User login
Patient benefits justify price of new lupus nephritis drugs
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction of Dialysis-Induced Metabolic Alkalosis
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
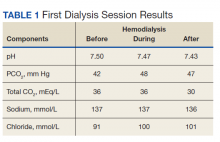
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
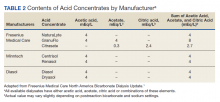
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
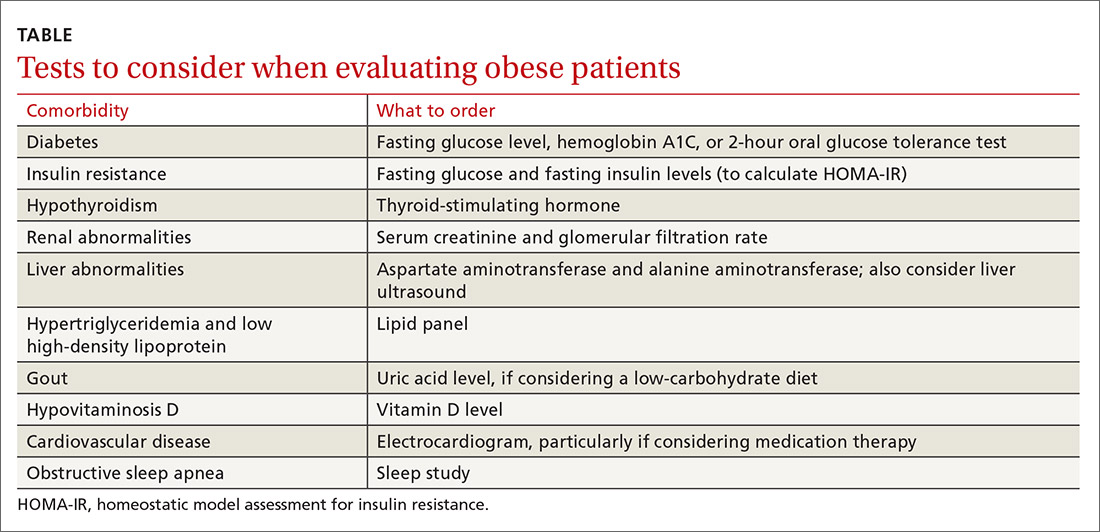
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Deaths tied to reprocessed urologic endoscopes, FDA warns
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is warning health care providers about the risk for potentially life-threatening infections associated with reprocessed endoscopes used for viewing the urinary tract, including cystoscopes, cystouerthroscopes, and ureteroscopes.
The federal agency is investigating more than 450 medical device reports, including three reports of deaths, received between Jan. 1, 2017, and Feb. 20, 2021, that describe post-procedure infections and other possible contamination problems associated with the reprocessing or cleaning and sterilization of the devices.
Although it’s early in the investigation, on the basis of available data, the FDA believes the risk for infection is low.
“We are very concerned about the three reported deaths – outside of the United States – associated with these infections, and we’re acting fast to communicate with health care providers and the public about what we know and what is still an emerging issue,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement released on April 1.
Manufacturer Olympus Corporation submitted three reports of deaths attributed to a bacterial infection. In two of those reports, the infection was linked to a forceps/irrigation plug, an accessory component used to control water flow and enable access to the working channel of the endoscope. Lab tests confirmed that the bacteria that caused the infection was present in the forceps/irrigation plug.
The FDA said the third victim’s death involved a cystoscope that did not pass a leak test. It is possible that the damaged device was a factor in the patient’s becoming infected.
It’s not known to what degree the reported infections or patient comorbidities played a part in the patient deaths. The FDA also hasn’t concluded that any specific manufacturer or brand of these devices is associated with higher risks than others.
The FDA released recommendations for processing and using these devices and emphasized the importance of following manufacturers’ labeling and reprocessing instructions to minimize the risk for infection.
In addition to following reprocessing instructions, the recommendations include not using a device that has failed a leak test, developing schedules for routine device inspection and maintenance, and discussing the potential benefits and risks associated with procedures involving reprocessed urologic endoscopes with patients.
The newly reported concerns with urologic endoscopes are similar to problems associated with reprocessed duodenoscopes. In 2018, the FDA warned about higher-than-expected contamination rates for reprocessed duodenoscopes. The FDA has taken action on infections related to the reprocessing of duodenoscopes. In 2015, it required postmarket safety studies and the updating of sampling and culturing protocols. In 2019, the FDA approved single-use duodenoscopes in an effort to curb infections.
A version of this article first appeared on Medscape.com.
Lenvatinib Plus Pembrolizumab Improves Outcomes in Previously Untreated Advanced Clear Cell Renal Cell Carcinoma
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
Bone loss common in kidney stone patients, yet rarely detected
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Missed visits during pandemic cause ‘detrimental ripple effects’
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
New skin papules
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
A 49-year-old woman with a history of end-stage renal disease, uncontrolled type 2 diabetes, and congestive heart failure visited the hospital for an acute heart failure exacerbation secondary to missed dialysis appointments. On admission, her provider noted that she had tender, pruritic lesions on the extensor surface of her arms. She said they had appeared 2 to 3 months after she started dialysis. She had attempted to control the pain and pruritus with over-the-counter topical hydrocortisone and oral diphenhydramine but nothing provided relief. She was recommended for follow-up at the hospital for further examination and biopsy of one of her lesions.
At this follow-up visit, the patient noted that the lesions had spread to her left knee. Multiple firm discrete papules and nodules, with central hyperkeratotic plugs, were noted along the extensor surfaces of her forearms, left extensor knee, and around her ankles (FIGURES 1A and 1B). Some of the lesions were tender. Examination of the rest of her skin was normal. A punch biopsy was obtained.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Kyrle disease
The patient’s end-stage renal disease and type 2 diabetes—along with findings from the physical examination—led us to suspect Kyrle disease. The punch biopsy, as well as the characteristic keratotic plugs (FIGURE 2) within epidermal invagination that was bordered by hyperkeratotic epidermis, confirmed the diagnosis.
Kyrle disease (also known as hyperkeratosis follicularis et follicularis in cutem penetrans) is a rare skin condition. It is 1 of 4 skin conditions that are classified as perforating skin disorders; the other 3 are elastosis perforans serpiginosa, reactive perforating collagenosis, and perforating folliculitis (TABLE1,2).3 Perforating skin disorders share the common characteristic of transepidermal elimination of material from the upper dermis.4 These disorders are typically classified based on the nature of the eliminated material and the type of epidermal disruption.5
There are 2 forms of Kyrle disease: an inherited form often seen in childhood that is not associated with systemic disease and an acquired form that occurs in adulthood, most commonly among women ages 35 to 70 years who have systemic disease.3,4,6 The acquired form of Kyrle disease is associated with diabetes and renal failure, but there is a lack of data on its pathogenesis.7,8
Characteristic findings include discrete pruritic, dry papules and nodules with central keratotic plugs that are occasionally tender. These can manifest over the extensor surface of the extremities, trunk, face, and scalp.4,7,9 Lesions most commonly manifest on the extensor surfaces of the lower extremities.
Other conditions that feature pruritic lesions
In addition to the other perforating skin disorders described in the TABLE,1,2 the differential for Kyrle disease includes the following:
Prurigo nodularis (PN) is a skin disorder in which the manifestation of extremely pruritic nodules leads to vigorous scratching and secondary infections. These lesions typically have a grouped and symmetrically distributed appearance. They often appear on extensor surfaces of upper and lower extremities.10 PN has no known etiology, but like Kyrle disease, is associated with renal failure. Biopsy can help to distinguish PN from Kyrle disease.
Continue to: Hypertrophic lichen planus
Hypertrophic lichen planus is a pruritic skin disorder characterized by the “6 Ps”: planar, purple, polygonal, pruritic, papules, and plaques. These lesions can mimic the early stages of Kyrle disease.11 However, in the later stages of Kyrle disease, discrete papules with hyperkeratotic plugs develop, whereas large plaques will be seen with lichen planus.
Keratosis pilaris (KP) is an extremely common, yet benign, disorder in which hair follicles become keratinized.12 KP can feature rough papules that are often described as “goosebumps” or having a sandpaper–like appearance. These papules often affect the upper arms. KP usually manifests in adolescents or young adults and tends to improve with age.12 The lesions are typically smaller than those seen in Kyrle disease and are asymptomatic. In addition, KP is not associated with systemic disease.
Target symptoms and any underlying conditions
In patients who have an acquired form of the disease, symptoms may improve by
For patients whose Kyrle disease is inherited or whose underlying condition is not easily treated, there are a number of treatment options to consider. First-line treatment includes topical keratolytics (salicylic acid and urea), topical retinoids, and ultraviolet light therapy.5,7 Systemic retinoids, topical steroids, cryotherapy, electrosurgery, CO2 laser surgery, and surgical excision have also been used with some success.7,14 Oral histamines and emollients also may help to relieve the pruritus. Lesions often recur upon discontinuation of therapy.
Our patient was referred to Dermatology for ultraviolet light therapy. She was also treated with topical 12% ammonium lactate twice daily. Within a few months, she reported improvement of her symptoms.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
1. Rapini R. Perforating disorders. Plastic Surgery Key. Published April 22, 2017. Accessed February 18, 2021. https://plasticsurgerykey.com/perforating-disorders/
2. Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581
3. Azad K, Hajirnis K, Sawant S, et al. Kyrle’s disease. Indian Dermatol Online J. 2013;4:378-379.
4. Arora K, Hajirnis KA, Sawant S, et al. Perforating disorders of the skin. Indian J Pathol Microbiol. 2013;56:355-358.
5. Ataseven A, Ozturk P, Kucukosmanoglu I, et al. Kyrle’s disease. BMJ Case Rep. 2014;2014: bcr2013009905.
6. Cunningham SR, Walsh M, Matthews R. Kyrle’s disease. J Am Acad Dermatol. 1987;16(pt 1):117-123.
7. Nair PA, Jivani NB, Diwan NG. Kyrle’s disease in a patient of diabetes mellitus and chronic renal failure on dialysis. J Family Med Prim Care. 2015;4:284-286.
8. Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
9. Kolla PK, Desai M, Pathapati RM, et al. Cutaneous manifestations in patients with chronic kidney disease on maintenance hemodialysis. ISRN Dermatol. 2012;2012:679619.
10. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-220.
11. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
12. Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
13. Chang P, Fernández V. Acquired perforating disease: report of nine cases. Int J Dermatol. 1993;32:874-876.
14. Wagner G, Sachse MM. Acquired reactive perforating dermatosis. J Dtsch Dermatol Ges. 2013;11:723-729.
FDA approves voclosporin for lupus nephritis
The Food and Drug Administration has approved voclosporin (Lupkynis) for the treatment of lupus nephritis, according to a Jan. 22 press release from manufacturer Aurinia Pharmaceuticals.
Lupkynis is a calcineurin-inhibitor immunosuppressant, and is the first oral medication to show effectiveness in lupus nephritis, according to the company. The drug is indicated for the treatment of adult patients with active lupus nephritis in combination with a background immunosuppressive therapy regimen, according to the drug label, which also has a boxed warning describing the increased risk of infections and malignancies, including lymphoma.
The approval of voclosporin was based on data from two studies, the AURORA phase 3 study and the AURA-LV phase 2 study. The studies included 533 adults with lupus nephritis who were randomized to 23.7 mg or placebo of voclosporin twice daily in the form of oral capsules, or placebo capsules, in addition to standard of care (mycophenolate mofetil plus low-dose glucocorticoids).
In the AURORA phase 3 study of 357 patients, close to twice as many patients in the treatment group showed a complete renal response, compared with the placebo group after 1 year (40.8% vs. 22.5%). In addition, patients treated with voclosporin more quickly achieved a significant reduction in urine protein to creatinine ratio, compared with the placebo patients (169 days vs. 372 days).
Severe adverse events were similar between the groups, including the most common complication of infection (10.1% and 11.2% for voclosporin and control groups, respectively). Other adverse reactions reported in at least 3% of the study participants included a decrease in glomerular filtration rate, hypertension, diarrhea, headache, anemia, cough, urinary tract infection, upper abdominal pain, dyspepsia, alopecia, renal impairment, abdominal pain, mouth ulceration, fatigue, tremor, acute kidney injury, and decreased appetite, according to the company press release.
Full clinical trial information for the AURORA study is available here.
The Food and Drug Administration has approved voclosporin (Lupkynis) for the treatment of lupus nephritis, according to a Jan. 22 press release from manufacturer Aurinia Pharmaceuticals.
Lupkynis is a calcineurin-inhibitor immunosuppressant, and is the first oral medication to show effectiveness in lupus nephritis, according to the company. The drug is indicated for the treatment of adult patients with active lupus nephritis in combination with a background immunosuppressive therapy regimen, according to the drug label, which also has a boxed warning describing the increased risk of infections and malignancies, including lymphoma.
The approval of voclosporin was based on data from two studies, the AURORA phase 3 study and the AURA-LV phase 2 study. The studies included 533 adults with lupus nephritis who were randomized to 23.7 mg or placebo of voclosporin twice daily in the form of oral capsules, or placebo capsules, in addition to standard of care (mycophenolate mofetil plus low-dose glucocorticoids).
In the AURORA phase 3 study of 357 patients, close to twice as many patients in the treatment group showed a complete renal response, compared with the placebo group after 1 year (40.8% vs. 22.5%). In addition, patients treated with voclosporin more quickly achieved a significant reduction in urine protein to creatinine ratio, compared with the placebo patients (169 days vs. 372 days).
Severe adverse events were similar between the groups, including the most common complication of infection (10.1% and 11.2% for voclosporin and control groups, respectively). Other adverse reactions reported in at least 3% of the study participants included a decrease in glomerular filtration rate, hypertension, diarrhea, headache, anemia, cough, urinary tract infection, upper abdominal pain, dyspepsia, alopecia, renal impairment, abdominal pain, mouth ulceration, fatigue, tremor, acute kidney injury, and decreased appetite, according to the company press release.
Full clinical trial information for the AURORA study is available here.
The Food and Drug Administration has approved voclosporin (Lupkynis) for the treatment of lupus nephritis, according to a Jan. 22 press release from manufacturer Aurinia Pharmaceuticals.
Lupkynis is a calcineurin-inhibitor immunosuppressant, and is the first oral medication to show effectiveness in lupus nephritis, according to the company. The drug is indicated for the treatment of adult patients with active lupus nephritis in combination with a background immunosuppressive therapy regimen, according to the drug label, which also has a boxed warning describing the increased risk of infections and malignancies, including lymphoma.
The approval of voclosporin was based on data from two studies, the AURORA phase 3 study and the AURA-LV phase 2 study. The studies included 533 adults with lupus nephritis who were randomized to 23.7 mg or placebo of voclosporin twice daily in the form of oral capsules, or placebo capsules, in addition to standard of care (mycophenolate mofetil plus low-dose glucocorticoids).
In the AURORA phase 3 study of 357 patients, close to twice as many patients in the treatment group showed a complete renal response, compared with the placebo group after 1 year (40.8% vs. 22.5%). In addition, patients treated with voclosporin more quickly achieved a significant reduction in urine protein to creatinine ratio, compared with the placebo patients (169 days vs. 372 days).
Severe adverse events were similar between the groups, including the most common complication of infection (10.1% and 11.2% for voclosporin and control groups, respectively). Other adverse reactions reported in at least 3% of the study participants included a decrease in glomerular filtration rate, hypertension, diarrhea, headache, anemia, cough, urinary tract infection, upper abdominal pain, dyspepsia, alopecia, renal impairment, abdominal pain, mouth ulceration, fatigue, tremor, acute kidney injury, and decreased appetite, according to the company press release.
Full clinical trial information for the AURORA study is available here.