User login
A Facility-Wide Plan to Increase Access to Medication for Opioid Use Disorder in Primary Care and General Mental Health Settings
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
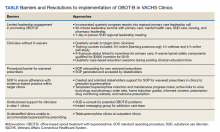
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
Depression rates up threefold since start of COVID-19
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
FROM LANCET REGIONAL HEALTH – AMERICAS
Old wives’ tales, traditional medicine, and science
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at alison.heru@cuanschutz.edu.
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at alison.heru@cuanschutz.edu.
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at alison.heru@cuanschutz.edu.
Should clinicians recommend vitamin D for psychiatric patients during COVID-19?
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
Amid a flurry of conflicting reports concerning the efficacy of vitamin D for COVID-19 patients, a sense of consternation has emerged in the health care sector regarding its overall utility.
Vitamin D plays a critical role in the restorative function of mental health. Low vitamin D levels correlate with mood disorders as well as the development of schizophrenia. In light of the rise in mental health dysfunction and the body of evidence examined to develop this article, we recommend that patients continue to incorporate regular vitamin D supplementation during the course of the pandemic with the goal of preventing deterioration of well-being. Recent studies have generally overlooked the role of vitamin D in mental health by primarily focusing on the immediacy of therapeutic management for medical disorders within the context of COVID-19.
What is the role of vitamin D in human physiology?
Vitamins play an integral role in homeostatic metabolism. Vitamin D, in particular, is intimately responsible for regulating the body’s underlying phosphorus and calcium balance, thereby facilitating bone mineralization.1 As an immunomodulatory hormone, vitamin D coordinates activities across innate and adaptive immune systems, providing defense against autoimmune diseases and miscellaneous infections.2
It is uncommon for people to be affected with vitamin D deficiency in equatorial zones, yet an Indonesian study uncovered low vitamin D effects (hypovitaminosis D) in virtually all of the patients in its COVID-19 case series.3
Likewise, a study conducted in Spain indicated that a whopping 82.2% of the COVID-19 patients endorsed clinically deficient levels of vitamin D, often within the context of severe presentation. Those patients also expressed elevated inflammatory markers, namely, D-dimer and ferritin.4
Comparable studies across the globe continue to support a correlative, if not causative, role for hypovitaminosis D and susceptibility to COVID-19. Mental health awareness entails healthy emotional interactions, preservation of well-being, and the ability to govern one’s thoughts and actions in accordance with societal expectations against the backdrop of ongoing psychosocial stressors. Such awareness helps ensure that people can make resourceful choices and meaningful associations, and can handle stress. We know that mental health is pivotal in dictating one’s overall health. This article provides a detailed exploration of the dynamics of mental health, COVID-19, and vitamin D.
The rationale for vitamin D supplementation therapy in COVID-19
When it comes to respiratory tract infections (RTI) such as COVID-19, influenza, and pneumonia, considerable interest has been generated with respect to the therapeutic efficacy of vitamin D in the acute setting. Vitamin D, as an inflammatory modulator, exerts a protective effect in patients with RTI, especially in those with deviations from baseline vitamin D levels.5
What is the rationale for administering vitamin D supplementation therapy for COVID-19? It has been noted that emergent cases of COVID-19 arise during the autumn months for European countries6 and there is also a firmly established connection between the amount of solar radiation/UV exposure (or the lack thereof) and influenza outbreaks,7 further underscoring the relevance of vitamin D levels. Despite those observations, wholesale implementation of vitamin D therapy should not be used in the acute setting for conditions such as COVID-19 or pneumonia as it is not supported by evidence-based practices. Despite the compound’s inherent antimicrobial actions,8 four randomized clinical trials involving pediatric subjects failed to demonstrate a significantly beneficial response (for example, radiographic resolution) to adjunctive supplementation during the course of acute pneumonia symptomatology.9 Likewise, data collected from a randomized controlled trial confirmed the suspicion that high-dose vitamin D therapy has no tangible effect, tied to mortality or otherwise, on moderate or severe presentations of COVID-19.10
Revisiting vitamin D supplementation therapy for mental health patients with COVID-19
It is clear that recent studies have undermined the overall applicability of vitamin D therapy with respect to acute presentations of COVID-19. However, our team would like to underscore the importance of vitamin D supplementation with respect to maintenance of the integrity of underlying mental health processes.
Numerous studies (for example, cross-sectional, cohort, case-control) have uncovered a statistically significant relationship between vitamin D deficiency and depression, including variants such as postpartum and antepartum depression. It should be noted that the pathophysiology for those variables is not entirely known and that the overall clinical utility of supplementation therapy has not previously been recommended because of existing gaps in the literature.11
In another prospective study involving a relatively small sample size, subjects with seasonal affective disorder (SAD) were either exposed to 10,000 IUs of vitamin D or phototherapy, and depression endpoints were evaluated via the Hamilton Rating Scale for Depression, the SIGH-SAD, and the SAD-8 depression scale. Improvements in 25-hydroxyvitamin D (25-OH D) levels correlated with improvements in depression metrics. However, subjects exposed to phototherapy sessions did not exhibit any meaningful improvements in clinical outcome.12
It is also possible that vitamin D deficiency is reflective of an overall poor nutritional status. People with schizophrenia have frequently been observed to have vitamin D deficiency with more than half of all patients also manifesting symptoms of osteoporosis, a condition that often necessitates vitamin D supplementation. The literature shows that the jury is still out regarding the applicability of vitamin D supplementation for schizophrenia patients, with numerous conflicting studies, including one randomized trial indicating an improvement in positive and negative symptoms as well as in the metabolic profile.13
However, in light of the rather large and growing body of evidence suggesting an increased risk of deterioration, psychological distress, and worsened prognosis during the pandemic coupled with the presence of medical and/or mental health morbidities, it would be sensible for psychiatric patients, especially those with preexisting deviations from baseline vitamin D levels, to consider vitamin D supplementation.
Vitamin D supplementation therapy, as a preventive, but not curative measure – one that is also low cost/high benefit – allows for the patient to be in a much better position from the perspective of her/his general health and nutritional status to tackle the ongoing psychosocial challenges of the pandemic and/or COVID-19 exposure.
Dr. Aman is a faculty member in the biology department at City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF) in Montreal; fellow, medical staff development, American Academy of Medical Management; and master online teacher (MOT) at the University of Illinois at Chicago. Dr. Aman disclosed no relevant relationships. Dr. Islam is a medical writer for the IMCHF and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical specialist. He disclosed no relevant financial relationships. Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston and is affiliated with Sturdy Memorial Hospital in Attleboro, Mass. He is on the speakers bureaus/advisory boards of Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience. Mr. Zaid Ulhaq Choudhry is a research assistant at the IMCHF. He has no disclosures. Dr. Zia Choudhry (Mr. Choudhry’s father) is chief scientific officer and head of the department of mental health and clinical research at the IMCHF. Dr. Choudhry has no disclosures.
References
1. van Driel M and van Leeuwen JPTM. Mol Cellular Endocrinol. 2017;453:46-51.
2. Charoenngam N and Holick MF. Nutrients. 2020 Jul 15;12(7):2097. doi: 103390/nu12072097.
3. Pinzon RT et al. Trop Med Health. 2020 Dec 20;48:102. doi: 10.1186/S41182-020-00277-w.
4. Hernández JL et al. J Clin Endocrinol Metab. 2021 Mar;106(3)e1343-53.
5. Martineau AR et al. BMJ. 2017;356:i6583. doi: 1136/bmj.i6583.
6. Walrand S. Sci Rep. 2021 Jan 21;11(1981). doi: 10.1038/s41598-021-81419-w.
7. Moan J. et al. Dermatoendocrinol. 2009 Nov-Dec;1(6):307-9.
8. Fabri M et al. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045.
9. Slow S et al. Sci Rep. 2018 Sep 14;8(1):13829. doi: 10.1038/s41598-018-32162-2.
10. Berman R. “Study confirms high doses of vitamin D have no effect on COVID-19.” Medical News Today. 2021 May 4.
11. Menon V et al. Indian J Psychol Med. 2020 Jan-Feb;42(1):11-21.
12. Gloth 3rd FM et al. Nutr Health Aging. 1999;3(1):5-7.
13. Cui X et al. Mol Psychiatry. 2021 Jan 26. doi:10.1038/s41380-021-01025-0.
MIND diet preserves cognition, new data show
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adherence to the MIND diet can improve memory and thinking skills of older adults, even in the presence of Alzheimer’s disease pathology, new data from the Rush Memory and Aging Project (MAP) show.
“The MIND diet was associated with better cognitive functions independently of brain pathologies related to Alzheimer’s disease, suggesting that diet may contribute to cognitive resilience, which ultimately indicates that it is never too late for dementia prevention,” lead author Klodian Dhana, MD, PhD, with the Rush Institute of Healthy Aging at Rush University, Chicago, said in an interview.
The study was published online Sept. 14, 2021, in the Journal of Alzheimer’s Disease.
Impact on brain pathology
“While previous investigations determined that the MIND diet is associated with a slower cognitive decline, the current study furthered the diet and brain health evidence by assessing the impact of brain pathology in the diet-cognition relationship,” Dr. Dhana said.
The MIND diet was pioneered by the late Martha Clare Morris, ScD, a Rush nutritional epidemiologist, who died in 2020 of cancer at age 64. A hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets, the MIND diet includes green leafy vegetables, fish, nuts, berries, beans, and whole grains and limits consumption of fried and fast foods, sweets, and pastries.
The current study focused on 569 older adults who died while participating in the MAP study, which began in 1997. Participants in the study were mostly White and were without known dementia. All of the participants agreed to undergo annual clinical evaluations. They also agreed to undergo brain autopsy after death.
Beginning in 2004, participants completed annual food frequency questionnaires, which were used to calculate a MIND diet score based on how often the participants ate specific foods.
The researchers used a series of regression analyses to examine associations of the MIND diet, dementia-related brain pathologies, and global cognition near the time of death. Analyses were adjusted for age, sex, education, apo E4, late-life cognitive activities, and total energy intake.
(beta, 0.119; P = .003).
Notably, the researchers said, neither the strength nor the significance of association changed markedly when AD pathology and other brain pathologies were included in the model (beta, 0.111; P = .003).
The relationship between better adherence to the MIND diet and better cognition remained significant when the analysis was restricted to individuals without mild cognitive impairment at baseline (beta, 0.121; P = .005) as well as to persons in whom a postmortem diagnosis of AD was made on the basis of NIA-Reagan consensus recommendations (beta, 0.114; P = .023).
The limitations of the study include the reliance on self-reported diet information and a sample made up of mostly White volunteers who agreed to annual evaluations and postmortem organ donation, thus limiting generalizability.
Strengths of the study include the prospective design with annual assessment of cognitive function using standardized tests and collection of the dietary information using validated questionnaires. Also, the neuropathologic evaluations were performed by examiners blinded to clinical data.
“Diet changes can impact cognitive functioning and risk of dementia, for better or worse. There are fairly simple diet and lifestyle changes a person could make that may help to slow cognitive decline with aging and contribute to brain health,” Dr. Dhana said in a news release.
Builds resilience
Weighing in on the study, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this “interesting study sheds light on the impact of nutrition on cognitive function.
“The findings add to the growing literature that lifestyle factors – like access to a heart-healthy diet – may help the brain be more resilient to disease-specific changes,” Snyder said in an interview.
“The Alzheimer’s Association’s US POINTER study is investigating how lifestyle interventions, including nutrition guidance, like the MIND diet, may impact a person’s risk of cognitive decline. An ancillary study of the US POINTER will include brain imaging to investigate how these lifestyle interventions impact the biology of the brain,” Dr. Snyder noted.
The research was supported by the National Institute on Aging of the National Institutes of Health. Dr. Dhana and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Military sexual trauma tied to risk for hypertension
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
COVID-19: Greater mortality among psych patients remains a mystery
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Relapse risk increased with antidepressant discontinuation
a new study shows.
The results of the Antidepressants to Prevent Relapse in Depression (ANTLER) trial also suggest that “many patients can discontinue their antidepressants safely in primary care without relapsing, when there is a tapering regime,” said lead investigator Gemma Lewis, PhD, from University College London, in an interview.
The multicenter, randomized, double-blind trial, which was published in the New England Journal of Medicine (2021;385:1257-67), included 478 patients, from 150 primary care practices in the United Kingdom.
The participants (73% female, average age 54 years) had a history of at least two depressive episodes or had been taking antidepressants (citalopram, fluoxetine, sertraline, or mirtazapine) for at least 2 years. The vast majority of patients – 70% – had been using the drugs for more than 3 years, the researchers wrote.
Study participants were randomized to either maintain their antidepressant regimen or to taper off for up to 2 months before switching to a placebo.
Over a follow-up of 52 weeks, relapse occurred in 56% of those who discontinued, compared with 39% of those who maintained their regimen (hazard ratio, 2.06; P < .001). Relapse also occurred sooner in the discontinuation group (13 weeks vs. 19 weeks).
The definition of relapse was answering yes to either of the following two questions:
- Have you had a spell of feeling sad, miserable, or depressed?
- Have you been unable to enjoy or take an interest in things as much as you usually do?
Patients also had to report that one of these experiences had lasted for 2 weeks or more, and having had at least one of the following symptoms: depressive thoughts, fatigue, loss of concentration, or sleep disturbance.
By the end of the trial, 39% of patients in the group who discontinued taking an antidepressant had returned to taking that type of drug.
“We found that remaining on antidepressants long-term does effectively reduce the risk of relapse. However, we also found that 44% of those who discontinued their antidepressants did not relapse after a full year,” Dr. Lewis said.
Who can stop medications without relapsing is unknown
“Many people can stop their medication without relapsing, though at present we cannot identify who those people are,” noted Dr. Lewis.
“Our study did not investigate who is at higher risk of relapse … but this is something we will focus on in the future,” she said.
For primary care clinicians whose patients are considering discontinuation of antidepressant medication, “current best practice is to engage with patients’ priorities and collaborate in coming to a decision,” she noted.
“For the individual patient, it is only possible to know about the average likelihood of relapse – and the severity of potential relapses will also be unpredictable. Our findings will give patients and clinicians an estimate of the likely benefits and harms of stopping long-term maintenance antidepressants to inform shared decision-making in primary care.”
Findings are ‘important’ but ‘disappointing’
In an editorial published alongside the study (N Engl J Med. 2021;385:1327-8), Jeffrey L. Jackson, MD, MPH, from the Zablocki VA Medical Center and the Medical College of Wisconsin in Milwaukee characterized the findings as “important but disappointing.”
“They confirm what most primary care physicians already knew or intuited. The frequency of relapse after the discontinuation of treatment is high, particularly among patients with several previous depressive episodes,” he explained.
Dr. Jackson also pointed out some unknowns about the trial, including the length trial participants had been in remission for depression.
“It is unclear whether the trial results are generalizable to primary care patients with a first episode of depression,” he said, and noted that participants with three or more previous depressive episodes were more than twice as likely to relapse, compared with participants with fewer episodes.
“I encourage patients with a single bout of depression, especially episodes that are triggered by a life event, such as loss of a loved one, to consider weaning antidepressant treatment after at least 6 months of remission,” he wrote. “For those with three or more previous bouts of depression, my practice has been to recommend that they anticipate medical treatment for life or, if they wish to stop taking medication, explore nonpharmacologic approaches, such as cognitive-behavior therapy.”
Protective effect of antidepressants was clear
“This is an important paper providing an evidence base to the often-cited recommendation that after two or more episodes of depression, antidepressant medication should be continued indefinitely,” said Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College, Thomas Jefferson University in Philadelphia, who was not involved in the study.
“The protective effect of antidepressant medication here was clear – those who discontinued antidepressant medication had a clinically and significantly higher rate of relapse at the end of a year.”
Side effects can be significant
“It is important to note, though, that in the discontinuation group, 44% of patients did not experience a relapse,” Dr. Skolnik said. “While antidepressants work without significant side effects for many patients, for others there are significant side effects that include adverse sexual side effects, effects on appetite and weight, nighttime sweats, and other side effects.”
“So, this study should not be confused to mean that all patients who have had recurrent depression should remain on antidepressants long term. The decision about whether to continue an antidepressant is influenced by many things and should be a shared decision-making process between clinician and patient, informed by the important results of this study, the current situation of the patient, and most importantly, the patient’s informed decision of what they would like to do,” he said.
The study was funded by the U.K. National Institute for Health Research
Dr. Lewis, Dr. Jackson, and Dr. Skolnik reported no conflicts of interest.
a new study shows.
The results of the Antidepressants to Prevent Relapse in Depression (ANTLER) trial also suggest that “many patients can discontinue their antidepressants safely in primary care without relapsing, when there is a tapering regime,” said lead investigator Gemma Lewis, PhD, from University College London, in an interview.
The multicenter, randomized, double-blind trial, which was published in the New England Journal of Medicine (2021;385:1257-67), included 478 patients, from 150 primary care practices in the United Kingdom.
The participants (73% female, average age 54 years) had a history of at least two depressive episodes or had been taking antidepressants (citalopram, fluoxetine, sertraline, or mirtazapine) for at least 2 years. The vast majority of patients – 70% – had been using the drugs for more than 3 years, the researchers wrote.
Study participants were randomized to either maintain their antidepressant regimen or to taper off for up to 2 months before switching to a placebo.
Over a follow-up of 52 weeks, relapse occurred in 56% of those who discontinued, compared with 39% of those who maintained their regimen (hazard ratio, 2.06; P < .001). Relapse also occurred sooner in the discontinuation group (13 weeks vs. 19 weeks).
The definition of relapse was answering yes to either of the following two questions:
- Have you had a spell of feeling sad, miserable, or depressed?
- Have you been unable to enjoy or take an interest in things as much as you usually do?
Patients also had to report that one of these experiences had lasted for 2 weeks or more, and having had at least one of the following symptoms: depressive thoughts, fatigue, loss of concentration, or sleep disturbance.
By the end of the trial, 39% of patients in the group who discontinued taking an antidepressant had returned to taking that type of drug.
“We found that remaining on antidepressants long-term does effectively reduce the risk of relapse. However, we also found that 44% of those who discontinued their antidepressants did not relapse after a full year,” Dr. Lewis said.
Who can stop medications without relapsing is unknown
“Many people can stop their medication without relapsing, though at present we cannot identify who those people are,” noted Dr. Lewis.
“Our study did not investigate who is at higher risk of relapse … but this is something we will focus on in the future,” she said.
For primary care clinicians whose patients are considering discontinuation of antidepressant medication, “current best practice is to engage with patients’ priorities and collaborate in coming to a decision,” she noted.
“For the individual patient, it is only possible to know about the average likelihood of relapse – and the severity of potential relapses will also be unpredictable. Our findings will give patients and clinicians an estimate of the likely benefits and harms of stopping long-term maintenance antidepressants to inform shared decision-making in primary care.”
Findings are ‘important’ but ‘disappointing’
In an editorial published alongside the study (N Engl J Med. 2021;385:1327-8), Jeffrey L. Jackson, MD, MPH, from the Zablocki VA Medical Center and the Medical College of Wisconsin in Milwaukee characterized the findings as “important but disappointing.”
“They confirm what most primary care physicians already knew or intuited. The frequency of relapse after the discontinuation of treatment is high, particularly among patients with several previous depressive episodes,” he explained.
Dr. Jackson also pointed out some unknowns about the trial, including the length trial participants had been in remission for depression.
“It is unclear whether the trial results are generalizable to primary care patients with a first episode of depression,” he said, and noted that participants with three or more previous depressive episodes were more than twice as likely to relapse, compared with participants with fewer episodes.
“I encourage patients with a single bout of depression, especially episodes that are triggered by a life event, such as loss of a loved one, to consider weaning antidepressant treatment after at least 6 months of remission,” he wrote. “For those with three or more previous bouts of depression, my practice has been to recommend that they anticipate medical treatment for life or, if they wish to stop taking medication, explore nonpharmacologic approaches, such as cognitive-behavior therapy.”
Protective effect of antidepressants was clear
“This is an important paper providing an evidence base to the often-cited recommendation that after two or more episodes of depression, antidepressant medication should be continued indefinitely,” said Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College, Thomas Jefferson University in Philadelphia, who was not involved in the study.
“The protective effect of antidepressant medication here was clear – those who discontinued antidepressant medication had a clinically and significantly higher rate of relapse at the end of a year.”
Side effects can be significant
“It is important to note, though, that in the discontinuation group, 44% of patients did not experience a relapse,” Dr. Skolnik said. “While antidepressants work without significant side effects for many patients, for others there are significant side effects that include adverse sexual side effects, effects on appetite and weight, nighttime sweats, and other side effects.”
“So, this study should not be confused to mean that all patients who have had recurrent depression should remain on antidepressants long term. The decision about whether to continue an antidepressant is influenced by many things and should be a shared decision-making process between clinician and patient, informed by the important results of this study, the current situation of the patient, and most importantly, the patient’s informed decision of what they would like to do,” he said.
The study was funded by the U.K. National Institute for Health Research
Dr. Lewis, Dr. Jackson, and Dr. Skolnik reported no conflicts of interest.
a new study shows.
The results of the Antidepressants to Prevent Relapse in Depression (ANTLER) trial also suggest that “many patients can discontinue their antidepressants safely in primary care without relapsing, when there is a tapering regime,” said lead investigator Gemma Lewis, PhD, from University College London, in an interview.
The multicenter, randomized, double-blind trial, which was published in the New England Journal of Medicine (2021;385:1257-67), included 478 patients, from 150 primary care practices in the United Kingdom.
The participants (73% female, average age 54 years) had a history of at least two depressive episodes or had been taking antidepressants (citalopram, fluoxetine, sertraline, or mirtazapine) for at least 2 years. The vast majority of patients – 70% – had been using the drugs for more than 3 years, the researchers wrote.
Study participants were randomized to either maintain their antidepressant regimen or to taper off for up to 2 months before switching to a placebo.
Over a follow-up of 52 weeks, relapse occurred in 56% of those who discontinued, compared with 39% of those who maintained their regimen (hazard ratio, 2.06; P < .001). Relapse also occurred sooner in the discontinuation group (13 weeks vs. 19 weeks).
The definition of relapse was answering yes to either of the following two questions:
- Have you had a spell of feeling sad, miserable, or depressed?
- Have you been unable to enjoy or take an interest in things as much as you usually do?
Patients also had to report that one of these experiences had lasted for 2 weeks or more, and having had at least one of the following symptoms: depressive thoughts, fatigue, loss of concentration, or sleep disturbance.
By the end of the trial, 39% of patients in the group who discontinued taking an antidepressant had returned to taking that type of drug.
“We found that remaining on antidepressants long-term does effectively reduce the risk of relapse. However, we also found that 44% of those who discontinued their antidepressants did not relapse after a full year,” Dr. Lewis said.
Who can stop medications without relapsing is unknown
“Many people can stop their medication without relapsing, though at present we cannot identify who those people are,” noted Dr. Lewis.
“Our study did not investigate who is at higher risk of relapse … but this is something we will focus on in the future,” she said.
For primary care clinicians whose patients are considering discontinuation of antidepressant medication, “current best practice is to engage with patients’ priorities and collaborate in coming to a decision,” she noted.
“For the individual patient, it is only possible to know about the average likelihood of relapse – and the severity of potential relapses will also be unpredictable. Our findings will give patients and clinicians an estimate of the likely benefits and harms of stopping long-term maintenance antidepressants to inform shared decision-making in primary care.”
Findings are ‘important’ but ‘disappointing’
In an editorial published alongside the study (N Engl J Med. 2021;385:1327-8), Jeffrey L. Jackson, MD, MPH, from the Zablocki VA Medical Center and the Medical College of Wisconsin in Milwaukee characterized the findings as “important but disappointing.”
“They confirm what most primary care physicians already knew or intuited. The frequency of relapse after the discontinuation of treatment is high, particularly among patients with several previous depressive episodes,” he explained.
Dr. Jackson also pointed out some unknowns about the trial, including the length trial participants had been in remission for depression.
“It is unclear whether the trial results are generalizable to primary care patients with a first episode of depression,” he said, and noted that participants with three or more previous depressive episodes were more than twice as likely to relapse, compared with participants with fewer episodes.
“I encourage patients with a single bout of depression, especially episodes that are triggered by a life event, such as loss of a loved one, to consider weaning antidepressant treatment after at least 6 months of remission,” he wrote. “For those with three or more previous bouts of depression, my practice has been to recommend that they anticipate medical treatment for life or, if they wish to stop taking medication, explore nonpharmacologic approaches, such as cognitive-behavior therapy.”
Protective effect of antidepressants was clear
“This is an important paper providing an evidence base to the often-cited recommendation that after two or more episodes of depression, antidepressant medication should be continued indefinitely,” said Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College, Thomas Jefferson University in Philadelphia, who was not involved in the study.
“The protective effect of antidepressant medication here was clear – those who discontinued antidepressant medication had a clinically and significantly higher rate of relapse at the end of a year.”
Side effects can be significant
“It is important to note, though, that in the discontinuation group, 44% of patients did not experience a relapse,” Dr. Skolnik said. “While antidepressants work without significant side effects for many patients, for others there are significant side effects that include adverse sexual side effects, effects on appetite and weight, nighttime sweats, and other side effects.”
“So, this study should not be confused to mean that all patients who have had recurrent depression should remain on antidepressants long term. The decision about whether to continue an antidepressant is influenced by many things and should be a shared decision-making process between clinician and patient, informed by the important results of this study, the current situation of the patient, and most importantly, the patient’s informed decision of what they would like to do,” he said.
The study was funded by the U.K. National Institute for Health Research
Dr. Lewis, Dr. Jackson, and Dr. Skolnik reported no conflicts of interest.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Antipsychotic effective for bipolar depression in phase 3 trial
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Nutritious meals, more fruits and vegetables boost children’s mental and emotional health
Good nutrition has long been linked to better behavior and academic performance in schoolchildren, as longstanding breakfast and lunch programs in U.S. schools attest. Now British researchers report that nutrition, a modifiable risk factor that can adversely impact mental health, should be part of public health strategies to boost children’s psychological wellness.
In a cross-sectional study published online Sept. 27 in BMJ Nutrition, Prevention & Health, a team from the University of East Anglia in Norwich, England, found a nutritious breakfast and lunch were linked to emotional well-being in schoolchildren of both primary and secondary school age. They also found that some school kids ate neither breakfast nor lunch.
In particular, eating more fruits and vegetables was significantly associated with better mental health in secondary schoolchildren, while a nutritious breakfast and lunch were linked to emotional well-being in students across the age spectrum, according to senior lecturer Richard P. Hayhoe, PhD, of East Anglia University and Anglia Ruskin University in Norwich and colleagues.
They found that primary school pupils who ate only a snack for breakfast had mental well-being scores 5.50 units lower than those eating a substantial breakfast, while having no lunch was tied to scores more than 6 units lower.
“The importance of good-quality nutrition for childhood growth and development is well established,” the authors wrote. “As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being.”
Their current analysis examined data on 7,570 secondary and 1,253 primary school children from 50 schools participating in the Norfolk Children and Young People Health and Well-being Survey 2017.
Multivariable linear regression measured the association between nutritional factors and mental well-being assessed by the Warwick-Edinburgh Mental Well-being Scale for secondary school pupils or by the Stirling Children’s Well-being Scale for primary school pupils. All analyses were adjusted for covariates including demographic, health variables, living/home situations, and adverse experiences.
“The 2017 survey provided a means for Norfolk children and young people to share their feelings on topics such as healthy lifestyles and nutrition, relationships, school experiences, bullying, and their mental well-being,” Dr. Hayhoe said in an interview. “Initial analysis of the data suggested an association between nutrition and well-being and so we decided to investigate this further.”
Dr. Hayhoe added that, as in the United States, youngsters in England get a high proportion of their daily calories from ultraprocessed convenience foods of lesser nutritional value.
“But what we didn’t know was whether the dietary habits of children in our survey had any association with their mental well-being,” he said. “Our current findings suggest that increasing fruit and vegetable consumption and ensuring all schoolchildren eat a nutritional breakfast and lunch may be of benefit to their mental well-being.”
His group cautions, however, that this is an observational study that cannot establish direct causation.
“This study provides the first insights into how fruit and vegetable intake affects children’s mental health, and contributes to the emerging evidence around ‘food and mood,’ ” said Sumantra Ray, MD, executive director of the NNEdPro Global Centre for Nutrition and Health in Cambridge, England.
“The findings are timely, not only because of the impact the pandemic has had on mental well-being, food security, and diet quality, especially in school children, but also in light of the recently published National Food Strategy for England, which highlighted gaps in school meal provision,” added Dr. Ray, who was not involved in the study.
Study results
In total, 10,853 schoolchildren completed the survey: 9% of Norfolk primary school children aged 9-11 and 22% of secondary school students, with approximately 6% of these in the 17- and 18-year-old age bracket. Comprehensive dietary questions explored fruit and vegetable intake, as well as type of breakfast and lunch eaten, alcohol intake, eligibility for free school meals, and satisfaction with weight.
The survey also gathered information on parameters ranging from having one’s own bedroom and bed and exposure to violence or discord in the home.
“Some of these were found to be associated with lower mental well-being scores, but we did not specifically investigate the interaction between these factors and the nutritional factors,” Dr. Hayhoe said. However, the difference in mental well-being between children who ate the most fruit and vegetables and those who ate the least was on a similar scale to those reporting daily, or almost daily, arguing or violence at home, he said.
Average mental health was assessed using validated age-appropriate measures. The mean mental health score of participants was 46.6 out of 70 for secondary school students and 46 out of 60 for primary school pupils.
Among the survey findings were:
- Just 25% of secondary school participants and 28.5% of primary school pupils reported eating the recommended five portions of fruits and vegetables a day, with 10% and 9%, respectively, eating none.
- 21% of secondary and 12% of primary school pupils consumed only a non–energy drink or nothing for breakfast, while 11.5% of secondary schoolchildren ate no lunch. In one high school class of 30, for example, four had nothing to eat or drink before starting classes in the morning, and three had nothing to eat or drink before starting classes in the afternoon.
- Higher combined fruit and vegetable intake was significantly associated in dose-related fashion with higher mental health scores: 3.73 (95% confidence interval, 2.94- 4.53) units higher in those consuming five or more fruits and vegetables (P < .001), compared with none.
- Breakfast or lunch type also correlated with significant differences in well-being scores. Compared with children consuming a conventional breakfast (porridge, toast, cereal, yogurt, fruit, or a cooked meal), those eating no breakfast had mean well-being scores that were 2.73 (95% CI, 2.11-3.35) units lower (P < .001). Those consuming only an energy drink scored even worse: 3.14 (95% CI, 1.20- 5.09) units lower (P = .002).
- Skipping lunch resulted in a 2.95-unit drop in well-being score (95% CI, 2.22-3.68, P < .001), compared with consuming a packed lunch.
In terms of the amounts of fruits and vegetables consumed, one or two daily portions were associated with a score 1.42 units higher, while three or four portions correlated with a score 2.34 units higher. Those eating five or more portions scored 3.73 units higher.
- For primary school pupils, eating only a snack for breakfast was associated with a score 5.50 units lower, and consuming only a non–energy drink was tied to a score 2.67 units lower than eating a conventional breakfast. Not eating any breakfast was associated with a score 3.62 units lower.
- Eating school food versus a packed lunch was associated with a score 1.27 units lower, although this wasn’t statistically significant. Having no lunch was associated with a score 6.08 units lower, although only a few children fell into this group.
“As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being,” the authors wrote, calling for further investigation of the association between nutrition and mental well-being.
This study was commissioned by Norfolk County Council Public Health and the Norfolk Safeguarding Children Board. The University of East Anglia and Social Care Partners provided funding to support Dr. Hayhoe’s work on this project.
Some coauthors are employed by the Norfolk County Council that commissioned the survey.
Good nutrition has long been linked to better behavior and academic performance in schoolchildren, as longstanding breakfast and lunch programs in U.S. schools attest. Now British researchers report that nutrition, a modifiable risk factor that can adversely impact mental health, should be part of public health strategies to boost children’s psychological wellness.
In a cross-sectional study published online Sept. 27 in BMJ Nutrition, Prevention & Health, a team from the University of East Anglia in Norwich, England, found a nutritious breakfast and lunch were linked to emotional well-being in schoolchildren of both primary and secondary school age. They also found that some school kids ate neither breakfast nor lunch.
In particular, eating more fruits and vegetables was significantly associated with better mental health in secondary schoolchildren, while a nutritious breakfast and lunch were linked to emotional well-being in students across the age spectrum, according to senior lecturer Richard P. Hayhoe, PhD, of East Anglia University and Anglia Ruskin University in Norwich and colleagues.
They found that primary school pupils who ate only a snack for breakfast had mental well-being scores 5.50 units lower than those eating a substantial breakfast, while having no lunch was tied to scores more than 6 units lower.
“The importance of good-quality nutrition for childhood growth and development is well established,” the authors wrote. “As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being.”
Their current analysis examined data on 7,570 secondary and 1,253 primary school children from 50 schools participating in the Norfolk Children and Young People Health and Well-being Survey 2017.
Multivariable linear regression measured the association between nutritional factors and mental well-being assessed by the Warwick-Edinburgh Mental Well-being Scale for secondary school pupils or by the Stirling Children’s Well-being Scale for primary school pupils. All analyses were adjusted for covariates including demographic, health variables, living/home situations, and adverse experiences.
“The 2017 survey provided a means for Norfolk children and young people to share their feelings on topics such as healthy lifestyles and nutrition, relationships, school experiences, bullying, and their mental well-being,” Dr. Hayhoe said in an interview. “Initial analysis of the data suggested an association between nutrition and well-being and so we decided to investigate this further.”
Dr. Hayhoe added that, as in the United States, youngsters in England get a high proportion of their daily calories from ultraprocessed convenience foods of lesser nutritional value.
“But what we didn’t know was whether the dietary habits of children in our survey had any association with their mental well-being,” he said. “Our current findings suggest that increasing fruit and vegetable consumption and ensuring all schoolchildren eat a nutritional breakfast and lunch may be of benefit to their mental well-being.”
His group cautions, however, that this is an observational study that cannot establish direct causation.
“This study provides the first insights into how fruit and vegetable intake affects children’s mental health, and contributes to the emerging evidence around ‘food and mood,’ ” said Sumantra Ray, MD, executive director of the NNEdPro Global Centre for Nutrition and Health in Cambridge, England.
“The findings are timely, not only because of the impact the pandemic has had on mental well-being, food security, and diet quality, especially in school children, but also in light of the recently published National Food Strategy for England, which highlighted gaps in school meal provision,” added Dr. Ray, who was not involved in the study.
Study results
In total, 10,853 schoolchildren completed the survey: 9% of Norfolk primary school children aged 9-11 and 22% of secondary school students, with approximately 6% of these in the 17- and 18-year-old age bracket. Comprehensive dietary questions explored fruit and vegetable intake, as well as type of breakfast and lunch eaten, alcohol intake, eligibility for free school meals, and satisfaction with weight.
The survey also gathered information on parameters ranging from having one’s own bedroom and bed and exposure to violence or discord in the home.
“Some of these were found to be associated with lower mental well-being scores, but we did not specifically investigate the interaction between these factors and the nutritional factors,” Dr. Hayhoe said. However, the difference in mental well-being between children who ate the most fruit and vegetables and those who ate the least was on a similar scale to those reporting daily, or almost daily, arguing or violence at home, he said.
Average mental health was assessed using validated age-appropriate measures. The mean mental health score of participants was 46.6 out of 70 for secondary school students and 46 out of 60 for primary school pupils.
Among the survey findings were:
- Just 25% of secondary school participants and 28.5% of primary school pupils reported eating the recommended five portions of fruits and vegetables a day, with 10% and 9%, respectively, eating none.
- 21% of secondary and 12% of primary school pupils consumed only a non–energy drink or nothing for breakfast, while 11.5% of secondary schoolchildren ate no lunch. In one high school class of 30, for example, four had nothing to eat or drink before starting classes in the morning, and three had nothing to eat or drink before starting classes in the afternoon.
- Higher combined fruit and vegetable intake was significantly associated in dose-related fashion with higher mental health scores: 3.73 (95% confidence interval, 2.94- 4.53) units higher in those consuming five or more fruits and vegetables (P < .001), compared with none.
- Breakfast or lunch type also correlated with significant differences in well-being scores. Compared with children consuming a conventional breakfast (porridge, toast, cereal, yogurt, fruit, or a cooked meal), those eating no breakfast had mean well-being scores that were 2.73 (95% CI, 2.11-3.35) units lower (P < .001). Those consuming only an energy drink scored even worse: 3.14 (95% CI, 1.20- 5.09) units lower (P = .002).
- Skipping lunch resulted in a 2.95-unit drop in well-being score (95% CI, 2.22-3.68, P < .001), compared with consuming a packed lunch.
In terms of the amounts of fruits and vegetables consumed, one or two daily portions were associated with a score 1.42 units higher, while three or four portions correlated with a score 2.34 units higher. Those eating five or more portions scored 3.73 units higher.
- For primary school pupils, eating only a snack for breakfast was associated with a score 5.50 units lower, and consuming only a non–energy drink was tied to a score 2.67 units lower than eating a conventional breakfast. Not eating any breakfast was associated with a score 3.62 units lower.
- Eating school food versus a packed lunch was associated with a score 1.27 units lower, although this wasn’t statistically significant. Having no lunch was associated with a score 6.08 units lower, although only a few children fell into this group.
“As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being,” the authors wrote, calling for further investigation of the association between nutrition and mental well-being.
This study was commissioned by Norfolk County Council Public Health and the Norfolk Safeguarding Children Board. The University of East Anglia and Social Care Partners provided funding to support Dr. Hayhoe’s work on this project.
Some coauthors are employed by the Norfolk County Council that commissioned the survey.
Good nutrition has long been linked to better behavior and academic performance in schoolchildren, as longstanding breakfast and lunch programs in U.S. schools attest. Now British researchers report that nutrition, a modifiable risk factor that can adversely impact mental health, should be part of public health strategies to boost children’s psychological wellness.
In a cross-sectional study published online Sept. 27 in BMJ Nutrition, Prevention & Health, a team from the University of East Anglia in Norwich, England, found a nutritious breakfast and lunch were linked to emotional well-being in schoolchildren of both primary and secondary school age. They also found that some school kids ate neither breakfast nor lunch.
In particular, eating more fruits and vegetables was significantly associated with better mental health in secondary schoolchildren, while a nutritious breakfast and lunch were linked to emotional well-being in students across the age spectrum, according to senior lecturer Richard P. Hayhoe, PhD, of East Anglia University and Anglia Ruskin University in Norwich and colleagues.
They found that primary school pupils who ate only a snack for breakfast had mental well-being scores 5.50 units lower than those eating a substantial breakfast, while having no lunch was tied to scores more than 6 units lower.
“The importance of good-quality nutrition for childhood growth and development is well established,” the authors wrote. “As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being.”
Their current analysis examined data on 7,570 secondary and 1,253 primary school children from 50 schools participating in the Norfolk Children and Young People Health and Well-being Survey 2017.
Multivariable linear regression measured the association between nutritional factors and mental well-being assessed by the Warwick-Edinburgh Mental Well-being Scale for secondary school pupils or by the Stirling Children’s Well-being Scale for primary school pupils. All analyses were adjusted for covariates including demographic, health variables, living/home situations, and adverse experiences.
“The 2017 survey provided a means for Norfolk children and young people to share their feelings on topics such as healthy lifestyles and nutrition, relationships, school experiences, bullying, and their mental well-being,” Dr. Hayhoe said in an interview. “Initial analysis of the data suggested an association between nutrition and well-being and so we decided to investigate this further.”
Dr. Hayhoe added that, as in the United States, youngsters in England get a high proportion of their daily calories from ultraprocessed convenience foods of lesser nutritional value.
“But what we didn’t know was whether the dietary habits of children in our survey had any association with their mental well-being,” he said. “Our current findings suggest that increasing fruit and vegetable consumption and ensuring all schoolchildren eat a nutritional breakfast and lunch may be of benefit to their mental well-being.”
His group cautions, however, that this is an observational study that cannot establish direct causation.
“This study provides the first insights into how fruit and vegetable intake affects children’s mental health, and contributes to the emerging evidence around ‘food and mood,’ ” said Sumantra Ray, MD, executive director of the NNEdPro Global Centre for Nutrition and Health in Cambridge, England.
“The findings are timely, not only because of the impact the pandemic has had on mental well-being, food security, and diet quality, especially in school children, but also in light of the recently published National Food Strategy for England, which highlighted gaps in school meal provision,” added Dr. Ray, who was not involved in the study.
Study results
In total, 10,853 schoolchildren completed the survey: 9% of Norfolk primary school children aged 9-11 and 22% of secondary school students, with approximately 6% of these in the 17- and 18-year-old age bracket. Comprehensive dietary questions explored fruit and vegetable intake, as well as type of breakfast and lunch eaten, alcohol intake, eligibility for free school meals, and satisfaction with weight.
The survey also gathered information on parameters ranging from having one’s own bedroom and bed and exposure to violence or discord in the home.
“Some of these were found to be associated with lower mental well-being scores, but we did not specifically investigate the interaction between these factors and the nutritional factors,” Dr. Hayhoe said. However, the difference in mental well-being between children who ate the most fruit and vegetables and those who ate the least was on a similar scale to those reporting daily, or almost daily, arguing or violence at home, he said.
Average mental health was assessed using validated age-appropriate measures. The mean mental health score of participants was 46.6 out of 70 for secondary school students and 46 out of 60 for primary school pupils.
Among the survey findings were:
- Just 25% of secondary school participants and 28.5% of primary school pupils reported eating the recommended five portions of fruits and vegetables a day, with 10% and 9%, respectively, eating none.
- 21% of secondary and 12% of primary school pupils consumed only a non–energy drink or nothing for breakfast, while 11.5% of secondary schoolchildren ate no lunch. In one high school class of 30, for example, four had nothing to eat or drink before starting classes in the morning, and three had nothing to eat or drink before starting classes in the afternoon.
- Higher combined fruit and vegetable intake was significantly associated in dose-related fashion with higher mental health scores: 3.73 (95% confidence interval, 2.94- 4.53) units higher in those consuming five or more fruits and vegetables (P < .001), compared with none.
- Breakfast or lunch type also correlated with significant differences in well-being scores. Compared with children consuming a conventional breakfast (porridge, toast, cereal, yogurt, fruit, or a cooked meal), those eating no breakfast had mean well-being scores that were 2.73 (95% CI, 2.11-3.35) units lower (P < .001). Those consuming only an energy drink scored even worse: 3.14 (95% CI, 1.20- 5.09) units lower (P = .002).
- Skipping lunch resulted in a 2.95-unit drop in well-being score (95% CI, 2.22-3.68, P < .001), compared with consuming a packed lunch.
In terms of the amounts of fruits and vegetables consumed, one or two daily portions were associated with a score 1.42 units higher, while three or four portions correlated with a score 2.34 units higher. Those eating five or more portions scored 3.73 units higher.
- For primary school pupils, eating only a snack for breakfast was associated with a score 5.50 units lower, and consuming only a non–energy drink was tied to a score 2.67 units lower than eating a conventional breakfast. Not eating any breakfast was associated with a score 3.62 units lower.
- Eating school food versus a packed lunch was associated with a score 1.27 units lower, although this wasn’t statistically significant. Having no lunch was associated with a score 6.08 units lower, although only a few children fell into this group.
“As a potentially modifiable factor, both at an individual and societal level, nutrition may therefore represent an important public health target for strategies to address childhood mental well-being,” the authors wrote, calling for further investigation of the association between nutrition and mental well-being.
This study was commissioned by Norfolk County Council Public Health and the Norfolk Safeguarding Children Board. The University of East Anglia and Social Care Partners provided funding to support Dr. Hayhoe’s work on this project.
Some coauthors are employed by the Norfolk County Council that commissioned the survey.
BMJ NUTRITION, PREVENTION & HEALTH