy: Beware of over-caution regarding treatment
MILAN—The news about multiple sclerosis (MS) and childbearing in women is largely good, a researcher told colleagues at the 9th Joint ECTRIMS-ACTRIMS Meeting. Evidence suggests that MS doesn’t disrupt fertility, pregnancy, birth, or lactation. However, there are still uncertainties about the timing of medical treatment for MS before, during, and after pregnancy.
Epidemiologist Emmanuelle Leray, PhD, of French School of Public Health in Rennes, urged neurologists to not be too eager to take women off medication—or too slow to put them back on it. “MS should not be undertreated due to a desire for pregnancy, as there are several options that are possible and compatible with pregnancy,” she said. As for after pregnancy, when women face a well-known high risk of MS rebound, “we can reasonably assume that women with active MS need to be advised to restart rapid, highly effective DMT [disease-modifying therapy] soon after delivery,” she said.
Women are more likely than men to develop MS, and they often do so during child-bearing years. Pregnancy among women with MS has become more common over the years: A 2018 Neurology study examined U.S. data from 2006 to 2014 and reported that the annual adjusted proportion of women with MS and pregnancy increased from 7.91% to 9.47%.
FEATURE
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
Many factors contribute to these shifting trends, a major factor being economic stress stemming from payment cuts in Medicare. Add in rising practice costs and administrative burdens, and more doctors than ever are seeking employment, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Continue to: LATEST NEWS...
LATEST NEWS
Three-quarters of menopausal women report unexpected symptoms
GLASGOW—Three-quarters of women going through perimenopause and menopause experience unexpected distressing, debilitating, and embarrassing symptoms but often fail to receive appropriate treatment, a large U.K.-based survey found.
“For too long, many people have thought of menopause as just hot flashes and vaginal dryness. But we know hormones work all over our body, so there are many symptoms beyond that,” said Daniel Reisel, MBBS, PhD, a gynecologist at University College London, who presented the survey findings at the 2023 annual meeting of the Royal College of General Practitioners.
Primary care physicians in the United Kingdom have seen an increase in cases of women presenting with symptoms associated with menopause at a time when the country’s Parliament is debating whether all women should have a menopause check-up in their early 40s, he said.
Still, only around 14% of menopausal women in the United Kingdom are prescribed hormone replacement therapy (HRT), despite national and international guidelines clearly stating the benefits of the treatment generally outweigh the risks.
Louise Newson, MBChB, who runs the U.K.’s largest menopause clinic, said many women with symptoms of menopause feel the medical system “gaslights” them—dismissing their concerns as trivial or even fabricated.
In her clinic, she typically sees many women with poor sleep, as well as muscle and joint pains. “Yet [when they visit their GPs], they are incorrectly told that it can’t be hormones because they’re still having periods,” she said.
Prescribed antidepressants often precede HRT
The new study sought to learn what women knew and experienced with respect to menopause symptoms and what they thought was important. Of the 5,744 women who responded to the survey, 79.4% were aged 40-60 years and 84.6% were White. “The survey respondents were not different from the distribution of ethnicities we see in NHS menopause care,” said Dr. Reisel, adding that “the barriers are greater for women in poorer areas and for those who are non-White.”
A total of 30.4% had two to five hospital consultations before the health care professional considered that symptoms were related to changing hormone levels; 38.5% were offered antidepressants before HRT. Nearly all (94.6%) said they had experienced negative mood changes and emotions since becoming perimenopausal or menopausal; of these, 19.1% were formally diagnosed with depression or a mood disorder.
“This all just highlights the frustrations I feel around menopause care,” Dr. Newson said. “Women are often not given the tools to properly understand what’s going on and then they don’t ask for the right treatment, and many are given antidepressants. It’s still medicalizing the menopause but in a different way.” ●
Two biomarkers promising for preeclampsia prediction
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
U.S. infant mortality rates rise for first time in 2 decades
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
RSV vaccination during pregnancy: Finally ready for prime time
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
The challenges of managing CMV infection during pregnancy
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18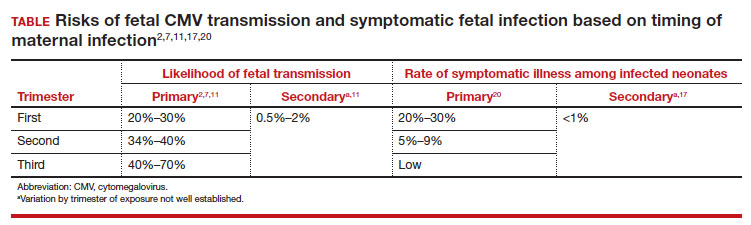
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.
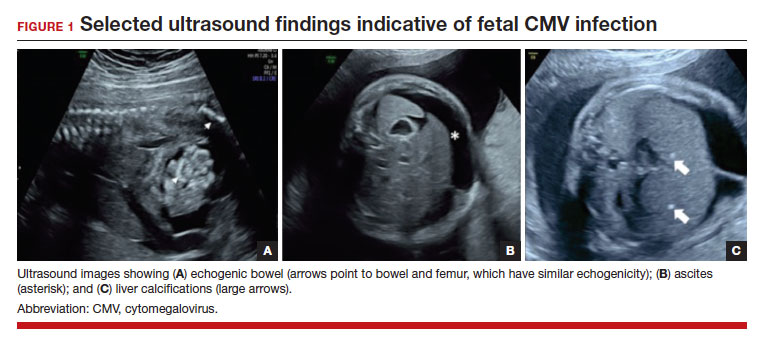
About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1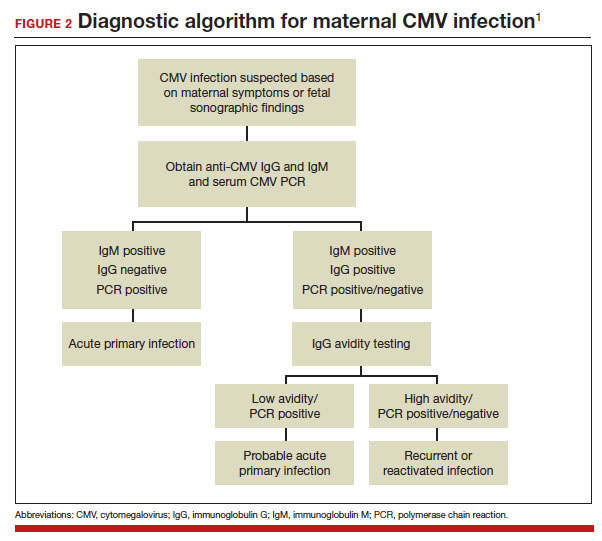
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
CASE Anomalous findings on fetal anatomic survey
A 27-year-old previously healthy primigravid woman is at 18 weeks’ gestation. She is a first-grade schoolteacher. On her fetal anatomic survey, the estimated fetal weight was in the eighth percentile. Echogenic bowel and a small amount of ascitic fluid were noted in the fetal abdomen. The lateral and third ventricles were mildly dilated, the head circumference was 2 standard deviations below normal, and the placenta was slightly thickened and edematous.
What is the most likely diagnosis?
What diagnostic tests are indicated?
What management options are available for this patient?
Cytomegalovirus (CMV) is the most common of the perinatally transmitted infections, affecting 1% to 4% of all pregnancies. Although the virus typically causes either asymptomatic infection or only mild illness in immunocompetent individuals, it can cause life-threatening disease in immunocompromised persons and in the developing fetus. In this article, we review the virology and epidemiology of CMV infection and then focus on the key methods to diagnose infection in the mother and fetus. We conclude by considering measures that may be of at least modest value in treating CMV in pregnancy.
Virology of CMV infection
Cytomegalovirus is a double-stranded DNA virus in the Herpesviridae family. This ubiquitous virus is present in virtually all secretions and excretions of an infected host, including blood, urine, saliva, breast milk, genital secretions, and tissues and organs used for donation. Infection is transmitted through direct contact with any of the substances listed; contact with infected urine or saliva is the most common mode of transmission. Disease occurrence does not show seasonal variation.
After exposure, an incubation period of 28 to 60 days ensues, followed by development of viremia and clinical symptoms. In the majority of exposed individuals, CMV establishes a lifelong latent infection, and recurrent episodes of illness can occur as a result of reactivation of latent virus (also known as secondary infection) or, more rarely, infection with a new viral strain. In fact, most CMV illness episodes in pregnancy represent a reactivation of a previous infection rather than a new infection.
Following initial infection, both IgM (immunoglobulin M) and IgG (immunoglobulin G) antibodies develop rapidly and can be detected in blood within 1 to 2 weeks. IgM levels typically wane within 30 to 60 days, although persistence for several months is not unusual, and levels also can increase with viral reactivation (secondary infection). IgG antibodies typically persist for many years after a primary infection.
Intrauterine CMV infection occurs through hematogenous transplacental passage during maternal viremia. The risk of transmission and severity of fetal effects depend on whether or not the infection is primary or secondary in nature as well as the gestational age at fetal exposure.1,2
Additionally, postnatal vertical transmission can occur through exposure to viral particles in genital secretions as well as breast milk. CMV acquired in the postnatal period rarely produces severe sequelae in a healthy term neonate, but it has been associated with an increased rate of complications in very low birth weight and premature newborns.3
Continue to: Who is at risk...
Who is at risk
Congenital CMV, which occurs in 2.1 to 7.7 per 10,000 live births in the United States, is both the most common congenital infection and the leading cause of nonhereditary congenital hearing loss in children.4,5 The main reservoir of CMV in the United States is young children in day care settings, with approximately 50% of this population showing evidence of viral shedding in saliva.1 Adult populations in North America have a high prevalence of CMV IgG antibodies indicative of prior infection, with rates reaching 50% to 80%. Among seronegative individuals aged 12 to 49, the rate of seroconversion is approximately 1 in 60 annually.6 Significant racial disparities have been noted in rates of seroprevalence and seroconversion, with higher rates of infection in non-Hispanic Black and Mexican American individuals.6 Overall, the rate of new CMV infection among pregnant women in the United States is 0.7% to 4%.7
Clinical manifestations
Manifestations of infection differ depending on whether or not infection is primary or recurrent (secondary) and whether or not the host is immunocompetent or has a compromised immune system. Unique manifestations develop in the fetus.
CMV infection in children and adults. Among individuals with a normal immune response, the typical course of CMV is either no symptoms or a mononucleosis-like illness. In symptomatic patients, the most common symptoms include malaise, fever, and night sweats, and the most common associated laboratory abnormalities are elevation in liver function tests and a decreased white blood cell count, with a predominance of lymphocytes.8
Immunocompromised individuals are at risk for significant morbidity and mortality resulting from CMV. Illness may be the result of reactivation of latent infection due to decreased immune function or may be acquired as a result of treatment such as transplantation of CMV-positive organs or tissues, including bone marrow. Virtually any organ system can be affected, with potential for permanent organ damage and death. Severe systemic infection also can occur.
CMV infection in the fetus and neonate. As noted previously, fetal infection develops as a result of transplacental passage coincident with maternal infection. The risk of CMV transmission to the fetus and the severity of fetal injury vary based on gestational age at fetal infection and whether or not maternal infection is primary or secondary.
In most studies, primary maternal infections are associated with higher rates of fetal infection and more severe fetal and neonatal disease manifestations.2,7,9,10 Primary infections carry an overall 30% to 40% risk of transmission to the fetus.7,11 The risk of fetal transmission is much lower with a recurrent infection and is usually less than 2%.11 Due to their greater overall incidence, secondary infections account for the majority of cases of fetal and neonatal CMV disease.7 Importantly, although secondary infections generally have been regarded as having a lower risk and lower severity of fetal and neonatal disease, several recent studies have demonstrated rates of complications similar to, and even exceeding, those of primary infections.12-15 The TABLE provides a summary of the risks of fetal transmission and symptomatic fetal infection based on trimester of pregnancy.2,11,16-18
In the fetus, CMV may affect multiple organ systems. Among sonographic and magnetic resonance imaging (MRI) findings, central nervous system (CNS) anomalies are the most common.19,20 These can include microcephaly, ventriculomegaly, and periventricular calcifications. The gastrointestinal system also is frequently affected, and findings include echogenic bowel, hepatosplenomegaly, and liver calcifications. Lastly, isolated effusions, placentomegaly, fetal growth restriction, and even frank hydrops can develop. More favorable neurologic outcomes have been demonstrated in infants with no prenatal brain imaging abnormalities.20,21 However, the role of MRI in prenatal prognosis currently is not well defined.
FIGURE 1 illustrates selected sonographic findings associated with fetal CMV infection.

About 85% to 90% of infants with congenital CMV that results from primary maternal infection have no symptoms at birth. Among the 10% to 15% of infants that do have symptoms, petechial rash, jaundice, and hepatosplenomegaly are the most common manifestations (“blueberry muffin baby”). Approximately 10% to 20% of infants in this group have evidence of chorioretinitis on ophthalmologic examination, and 50% show either microcephaly or low birth weight.22Among survivors of symptomatic congenital CMV, more than 50% have long-term neurologic morbidities that may include sensorineural hearing loss, seizures, vision impairment, and developmental disabilities. Note that even when neonates appear asymptomatic at birth (regardless of whether infection is primary or secondary), 5% may develop microcephaly and motor deficits, 10% go on to develop sensorineural hearing loss, and the overall rate of neurologic morbidity reaches 13% to 15%.12,23 Some of the observed deficits manifest at several years of age, and, currently, no models exist for prediction of outcome.
Continue to: Diagnosing CMV infection...
Diagnosing CMV infection
Maternal infection
If maternal CMV infection is suspected based on a symptomatic illness or an abnormal fetal ultrasound exam, the first diagnostic test should be an assessment of IgM and IgG serology. If the former test results are positive and the latter negative, the diagnosis of acute CMV infection is confirmed. A positive serum CMV DNA polymerase chain reaction (PCR) test adds additional assurance that the diagnosis is correct. Primary infection, as noted above, poses the greatest risk of serious injury to the fetus.1
A frequent diagnostic dilemma arises when both the IgM and IgG antibody are positive. Remember that CMV IgM antibody can remain positive for 9 to 12 months after a primary infection and can reappear in the maternal serum in the face of a recurrent or reactivated infection. When confronted by both a positive IgM and positive IgG result, the clinician should then order IgG avidity testing. If the avidity is low to moderate, which reflects poor binding of antibody to the virus, the patient likely has an acute infection. If the avidity is high, which reflects enhanced binding of antibody to virus, the patient probably has a recurrent or reactivated infection; this scenario poses less danger to the developing fetus. The presence of CMV DNA in serum is also more consistent with acute infection, although viremia still can occur with recurrent infection. FIGURE 2 presents a suggested algorithm for the diagnosis of CMV in the pregnant patient.1
If a diagnosis of maternal CMV infection is confirmed, liver function tests should be obtained to determine if CMV hepatitis is present. If the liver function tests are abnormal, a coagulation profile also should be performed to identify the mother who might be at risk for peripartum hemorrhage.
Fetal infection
The single best test for confirmation of congenital CMV infection is detection of viral DNA and quantitation of viral load in the amniotic fluid by PCR. If the amniocentesis is performed prior to 20 weeks’ gestation and is negative, the test should be repeated in approximately 4 weeks.1,19,24
Detection of viral DNA indicates congenital infection. The ultimate task, however, is to determine if the infection has injured the fetus. Detailed ultrasound examination is the key to identifying fetal injury. As noted previously, the principal ultrasonographic findings that suggest congenital CMV infection include2,19,20,21,25:
- hydropic placenta
- fetal growth restriction
- microcephaly (head circumference more than 3 standard deviations below the mean)
- periventricular calcifications
- enlarged liver
- echogenic bowel
- ascites
- fetal hydrops.
Management: Evidence on CMV hyperimmune globulin, valacyclovir
If the immunocompetent mother has clinical manifestations of infection, she should receive symptomatic treatment. She should be encouraged to rest as much as possible, stay well hydrated, and use acetaminophen (1,000 mg every 6 to 8 hours) as needed for malaise and fever.
However, if the mother is immunocompromised and has signs of serious complications, such as chorioretinitis, hepatitis, or pneumonia, more aggressive therapy is indicated. Drugs used in this setting include foscarnet and ganciclovir and are best prescribed in consultation with a medical infectious disease specialist.
At this time, no consistently effective therapy for congenital infection is available. Therefore, if a patient has primary CMV infection in the first half of pregnancy, particularly in the first trimester, she should be counseled that the risk of fetal infection is approximately 40% and that approximately 5% to 15% of infants will be severely affected at birth. Given this information, some patients may opt for pregnancy termination.
In 2005, a report from Nigro and colleagues stimulated great hope that CMV-specific hyperimmune globulin (CytoGam) might be of value for both treatment and prophylaxis for congenital infection.26 These authors studied 157 women with confirmed primary CMV infection. One-hundred forty-eight women were asymptomatic and were identified by routine serologic screening, 8 had symptomatic infection, and 1 was identified because of abnormal fetal ultrasound findings. Forty-five women had CMV detected in amniotic fluid by PCR or culture more than 6 weeks before study enrollment. Thirty-one of these women were treated with intravenous hyperimmune globulin (200 U or 200 mg/kg maternal body weight); 14 declined treatment. Seven of the latter women had infants who were acutely symptomatic at the time of delivery; only 1 of the 31 treated women had an affected neonate (adjusted odds ratio [OR], 0.02; P<.001). In this same study, 84 women did not have a diagnostic amniocentesis because their infection occurred within 6 weeks of enrollment, their gestational age was less than 20 weeks, or they declined the procedure. Thirty-seven of these women received hyperimmune globulin (100 U or 100 mg/kg) every month until delivery, and 47 declined treatment. Six of the treated women delivered infected infants compared with 19 of the untreated women (adjusted OR, 0.32; P<.04).
Although these results were quite encouraging, several problems existed with the study’s design, as noted in an editorial that accompanied the study’s publication.27 First, the study was not randomized or placebo controlled. Second, patients were not stratified based on the severity of fetal ultrasound abnormalities. Third, the dosing of hyperimmune globulin varied; 9 of the 31 patients in the treatment group received additional infusions of drug into either the amniotic fluid or fetal umbilical vein. Moreover, patients in the prophylaxis group actually received a higher cumulative dose of hyperimmune globulin than patients in the treatment group.
Two subsequent investigations that were better designed were unable to verify the effectiveness of hyperimmune globulin. In 2014, Revello and colleagues reported the results of a prospective, randomized, placebo-controlled, double-blinded study of 124 women at 5 to 26 weeks’ gestation with confirmed primary CMV infection.28 The rate of congenital infection was 30% in the group treated with hyperimmune globulin and 44% in the placebo group (P=.13). There also was no significant difference in the concentration of serum CMV DNA in treated versus untreated mothers. Moreover, the number of adverse obstetric events (preterm delivery, fetal growth restriction, intrahepatic cholestasis of pregnancy, and postpartum preeclampsia) in the treatment group was higher than in the placebo group, 13% versus 2%.
In 2021, Hughes and colleagues published the results of a multicenter, double-blind trial in 399 women who had a diagnosis of primary CMV infection before 23 weeks’ gestation.29 The primary outcome was defined as a composite of congenital CMV infection or fetal/neonatal death. An adverse primary outcome occurred in 22.7% of the patients who received hyperimmune globulin and 19.4% of those who received placebo (relative risk, 1.17; 95% confidence interval [CI], 0.80–1.72; P=.42).
Continue to: Jacquemard and colleagues...
Jacquemard and colleagues then proposed a different approach.30 In a small pilot study of 20 patients, these authors used high doses of oral valacylovir (2 g 4 times daily) and documented therapeutic drug concentrations and a decline in CMV viral load in fetal serum. Patients were not stratified by severity of fetal injury at onset of treatment, so the authors were unable to define which fetuses were most likely to benefit from treatment.
In a follow-up investigation, Leruez-Ville and colleagues reported another small series in which high-dose oral valacyclovir (8 g daily) was used for treatment.31 They excluded fetuses with severe brain anomalies and fetuses with no sonographic evidence of injury. The median gestational age at diagnosis was 26 weeks. Thirty-four of 43 treated fetuses were free of injury at birth. In addition, the viral load in the neonate’s serum decreased significantly after treatment, and the platelet count increased. The authors then compared these outcomes to a historical cohort and confirmed that treatment increased the proportion of asymptomatic neonates from 43% without treatment to 82% with treatment (P<.05 with no overlapping confidence intervals).
We conclude from these investigations that hyperimmune globulin is unlikely to be of value in treating congenital CMV infection, especially if the fetus already has sonographic findings of severe injury. High-dose oral valacyclovir also is unlikely to be of value in severely affected fetuses, particularly those with evidence of CNS injury. However, antiviral therapy may be of modest value in situations when the fetus is less severely injured.
Preventive measures
Since no definitive treatment is available for congenital CMV infection, our efforts as clinicians should focus on measures that may prevent transmission of infection to the pregnant patient. These measures include:
- Encouraging patients to use careful handwashing techniques when handling infant diapers and toys.
- Encouraging patients to adopt safe sexual practices if not already engaged in a mutually faithful, monogamous relationship.
- Using CMV-negative blood when transfusing a pregnant woman or a fetus.
At the present time, unfortunately, a readily available and highly effective therapy for prevention of CMV infection is not available.
CASE Congenital infection diagnosed
The ultrasound findings are most consistent with congenital CMV infection, especially given the patient’s work as an elementary schoolteacher. The diagnosis of maternal infection is best established by conventional serology (positive IgM, negative IgM) and detection of viral DNA in maternal blood by PCR testing. The diagnosis of congenital infection is best confirmed by documentation of viral DNA in the amniotic fluid by PCR testing. Given that this fetus already has evidence of moderate to severe injury, no treatment is likely to be effective in reversing the abnormal ultrasound findings. Pregnancy termination may be an option, depending upon the patient’s desires and the legal restrictions prevalent in the patient’s geographic area. ●
- Cytomegalovirus infection is the most common of the perinatally transmitted infections.
- Maternal infection is often asymptomatic. When symptoms are present, they resemble those of an influenza-like illness. In immunocompromised persons, however, CMV may cause serious complications, including pneumonia, hepatitis, and chorioretinitis.
- The virus is transmitted by contact with contaminated body fluids, such as saliva, urine, blood, and genital secretions.
- The greatest risk of severe fetal injury results from primary maternal infection in the first trimester of pregnancy.
- Manifestations of severe congenital CMV infection include growth restriction, microcephaly, ventriculomegaly, hepatosplenomegaly, ascites, chorioretinitis, thrombocytopenia, purpura, and hydrops (“blueberry muffin baby”).
- Late manifestations of infection, which usually follow recurrent maternal infection, may appear as a child enters elementary school and include visual and auditory deficits, developmental delays, and learning disabilities.
- The diagnosis of maternal infection is confirmed by serology and detection of viral DNA in the serum by PCR testing.
- The diagnosis of fetal infection is best made by a combination of abnormal ultrasound findings and detection of CMV DNA in amniotic fluid. The characteristic ultrasound findings include placentomegaly, microcephaly, ventriculomegaly, growth restriction, echogenic bowel, and serous effusions/hydrops.
- Treatment of the mother with antiviral medications such as valacyclovir may be of modest value in reducing placental edema, decreasing viral load in the fetus, and hastening the resolution of some ultrasound findings, such as echogenic bowel.
- While initial studies seemed promising, the use of hyperimmune globulin has not proven to be consistently effective in treating congenital infection.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. 2019:888-890.
- Chatzakis C, Ville Y, Makrydimas G, et al. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am J Obstet Gynecol. 2020;223:870-883.e11. doi:10.1016/j.ajog.2020.05.038
- Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:e153785. doi:10.1001 /jamapediatrics.2015.3785
- Messinger CJ, Lipsitch M, Bateman BT, et al. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020;174:1159-1167. doi:10.1001/jamapediatrics.2020.3009
- De Cuyper E, Acke F, Keymeulen A, et al. Risk factors for hearing loss at birth in newborns with congenital cytomegalovirus infection. JAMA Otolaryngol Head Neck Surg. 2023;149:122-130. doi:10.1001/jamaoto.2022.4109
- Colugnati FA, Staras SA, Dollard SC, et al. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi:10.1186/1471-2334-7-71
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904-1908.
- Wreghitt TG, Teare EL, Sule O, et al. Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2003;37:1603-1606. doi:10.1086/379711
- Fowler KB, Stagno S, Pass RF, et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663-667. doi:10.1056 /NEJM199203053261003
- Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526-1532. doi:10.1093/ cid/ciy1128
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-276. doi:10.1002/ rmv.535
- Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93-99. doi:10.1097/00006454-199202000-00007
- Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332-336. doi:10.1016/j.jpeds.2005.09.003
- Zalel Y, Gilboa Y, Berkenshtat M, et al. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet Gynecol. 31:417-420. doi:10.1002/uog.5255
- Scaramuzzino F, Di Pastena M, Chiurchiu S, et al. Secondary cytomegalovirus infections: how much do we still not know? Comparison of children with symptomatic congenital cytomegalovirus born to mothers with primary and secondary infection. Front Pediatr. 2022;10:885926. doi:10.3389/fped.2022.885926
- Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830-835. doi:10.1111/j.1471-0528.2007.01651.x
- Hadar E, Dorfman E, Bardin R, et al. Symptomatic congenital cytomegalovirus disease following non-primary maternal infection: a retrospective cohort study. BMC Infect Dis. 2017;17:31. doi:10.1186/s12879-016-2161-3
- Elkan Miller T, Weisz B, Yinon Y, et al. Congenital cytomegalovirus infection following second and third trimester maternal infection is associated with mild childhood adverse outcome not predicted by prenatal imaging. J Pediatric Infect Dis Soc. 2021;10:562-568. doi:10.1093/jpids/ piaa154
- Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirusassociated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol. 2013;41:508-514. doi:10.1002/uog.12377
- Lipitz S, Elkan Miller T, Yinon Y, et al. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet Gynecol. 2020;56:572-578. doi:10.1002/uog.21946
- Buca D, Di Mascio D, Rizzo G, et al. Outcome of fetuses with congenital cytomegalovirus infection and normal ultrasound at diagnosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021;57:551-559. doi:10.1002/uog.23143
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57 (suppl 4):S178-S181. doi:10.1093/cid/cit629
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. doi:10.1002/rmv.544
- Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol. 2016;214:B5-11. doi:10.1016 /j.ajog.2016.02.042
- Rouse DJ, Fette LM, Hughes BL, et al. Noninvasive prediction of congenital cytomegalovirus infection after maternal primary infection. Obstet Gynecol. 2022;139:400-406. doi:10.1097/AOG.0000000000004691
- Nigro G, Adler SP, La Torre R, et al; Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350-1362. doi:10.1056/NEJMoa043337
- Duff P. Immunotherapy for congenital cytomegalovirus infection. N Engl J Med. 2005;355:1402-1404. doi:10.1056 /NEJMe058172
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. doi:10.1056/NEJMoa1310214
- Hughes BL, Clifton RG, Rouse DJ, et al. A trial of hyperimmune globulin to prevent congenital cytomegalovirus infection. N Engl J Med. 2021;385:436-444. doi:10.1056/NEJMoa1913569
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113-1121. doi:10.1111/j.1471-0528.2007.01308.x
- Leruez-Ville M, Ghout I, Bussières L, et al. In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol. 2016;215:462.e1-462.e10. doi:10.1016/j.ajog.2016.04.003
Test all perinatally exposed infants for HCV: CDC
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
In utero–exposed infants should be tested at 2-6 months of life, much earlier than the current strategy of testing at 18 months.
HCV infection, which can lead to liver fibrosis and cirrhosis, liver failure, hepatic cancer, and transplant, will develop in 6%-7% of all perinatally exposed infants and children. Curative therapy with direct-acting antivirals can be administered starting at age 3, the CDC noted in Morbidity and Mortality Week Report (MMWR).
About 70% of children 18 months and older are not being tested with the current strategy of anti-HCV testing.
This current MMWR report supplements the 2020 CDC recommendations for adult HCV screening, which includes universal screening among pregnant persons during each pregnancy.
The new recommendations
- Perinatally exposed infants should receive a nucleic acid amplification test for HCV RNA at 2-6 months of age to identify those who might develop chronic HCV infection if not treated.
- Those with detectable HCV RNA should be managed in consultation with an expert in pediatric HCV.
- Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
“Testing perinatally exposed infants beginning at age 2 months with a NAT for HCV RNA is cost-effective and allows for earlier linkage to care, appropriate evaluation, and the opportunity to provide curative, life-saving therapy,” the MMWR report said.
A growing problem
The CDC noted that rates of HCV infections during pregnancy are on the rise, corresponding with the ongoing opioid crisis and intravenous drug use.
Yet most perinatally exposed children are not tested for HCV infection and are not referred for hepatitis C care. Reasons might include lack of awareness of perinatal exposure by pediatric providers, lack of regular pediatric care among exposed children, and switching of health care providers before the former recommended testing age of 18 months.
The CDC’s testing recommendation is welcome news to Dawnette A. Lewis, MD, a maternal fetal medicine specialist at Northwell Health in New Hyde Park, N.Y. “As opposed to data for hep B and HIV, we have traditionally had little information and experience regarding the transmission and impact of hep C in pregnant women and their babies. We’ve been having that conversation about the lack of information for some time, and now there’s an opportunity to get evolving data on hep C and how it affects the baby, ” she said.
In her view, mothers will likely be quite accepting of testing for their infants. “It could be integrated into the routine newborn screening panel, so there should not be barriers to accessibility if they’re getting prenatal and neonatal care.”
Commenting on HCV testing for babies in an interview at his institution, Ravi R. Jhaveri, MD, division head of pediatric infectious diseases at Northwestern Medicine’s Ann & Robert H. Lurie Children’s Hospital of Chicago, said, “This is a terrific way to capitalize on the fact that infants already come to the doctor for many visits during the first months of life for their vaccines and their well-child check. And so this should be an easy way to streamline our testing strategy and hopefully lose many fewer patients.”
Northwestern Medicine is an innovative clinic offering HCV testing and treatment outside of clinical trials for pregnant women and their infants with the goal of preventing transmission from mother to child.
Northwestern is launching a clinical trial of treatment for HCV-positive pregnant patients during regular prenatal care. “With very simple treatments similar to taking a prenatal vitamin, it would be easy and seamless to fit into the existing schedule,” said Lyn Yee, MD, a Northwestern maternal-fetal medicine specialist.
Dr. Yee stressed that eliminating hepatitis C will likely be one of the most significant health advancements of the decade.
Dr. Lewis, Dr. Jhaveri, and Dr. Yee had no relevant conflicts of interest to declare with regard to their comments.
Adverse events related to embryo transfer catheters may be underreported to the FDA
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the American Society for Reproductive Medicine’s 2023 meeting.
ETCs are medical devices used routinely in assisted reproduction. The findings highlight the need for increased vigilance in tracking and reporting adverse events associated with these devices, according to the investigators.
“With hundreds of thousands of embryo transfers being performed per year, surveillance of the safety, performance, and quality of embryo transfer catheter devices is critical and should not be taken for granted,” said Anita Madison, MD, MPH, from the division of reproductive endocrinology and infertility at Johns Hopkins School of Medicine, Baltimore, who led the study. “There are a variety of transfer catheters with different indications, with little data on the superiority and safety of the brands compared to one another.”
Although the number of reported adverse events associated with ETCs is relatively small, the problems can significantly affect patient care, the researchers said.
Dr. Madison and her colleagues used the Manufacturer and User Facility Device Experience (MAUDE) database to identify adverse events associated with ETC devices. The MAUDE database is a voluntary reporting system that holds hundreds of thousands of medical device reports of suspected device-associated deaths, injuries, and malfunctions reported to the FDA annually.
For each adverse event in the database linked to an ECT, the researchers collected information related to the brand of the device, the nature of the event, and the nature of the reporter. The researchers omitted the device and manufacturer names from the presentation of the study findings, delineating them only as “Brand 1,” “Brand 2,” “Brand 3,” “Brand 4,” or “Other.”
Problems with devices included contamination, packaging problems, malfunction, mechanical flaws, and material separation. Patient-level adverse events included retaining of foreign body, trauma, malfunction, or failed embryo transfer.
Between 2014 and 2023, Dr. Madison and her colleagues identified 101 adverse events associated with ECTs in the database. About 25% of these occurred in 2018, with 27 cases reported. Contamination was the most prevalent problem, found in 68 reports; oil was the most common contaminant.
The distribution of types of adverse events varied, depending on ETC brand. A breakdown of occurrences revealed high numbers for Brand 2, with 52 adverse events. Although Brand 3 accounted for only 16 adverse events, the majority of these were related to device separation.
“That finding stood out,” Dr. Madison said.
Nearly 1 in 4 (22%) of all reported incidents led to overt patient harm. Retention of a foreign body was the prime type of injury, occurring in 12 cases. Malfunction and injury were found in four cases each, with two failed embryo transfers reported, Dr. Madison said.
Because the majority of these adverse event reports were submitted by manufacturers (87%) and were rarely submitted by end users (for example, physicians, lab staff), the researchers said their findings likely underestimate such problems.
“I’m surprised the [number of reported adverse events] is as low as it is,” said Kimball Pomeroy, PhD, scientific director at the World Egg and Sperm Bank, Scottsdale, Ariz., who was not part of the study team. “Laboratories are required to report failed devices; they have to have a plan for that.”
“It just comes down to underreporting,” added Valerie L. Baker, MD, director in the Division of Reproductive Endocrinology and Infertility at Johns Hopkins Medicine, Lutherville, Md., who was not affiliated with the study.
“In two of these reports, they failed to transfer the embryo; they actually lost the embryo,” Dr. Pomeroy added. “That’s drastic for those patients; it’s a serious problem that needs to be addressed.”
Citing these findings, the authors underscored the need for heightened surveillance of ETC devices and recommend further studies to assess the sensitivity of these procedures for attempting pregnancy. They urge physicians and lab staff involved in these procedures to exercise continued vigilance and to improve the reporting of problems with ETC devices.
Dr. Madison, Dr. Baker, and Dr. Pomeroy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASRM 2023