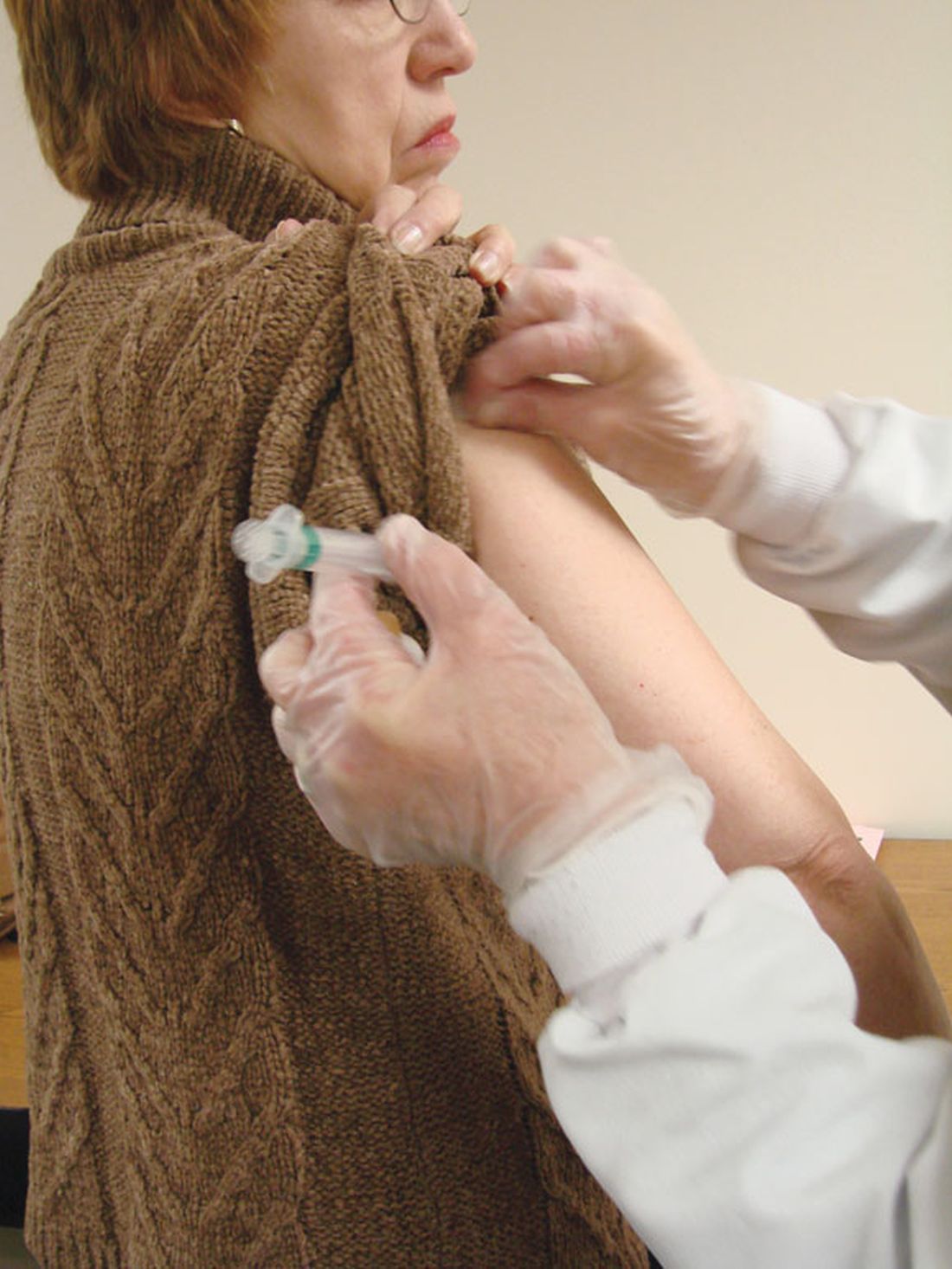
User login
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Bempedoic acid cuts CV events in statin-intolerant patients: CLEAR Outcomes
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
New AHA statement urges focus on CV risk before pregnancy
Increased public health and research efforts to optimize prepregnancy cardiovascular health are needed, particularly among those in under-represented racial and ethnic groups, according to a new scientific statement from the American Heart Association.
“We have released this statement at this time because there is a maternal health crisis in the U.S. with rising maternal morbidity and mortality rates, which are the highest among high-income countries,” chair of the scientific statement writing group, Sadiya S. Khan, MD, told this news organization.
Cardiovascular disease (CVD) is the leading cause of death during pregnancy and the postpartum period and represents 26.5% of pregnancy-related deaths, the statement reports.
“While there is a lot of emphasis in trying to reduce cardiovascular risk during the period of actual pregnancy, much of that risk has often already developed and the women have been living with it for some time, so interventions during pregnancy may be too late,” Dr. Khan, assistant professor of medicine and preventive medicine at Northwestern University, Chicago, said.
“We wanted to try and emphasize the importance of starting to reduce cardiovascular risk earlier before pregnancy. In terms of improving cardiovascular health, this should have benefits both for the mother and the child,” she added.
The statement, “Optimizing Prepregnancy Cardiovascular Health to Improve Outcomes in Pregnant and Postpartum Individuals and Offspring” was published online in a “Go Red For Women” spotlight issue of the AHA publication Circulation.
Currently, nearly one in five births are complicated by such an adverse pregnancy outcome, and there is a strong association between these complications and risk for subsequent cardiovascular disease.
Prepregnancy window
Over the past decade, rates of adverse pregnancy outcomes have increased significantly in the United States, with a near doubling in rates of hypertensive disorders of pregnancy, and there are persistent disparities, with Black individuals significantly more likely to experience adverse pregnancy outcomes, the statement notes.
Emerging data suggest that these complications have, at least in part, prepregnancy origins. Thus, the prepregnancy period may be a critical window during which interventions have a great potential for benefit in both women and their offspring, it says.
The authors suggest a life-course approach to measure, modify, and monitor prepregnancy cardiovascular health, with all clinicians who interact with pregnancy-capable individuals emphasizing optimization of cardiovascular health beginning early in childhood.
“Leveraging these opportunities to target cardiovascular health has the potential to improve health across the life course and for subsequent generations,” they add.
Critical research gap
Despite the evidence linking an individual’s prepregnancy health to their offspring’s health, there are no large trials to test whether improving overall cardiovascular health before pregnancy will reduce pregnancy complications, pregnancy-related cardiovascular death, or cardiovascular risk for offspring. The statement authors suggest that such a trial should be considered.
“This would be a big undertaking, but it could be feasible and could be really impactful,” Dr. Khan said. “Of course it would be challenging to recruit women who are planning a pregnancy and to follow them to see if they do get pregnant and consider interventions and outcomes, but given the importance of the need, we think this is something that should be invested in.”
She pointed out that the main way to improve the cardiovascular health of this cohort would be through behavioral counseling on physical activity and diet. “We need to develop strategies tailored to this age group – young women and those who may already have young children – and often the last thing they are thinking about is themselves and their own health.”
She explained that while it is presumed that controlling cardiovascular risk factors will be beneficial, the bigger question is how that can be achieved. “Behavioral interventions are difficult to achieve and often have low adherence, so the focus of the trials should be on strategies on how to deliver behavioral counseling to achieve better cardiovascular health in this population.”
Dr. Khan stressed that any approaches to improving prepregnancy cardiovascular health must address the current racial disparities that are present. “We must make sure that our policies are successful not just in improving cardiovascular health but to ensure it is done equitably. We must find ways to ensure all individuals can access care.”
A version of this article first appeared on Medscape.com.
Increased public health and research efforts to optimize prepregnancy cardiovascular health are needed, particularly among those in under-represented racial and ethnic groups, according to a new scientific statement from the American Heart Association.
“We have released this statement at this time because there is a maternal health crisis in the U.S. with rising maternal morbidity and mortality rates, which are the highest among high-income countries,” chair of the scientific statement writing group, Sadiya S. Khan, MD, told this news organization.
Cardiovascular disease (CVD) is the leading cause of death during pregnancy and the postpartum period and represents 26.5% of pregnancy-related deaths, the statement reports.
“While there is a lot of emphasis in trying to reduce cardiovascular risk during the period of actual pregnancy, much of that risk has often already developed and the women have been living with it for some time, so interventions during pregnancy may be too late,” Dr. Khan, assistant professor of medicine and preventive medicine at Northwestern University, Chicago, said.
“We wanted to try and emphasize the importance of starting to reduce cardiovascular risk earlier before pregnancy. In terms of improving cardiovascular health, this should have benefits both for the mother and the child,” she added.
The statement, “Optimizing Prepregnancy Cardiovascular Health to Improve Outcomes in Pregnant and Postpartum Individuals and Offspring” was published online in a “Go Red For Women” spotlight issue of the AHA publication Circulation.
Currently, nearly one in five births are complicated by such an adverse pregnancy outcome, and there is a strong association between these complications and risk for subsequent cardiovascular disease.
Prepregnancy window
Over the past decade, rates of adverse pregnancy outcomes have increased significantly in the United States, with a near doubling in rates of hypertensive disorders of pregnancy, and there are persistent disparities, with Black individuals significantly more likely to experience adverse pregnancy outcomes, the statement notes.
Emerging data suggest that these complications have, at least in part, prepregnancy origins. Thus, the prepregnancy period may be a critical window during which interventions have a great potential for benefit in both women and their offspring, it says.
The authors suggest a life-course approach to measure, modify, and monitor prepregnancy cardiovascular health, with all clinicians who interact with pregnancy-capable individuals emphasizing optimization of cardiovascular health beginning early in childhood.
“Leveraging these opportunities to target cardiovascular health has the potential to improve health across the life course and for subsequent generations,” they add.
Critical research gap
Despite the evidence linking an individual’s prepregnancy health to their offspring’s health, there are no large trials to test whether improving overall cardiovascular health before pregnancy will reduce pregnancy complications, pregnancy-related cardiovascular death, or cardiovascular risk for offspring. The statement authors suggest that such a trial should be considered.
“This would be a big undertaking, but it could be feasible and could be really impactful,” Dr. Khan said. “Of course it would be challenging to recruit women who are planning a pregnancy and to follow them to see if they do get pregnant and consider interventions and outcomes, but given the importance of the need, we think this is something that should be invested in.”
She pointed out that the main way to improve the cardiovascular health of this cohort would be through behavioral counseling on physical activity and diet. “We need to develop strategies tailored to this age group – young women and those who may already have young children – and often the last thing they are thinking about is themselves and their own health.”
She explained that while it is presumed that controlling cardiovascular risk factors will be beneficial, the bigger question is how that can be achieved. “Behavioral interventions are difficult to achieve and often have low adherence, so the focus of the trials should be on strategies on how to deliver behavioral counseling to achieve better cardiovascular health in this population.”
Dr. Khan stressed that any approaches to improving prepregnancy cardiovascular health must address the current racial disparities that are present. “We must make sure that our policies are successful not just in improving cardiovascular health but to ensure it is done equitably. We must find ways to ensure all individuals can access care.”
A version of this article first appeared on Medscape.com.
Increased public health and research efforts to optimize prepregnancy cardiovascular health are needed, particularly among those in under-represented racial and ethnic groups, according to a new scientific statement from the American Heart Association.
“We have released this statement at this time because there is a maternal health crisis in the U.S. with rising maternal morbidity and mortality rates, which are the highest among high-income countries,” chair of the scientific statement writing group, Sadiya S. Khan, MD, told this news organization.
Cardiovascular disease (CVD) is the leading cause of death during pregnancy and the postpartum period and represents 26.5% of pregnancy-related deaths, the statement reports.
“While there is a lot of emphasis in trying to reduce cardiovascular risk during the period of actual pregnancy, much of that risk has often already developed and the women have been living with it for some time, so interventions during pregnancy may be too late,” Dr. Khan, assistant professor of medicine and preventive medicine at Northwestern University, Chicago, said.
“We wanted to try and emphasize the importance of starting to reduce cardiovascular risk earlier before pregnancy. In terms of improving cardiovascular health, this should have benefits both for the mother and the child,” she added.
The statement, “Optimizing Prepregnancy Cardiovascular Health to Improve Outcomes in Pregnant and Postpartum Individuals and Offspring” was published online in a “Go Red For Women” spotlight issue of the AHA publication Circulation.
Currently, nearly one in five births are complicated by such an adverse pregnancy outcome, and there is a strong association between these complications and risk for subsequent cardiovascular disease.
Prepregnancy window
Over the past decade, rates of adverse pregnancy outcomes have increased significantly in the United States, with a near doubling in rates of hypertensive disorders of pregnancy, and there are persistent disparities, with Black individuals significantly more likely to experience adverse pregnancy outcomes, the statement notes.
Emerging data suggest that these complications have, at least in part, prepregnancy origins. Thus, the prepregnancy period may be a critical window during which interventions have a great potential for benefit in both women and their offspring, it says.
The authors suggest a life-course approach to measure, modify, and monitor prepregnancy cardiovascular health, with all clinicians who interact with pregnancy-capable individuals emphasizing optimization of cardiovascular health beginning early in childhood.
“Leveraging these opportunities to target cardiovascular health has the potential to improve health across the life course and for subsequent generations,” they add.
Critical research gap
Despite the evidence linking an individual’s prepregnancy health to their offspring’s health, there are no large trials to test whether improving overall cardiovascular health before pregnancy will reduce pregnancy complications, pregnancy-related cardiovascular death, or cardiovascular risk for offspring. The statement authors suggest that such a trial should be considered.
“This would be a big undertaking, but it could be feasible and could be really impactful,” Dr. Khan said. “Of course it would be challenging to recruit women who are planning a pregnancy and to follow them to see if they do get pregnant and consider interventions and outcomes, but given the importance of the need, we think this is something that should be invested in.”
She pointed out that the main way to improve the cardiovascular health of this cohort would be through behavioral counseling on physical activity and diet. “We need to develop strategies tailored to this age group – young women and those who may already have young children – and often the last thing they are thinking about is themselves and their own health.”
She explained that while it is presumed that controlling cardiovascular risk factors will be beneficial, the bigger question is how that can be achieved. “Behavioral interventions are difficult to achieve and often have low adherence, so the focus of the trials should be on strategies on how to deliver behavioral counseling to achieve better cardiovascular health in this population.”
Dr. Khan stressed that any approaches to improving prepregnancy cardiovascular health must address the current racial disparities that are present. “We must make sure that our policies are successful not just in improving cardiovascular health but to ensure it is done equitably. We must find ways to ensure all individuals can access care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
New ACC, AHA, SCAI interventional cardiology training guidance
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
Intermittent fasting plus exercise a good option for fatty liver
However, the combined approach did not give significantly added benefit, compared with fasting alone, the researchers report.
Eighty patients with NAFLD were randomized to one of four lifestyle strategies (alternate-day fasting, aerobic exercise, both, or neither) for 3 months.
The primary outcome was change in intrahepatic triglyceride (IHTG) content from baseline to study end, measured by magnetic resonance imaging proton density fat fraction.
The results suggest that “combining intermittent fasting with exercise is effective for reducing hepatic steatosis [fatty liver] in patients with NAFLD but may offer no additional benefit versus fasting alone,” Mark Ezpeleta, PhD, formerly at the University of Illinois, Chicago, and now at the University of Colorado Anschutz Medical Campus, and colleagues conclude.
“Our findings also indicate that the combination intervention was effective for reducing body weight, fat mass, waist circumference, [the liver enzyme alanine transaminase (ALT)], fasting insulin, [and] insulin resistance and increasing insulin sensitivity, among patients with obesity and NAFLD versus controls,” the group reports.
“When we compared the results of our study groups, we saw clearly that the most improved patients were in the group that followed the alternate-day fasting diet and exercised 5 days a week,” senior author Krista A. Varady, PhD, professor of nutrition, University of Illinois, said in a press release from the university.
“The people who only dieted or only exercised did not see the same improvements,” she added, “which reinforces the importance of these two relatively inexpensive lifestyle modifications on overall health and on combating chronic diseases like fatty liver disease.”
Moreover, “alternate-day fasting and exercise interventions can be difficult for people to stick to, and in prior studies we have seen significant dropout,” she noted. “It was very interesting to see that in this trial we had very high adherence to the interventions.”
The study was recently published in Cell Metabolism.
An estimated 65% of people with obesity have NAFLD, or fat in the liver that is not the result of excessive alcohol consumption, which is strongly related to the development of insulin resistance and type 2 diabetes, the group writes.
Thiazolidinediones such as pioglitazone reduce hepatic steatosis, but there is mounting concern about the weight-gaining effect of these compounds.
Recent attention has focused on lifestyle interventions to resolve hepatic steatosis, and previous trials showed that alternate-day fasting was effective for certain outcomes in NAFLD, but those studies did not measure changes in IHTG content or include an exercise intervention.
The researchers enrolled 80 adults with obesity and NAFLD and randomized them to one of four groups for 3 months:
- Alternate day fasting group: Participants were instructed to consume 600 kcal at dinner between 5 PM and 8 PM on a fasting day alternating with food as desired on a feasting day.
- Exercise group: A 60-minute moderate-intensity aerobic exercise session 5 times a week.
- Fasting plus exercise group.
- Control group (no intervention).
Participants were age 23-65 (mean age, 44) and 81% were women.
Half were Hispanic, and the rest were Black (30%), White (11%), or Asian (9%).
They had a mean weight of 99 kg (218 lb) and a mean body mass index of 36 kg/m2.
Dropout rates were minimal in the combination group (0%) and fasting groups (5%) and moderately high in the exercise group (25%).
IHTG content was reduced by a significantly greater amount in the combination group (–5.48%) than in the exercise alone group (–1.30%; P = .02) or in the control group (–0.17%; P < .01) and by a greater amount than in the fasting alone group, although this was not significant (–2.25%; P = .05).
Lean mass, aspartate transaminase (AST), A1c, blood pressure, plasma lipids, liver fibrosis score, and hepatokines (fetuin-A, FGF-21, and selenoprotein P) did not differ between groups.
Researchers acknowledge that although the combination intervention resulted in improved NAFLD parameters, IHTG and ALT did not reach the normal range.
Participants likely had early stage NAFLD (their baseline IHTG was in the 16% to 18% range, where 5% to 33% is mild steatosis) and they were likely highly motivated (indicated by the low dropout rate), so the findings may not be generalizable.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Dr. Varady has reported receiving author fees from the Hachette Book Group for the book entitled “The Every Other Day Diet.” The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, the combined approach did not give significantly added benefit, compared with fasting alone, the researchers report.
Eighty patients with NAFLD were randomized to one of four lifestyle strategies (alternate-day fasting, aerobic exercise, both, or neither) for 3 months.
The primary outcome was change in intrahepatic triglyceride (IHTG) content from baseline to study end, measured by magnetic resonance imaging proton density fat fraction.
The results suggest that “combining intermittent fasting with exercise is effective for reducing hepatic steatosis [fatty liver] in patients with NAFLD but may offer no additional benefit versus fasting alone,” Mark Ezpeleta, PhD, formerly at the University of Illinois, Chicago, and now at the University of Colorado Anschutz Medical Campus, and colleagues conclude.
“Our findings also indicate that the combination intervention was effective for reducing body weight, fat mass, waist circumference, [the liver enzyme alanine transaminase (ALT)], fasting insulin, [and] insulin resistance and increasing insulin sensitivity, among patients with obesity and NAFLD versus controls,” the group reports.
“When we compared the results of our study groups, we saw clearly that the most improved patients were in the group that followed the alternate-day fasting diet and exercised 5 days a week,” senior author Krista A. Varady, PhD, professor of nutrition, University of Illinois, said in a press release from the university.
“The people who only dieted or only exercised did not see the same improvements,” she added, “which reinforces the importance of these two relatively inexpensive lifestyle modifications on overall health and on combating chronic diseases like fatty liver disease.”
Moreover, “alternate-day fasting and exercise interventions can be difficult for people to stick to, and in prior studies we have seen significant dropout,” she noted. “It was very interesting to see that in this trial we had very high adherence to the interventions.”
The study was recently published in Cell Metabolism.
An estimated 65% of people with obesity have NAFLD, or fat in the liver that is not the result of excessive alcohol consumption, which is strongly related to the development of insulin resistance and type 2 diabetes, the group writes.
Thiazolidinediones such as pioglitazone reduce hepatic steatosis, but there is mounting concern about the weight-gaining effect of these compounds.
Recent attention has focused on lifestyle interventions to resolve hepatic steatosis, and previous trials showed that alternate-day fasting was effective for certain outcomes in NAFLD, but those studies did not measure changes in IHTG content or include an exercise intervention.
The researchers enrolled 80 adults with obesity and NAFLD and randomized them to one of four groups for 3 months:
- Alternate day fasting group: Participants were instructed to consume 600 kcal at dinner between 5 PM and 8 PM on a fasting day alternating with food as desired on a feasting day.
- Exercise group: A 60-minute moderate-intensity aerobic exercise session 5 times a week.
- Fasting plus exercise group.
- Control group (no intervention).
Participants were age 23-65 (mean age, 44) and 81% were women.
Half were Hispanic, and the rest were Black (30%), White (11%), or Asian (9%).
They had a mean weight of 99 kg (218 lb) and a mean body mass index of 36 kg/m2.
Dropout rates were minimal in the combination group (0%) and fasting groups (5%) and moderately high in the exercise group (25%).
IHTG content was reduced by a significantly greater amount in the combination group (–5.48%) than in the exercise alone group (–1.30%; P = .02) or in the control group (–0.17%; P < .01) and by a greater amount than in the fasting alone group, although this was not significant (–2.25%; P = .05).
Lean mass, aspartate transaminase (AST), A1c, blood pressure, plasma lipids, liver fibrosis score, and hepatokines (fetuin-A, FGF-21, and selenoprotein P) did not differ between groups.
Researchers acknowledge that although the combination intervention resulted in improved NAFLD parameters, IHTG and ALT did not reach the normal range.
Participants likely had early stage NAFLD (their baseline IHTG was in the 16% to 18% range, where 5% to 33% is mild steatosis) and they were likely highly motivated (indicated by the low dropout rate), so the findings may not be generalizable.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Dr. Varady has reported receiving author fees from the Hachette Book Group for the book entitled “The Every Other Day Diet.” The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, the combined approach did not give significantly added benefit, compared with fasting alone, the researchers report.
Eighty patients with NAFLD were randomized to one of four lifestyle strategies (alternate-day fasting, aerobic exercise, both, or neither) for 3 months.
The primary outcome was change in intrahepatic triglyceride (IHTG) content from baseline to study end, measured by magnetic resonance imaging proton density fat fraction.
The results suggest that “combining intermittent fasting with exercise is effective for reducing hepatic steatosis [fatty liver] in patients with NAFLD but may offer no additional benefit versus fasting alone,” Mark Ezpeleta, PhD, formerly at the University of Illinois, Chicago, and now at the University of Colorado Anschutz Medical Campus, and colleagues conclude.
“Our findings also indicate that the combination intervention was effective for reducing body weight, fat mass, waist circumference, [the liver enzyme alanine transaminase (ALT)], fasting insulin, [and] insulin resistance and increasing insulin sensitivity, among patients with obesity and NAFLD versus controls,” the group reports.
“When we compared the results of our study groups, we saw clearly that the most improved patients were in the group that followed the alternate-day fasting diet and exercised 5 days a week,” senior author Krista A. Varady, PhD, professor of nutrition, University of Illinois, said in a press release from the university.
“The people who only dieted or only exercised did not see the same improvements,” she added, “which reinforces the importance of these two relatively inexpensive lifestyle modifications on overall health and on combating chronic diseases like fatty liver disease.”
Moreover, “alternate-day fasting and exercise interventions can be difficult for people to stick to, and in prior studies we have seen significant dropout,” she noted. “It was very interesting to see that in this trial we had very high adherence to the interventions.”
The study was recently published in Cell Metabolism.
An estimated 65% of people with obesity have NAFLD, or fat in the liver that is not the result of excessive alcohol consumption, which is strongly related to the development of insulin resistance and type 2 diabetes, the group writes.
Thiazolidinediones such as pioglitazone reduce hepatic steatosis, but there is mounting concern about the weight-gaining effect of these compounds.
Recent attention has focused on lifestyle interventions to resolve hepatic steatosis, and previous trials showed that alternate-day fasting was effective for certain outcomes in NAFLD, but those studies did not measure changes in IHTG content or include an exercise intervention.
The researchers enrolled 80 adults with obesity and NAFLD and randomized them to one of four groups for 3 months:
- Alternate day fasting group: Participants were instructed to consume 600 kcal at dinner between 5 PM and 8 PM on a fasting day alternating with food as desired on a feasting day.
- Exercise group: A 60-minute moderate-intensity aerobic exercise session 5 times a week.
- Fasting plus exercise group.
- Control group (no intervention).
Participants were age 23-65 (mean age, 44) and 81% were women.
Half were Hispanic, and the rest were Black (30%), White (11%), or Asian (9%).
They had a mean weight of 99 kg (218 lb) and a mean body mass index of 36 kg/m2.
Dropout rates were minimal in the combination group (0%) and fasting groups (5%) and moderately high in the exercise group (25%).
IHTG content was reduced by a significantly greater amount in the combination group (–5.48%) than in the exercise alone group (–1.30%; P = .02) or in the control group (–0.17%; P < .01) and by a greater amount than in the fasting alone group, although this was not significant (–2.25%; P = .05).
Lean mass, aspartate transaminase (AST), A1c, blood pressure, plasma lipids, liver fibrosis score, and hepatokines (fetuin-A, FGF-21, and selenoprotein P) did not differ between groups.
Researchers acknowledge that although the combination intervention resulted in improved NAFLD parameters, IHTG and ALT did not reach the normal range.
Participants likely had early stage NAFLD (their baseline IHTG was in the 16% to 18% range, where 5% to 33% is mild steatosis) and they were likely highly motivated (indicated by the low dropout rate), so the findings may not be generalizable.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Dr. Varady has reported receiving author fees from the Hachette Book Group for the book entitled “The Every Other Day Diet.” The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL METABOLISM
Meta-analysis throws more shade aspirin’s way
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
A new meta-analysis has added evidence questioning the utility and efficacy of prophylactic low-dose aspirin for preventing cardiovascular events in people who don’t have atherosclerotic cardiovascular disease (ASCVD), whether or not they’re also taking statins, and finds that at every level of ASCVD risk the aspirin carries a risk of major bleeding that exceeds its potentially protective benefits.
In a study published online in JACC: Advances, the researchers, led by Safi U. Khan, MD, MS, analyzed data from 16 trials with 171,215 individuals, with a median age of 64 years. Of the population analyzed, 35% were taking statins.
“This study focused on patients without ASCVD who are taking aspirin with or without statin therapy to prevent ASCVD events,” Dr. Khan, a cardiovascular disease fellow at Houston Methodist DeBakey Heart and Vascular Institute, told this news organization. “We noted that the absolute risk of major bleeding in this patient population exceeds the absolute reduction in MI by aspirin across different ASCVD risk categories. Furthermore, concomitant statin therapy use further diminishes aspirin’s cardiovascular effects without influencing bleeding risk.”
Across the 16 studies, people taking aspirin had a relative risk reduction of 15% for MI vs. controls (RR .85; 95% confidence interval [CI], .77 to .95; P < .001). However, they had a 48% greater risk of major bleeding (RR, 1.48; 95% CI, 1.31-1.66; P < .001).
The meta-analysis also found that aspirin, either as monotherapy or with a statin, carried a slight to significant benefit depending on the estimated risk of developing ASCVD. The risk of major bleeding exceeded the benefit across all three risk-stratified groups. The greatest benefit, and greatest risk, was in the groups with high to very-high ASCVD risk groups, defined as a 20%-30% and 30% or greater ASCVD risk, respectively: 20-37 fewer MIs per 10,000 with monotherapy and 27-49 fewer with statin, but 78-98 more major bleeding events with monotherapy and 74-95 more with statin.
And aspirin, either as monotherapy or with statin, didn’t reduce the risk of other key endpoints: stroke, all-cause mortality, or cardiovascular mortality. While aspirin was associated with a lower risk of nonfatal MI (RR, .82; 95% CI, .72 to .94; P ≤. 001), it wasn’t associated with reducing the risk of nonfatal stroke. Aspirin patients had a significantly 32% greater risk of intracranial hemorrhage (RR, 1.32; 95% CI, 1.12-1.55; P ≤ .001) and 51% increased risk of gastrointestinal bleeding (RR, 1.51; 95% CI, 1.33-1.72; P ≤ .001).
“We used randomized data from all key primary prevention of aspirin trials and estimated the absolute effects of aspirin therapy with or without concomitant statin across different baseline risks of the patients,” Dr. Khan said. “This approach allowed us to identify aspirin therapy’s risk-benefit equilibrium, which is tilted towards more harm than benefit.”
He acknowledged study limitations included using study-level rather than patient-level meta-analysis, and the inability to calculate effects in younger populations at high absolute risk.
The investigators acknowledged the controversy surrounding aspirin use to prevent ASCVD, noting the three major guidelines: the 2019 American College of Cardiology/American Heart Association and the 2021 European Society of Cardiology guidelines for aspirin only among asymptomatic individuals with high risk of ASCVD events, low bleeding risk, and age 70 years and younger; and the United States Preventive Services Task Force guidelines, updated in 2022, recommending individualized low-dose aspirin only among adults ages 40-59 years with 10-year ASCVD risk of 10% or greater and a low bleeding risk.
The findings are not a clarion call to halt aspirin therapy, Dr. Khan said. “This research focuses only on patients who do not have ASCVD,” he said. “Patients who do have ASCVD should continue with aspirin and statin therapy. However, we noted that aspirin has a limited role for patients who do not have ASCVD beyond lifestyle modifications, smoking cessation, exercise, and preventive statin therapy. Therefore, they should only consider using aspirin if their physicians suggest that the risk of having a cardiovascular event exceeds their bleeding risk. Otherwise, they should discuss with their physicians about omitting aspirin.”
The study confirms the move away from low-dose aspirin to prevent ASCVD, said Tahmid Rahman, MD, cardiologist and associate director of the Center for Advanced Lipid Management at Stony Brook (N.Y.) Heart Institute. “The study really continues to add to essentially what we already know,” he said. “There was a big push that aspirin, initially before the major statin trials, was the way to go to prevent heart disease, but with later studies, and especially now with newer antiplatelet therapies and longer duration of medication for people with both secondary prevention and primary prevention, we are getting away from routine aspirin, especially in primary prevention.”
Lowering LDL cholesterol is the definitive target for lowering risk for MI and stroke, Dr. Rahman said. “Statins don’t lead to a bleeding risk,” he said, “so my recommendation is to be aggressive with lowering your cholesterol and getting the LDL as low possible to really reduce outcomes, especially in secondary prevention, as well as in high-risk patients for primary prevention, especially diabetics.”
He added, however, lifestyle modification also has a key role for preventing ASCVD. “No matter what we have with medication, the most important thing is following a proper diet, especially something like the Mediterranean diet, as well as exercising regularly,” he said.
Dr. Khan and Dr. Rahman have no relevant disclosures.
FROM JACC: ADVANCES
Exercise training reduces liver fat in patients with NAFLD, even without weight loss
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), according to a new systematic review and meta-analysis.
An exercise dose of 750 metabolic equivalents of task (MET)–minutes per week – or 150 minutes per week of brisk walking – was required to achieve a treatment response, independently of weight loss.
“In the absence of a regulatory agency–approved drug treatment or a cure, lifestyle modification with dietary change and increased exercise is recommended for all patients with NAFLD,” first author Jonathan Stine, MD, an associate professor of medicine and public health sciences and director of the fatty liver program at the Penn State Health Milton S. Hershey Medical Center, Hershey, said in an interview.
“With that said, there are many key unanswered questions about how to best prescribe exercise as medicine to our patients with NAFLD, including whether the liver-specific benefit of exercise can be seen without any body weight loss,” Dr. Stine said. “And if found, what dose of exercise is required in order to achieve clinically meaningful benefit?” He noted that this analysis is a step toward helping to answer these questions.
The study by Dr. Stine and colleagues was published online in The American Journal of Gastroenterology.
Analyzing studies
Exercise training, which includes planned and structured physical activity intended to improve physical fitness, has been shown to provide multiple benefits for patients with NAFLD, the study authors wrote. The gains include improvements in liver fat, physical fitness, body composition, vascular biology, and health-related quality of life.
However, it has been unclear whether exercise training achieves a 30% or more relative reduction in liver fat, which is considered the minimal clinically important difference and is a surrogate for histologic response or improvement in liver fibrosis.
In their systematic review and meta-analysis, Dr. Stine and colleagues analyzed the evidence for MRI-measured liver reduction in response to exercise training across different doses, with a 30% or more relative reduction serving as the primary outcome. They included randomized controlled trials in adults with NAFLD who participated in exercise training programs.
The 14 studies included a total of 551 participants. The average age of the participants was 53 years, and the average body mass index was 31 kg/mg2. The duration of the interventions ranged from 4 to 52 weeks and included different types of exercise, such as aerobic, high-intensity interval, resistance, and aerobic plus resistance training.
No study yielded the clinically significant weight loss required for histologic response (7%-10%). The average weight loss was about 2.8% among those who participated in exercise training.
Overall, seven studies with 152 participants had data for the 30% or more relative reduction in MRI-measured liver fat. The pooled rate was 34% for exercise training and 13% for the control condition.
In general, those who participated in exercise training were 3.5 times more likely to achieve a 30% or more relative reduction in MRI-measured liver fat than those in the control condition.
Among all participants, the mean change in absolute liver fat was –6.7% for the 338 participants enrolled in exercise training, compared with –0.8% for the 213 participants under the control condition. The pooled mean difference in absolute change in MRI-measured liver fat for exercise training versus the control was –5.8%.
For relative change in MRI-measured liver fat, researchers analyzed nine studies with 195 participants – 118 participants in exercise training, and 77 control participants. The mean relative change was –24.1% among the exercise training group and 7.3% among the control group. The pooled mean difference in relative change for exercise training versus the control was –26.4%.
For all 14 studies, an exercise dose of 750 or more MET-minutes per week resulted in a significant treatment response. This equates to 150 minutes per week of moderate-intensity exercise, such as brisk walking, or 75 minutes per week of vigorous-intensity exercise, such as jogging or cycling.
Among participants who had 750 MET-minutes per week, there was a –8% absolute and –28.9% relative mean difference in MRI-measured liver fat, compared with –4.1% and –22.8%, respectively, among those who had fewer than 750 MET-minutes per week.
An exercise dose of 750 or more MET-minutes per week led to a 30% or more relative reduction in MRI-measured liver fat in 39.3% of participants, compared with 25.7% who had fewer than that threshold.
The treatment response was independent of clinically significant body weight loss of more than 5%.
“Prior to our study, it was felt that body weight loss of at least 5% was required in order to significantly improve liver histology,” Dr. Stine said. “Our findings challenge this thought in that exercise training achieved rates of clinically significant liver fat reduction.”
Ongoing research
Dr. Stine and colleagues are continuing their research and are directly comparing exercise doses of 750 MET-minutes per week and 1,000 MET-minutes per week to standard clinical care in adults with biopsy-proven nonalcoholic steatohepatitis, or the progressive type of NAFLD.
“Importantly, this new study we’re undertaking is designed to mimic a real-world setting in which people’s daily schedules are highly variable,” he said. “Our experienced team of exercise professionals may vary frequency and time of exercise in a week so long as our study participant achieves the prescribed dose of exercise.”
Currently, leading professional societies have not reached consensus regarding the optimal physical activity program for patients with NAFLD, the study authors wrote. However, most clinical guidelines support at least 150 minutes per week of moderate-intensity aerobic activity.
Although more head-to-head clinical trials are needed, exercise training appears to reduce liver fat and provides other benefits, such as cardiorespiratory fitness, body composition changes, and improvements in vascular biology, they wrote.
“The important piece here is that this review shows that there does not have to be weight loss for improvements in fatty liver,” Jill Kanaley, PhD, a professor of nutrition and exercise physiology at University of Missouri–Columbia, said in an interview.
Dr. Kanaley, who wasn’t involved with this study, has researched exercise training among patients with NAFLD. She and her colleagues have found that moderate-and high-intensity exercise can decrease intrahepatic lipid content and NAFLD risk factors, independently of abdominal fat or body mass reductions.
“So often, people get frustrated with exercise if they do not see weight loss,” she said. “But in this case, there seems to be benefits of the exercise, even without weight loss.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have received research funding and have had consultant roles with numerous pharmaceutical companies. Dr. Kanaley reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Keto for life? Reasons to think twice
Is the ketogenic diet the only way to lose weight? Of course not! Keep track of calories in vs. calories out and almost anyone can lose weight. The problem is keeping it off. To understand that, we need to look at metabolic adaptation and the biology of obesity.
Our bodies have a “set point” that is epigenetically latched onto the environment the brain senses, just as the fetal environment responds to the maternal environment.
If food is plentiful, our hormones force us to eat until our bodies feel that there are enough fat stores to survive. Because of environmental influences such as highly processed food, preservatives, climate change, and regulation of temperature, our brains have decided that we need more adipose tissue than we did 50-100 years ago. It could be that an element in food has caused a dysfunction of the pathways that regulate our body weight, and most of us “defend” a higher body weight in this environment.
How to counteract that? Not easily. The ketogenic diet works temporarily just like any other diet where calorie intake is lower than usual. It seems to be agreeable to many people because they say they feel full after eating protein, fat, and perhaps some vegetables. Protein and fat are certainly more satiating than simple carbohydrates.
If strictly followed, a ketogenic diet will force the body to burn fat and go into ketosis. Without a source for glucose, the brain will burn ketones from fat stores. Owen and colleagues discovered this in 1969 when they did their now-famous studies of fasting in inpatients at Brigham and Women’s hospital, using IV amino acids to protect muscle mass.
Keto for life?
Is the ketogenic diet a healthy diet for the long term? That is a different question.
Of course not – we need high-fiber carbohydrate sources such as whole grains, fruits, and vegetables to keep the colon healthy and obtain the vitamins and minerals needed to make the Krebs cycle, or citric acid cycle, work at its best.
Why, then, are we promoting ketogenic diets for those with obesity and type 2 diabetes? Ketogenic or low-carbohydrate diets are easy to teach and can rapidly help patients lose weight and return their blood glucose, blood pressure, and other metabolic parameters to normal.
The patient will be instructed to avoid all highly processed foods. Studies have shown that highly processed foods, created to maximize flavor, “coerce” people to eat more calories than when presented with the same number of calories in unprocessed foods, a way to fool the brain.
Why are we fooling the brain?
We circumvent the natural satiety mechanisms that start with the gut. When we eat, our gastric fundus and intestinal stretch receptors start the process that informs the hypothalamus about food intake. Highly processed foods are usually devoid of fiber and volume, and pack in the calories in small volumes so that the stretch receptors are not activated until more calories are ingested. The study mentioned above developed two ad lib diets with the same number of calories, sugar, fat, and carbohydrate content – one ultraprocessed and the other unprocessed.
That explanation is just the tip of the iceberg, because a lot more than primitive stretch receptors is informing the brain. There are gut hormones that are secreted before and after meals, such as ghrelin, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and cholecystokinin (CCK), among a slew of others. These peptide hormones are all secreted from gut cells into the blood or vagus nerve, or both, and alert the brain that there is or is not enough food to maintain body weight at its set point.
It’s a highly regulated and precise system that regulates body weight for survival of the species in this environment. However, the environment has changed over the past 100 years but our genetic makeup for survival of the fittest has not. The mechanism of action for defense of a higher body weight set point in this new environment has not been elucidated as yet. Most likely, there are many players or instigators involved, such as food-supply changes, sedentary lifestyle, ambient temperature, fetal programming, air quality, and global warming and climate change, to name a few.
The goal of obesity researchers is to investigate the underlying mechanisms of the increased prevalence of obesity over the past 100 years. The goal of obesity medicine specialists is to treat obesity in adults and children, and to prevent obesity as much as possible with lifestyle change and medications that have been shown to help “reverse” the metabolic adaptation to this environment. Our newest GLP-1/GIP receptor agonists have been shown in animal models to hit several pathways that lead to obesity. They are not just appetite suppressants. Yes, they do modulate appetite and satiety, but they also affect energy expenditure. The body’s normal reaction to a lack of calorie intake is to reduce resting energy expenditure until body weight increases back to “set point levels.” These agonists prevent that metabolic adaptation. That is why they are true agents that can treat obesity – the disease.
Back to the ketogenic diet. The ketogenic diet can fool the brain temporarily by using protein and fat to elicit satiety with less food intake in calories. After a while, however, gut hormones and other factors begin to counteract the weight loss with a reduction in resting energy and total energy expenditure, and other metabolic measures, to get the body back to a certain body weight set point.
The ketogenic diet also can help dieters avoid ultra- and highly processed foods. In the end, any type of diet that lowers caloric intake will work for weight loss, but it’s the maintenance of that weight loss that makes a long-term difference, and that involves closing the metabolic gap that the body generates to defend fat mass. Understanding this pathophysiology will allow obesity medicine specialists to assist patients with obesity to lose weight and keep it off.
Dr. Apovian is in the department of medicine, division of endocrinology, diabetes, and hypertension, and codirector, Center for Weight Management and Wellness, Harvard Medical School, Boston. She disclosed ties with Altimmune, Cowen and Company, Currax Pharmaceuticals, EPG Communication Holdings, Gelesis Srl, L-Nutra, NeuroBo Pharmaceuticals, National Institutes of Health, Patient-Centered Outcomes Research Institute, GI Dynamics, and Novo Nordisk. A version of this article first appeared on Medscape.com.
Is the ketogenic diet the only way to lose weight? Of course not! Keep track of calories in vs. calories out and almost anyone can lose weight. The problem is keeping it off. To understand that, we need to look at metabolic adaptation and the biology of obesity.
Our bodies have a “set point” that is epigenetically latched onto the environment the brain senses, just as the fetal environment responds to the maternal environment.
If food is plentiful, our hormones force us to eat until our bodies feel that there are enough fat stores to survive. Because of environmental influences such as highly processed food, preservatives, climate change, and regulation of temperature, our brains have decided that we need more adipose tissue than we did 50-100 years ago. It could be that an element in food has caused a dysfunction of the pathways that regulate our body weight, and most of us “defend” a higher body weight in this environment.
How to counteract that? Not easily. The ketogenic diet works temporarily just like any other diet where calorie intake is lower than usual. It seems to be agreeable to many people because they say they feel full after eating protein, fat, and perhaps some vegetables. Protein and fat are certainly more satiating than simple carbohydrates.
If strictly followed, a ketogenic diet will force the body to burn fat and go into ketosis. Without a source for glucose, the brain will burn ketones from fat stores. Owen and colleagues discovered this in 1969 when they did their now-famous studies of fasting in inpatients at Brigham and Women’s hospital, using IV amino acids to protect muscle mass.
Keto for life?
Is the ketogenic diet a healthy diet for the long term? That is a different question.
Of course not – we need high-fiber carbohydrate sources such as whole grains, fruits, and vegetables to keep the colon healthy and obtain the vitamins and minerals needed to make the Krebs cycle, or citric acid cycle, work at its best.
Why, then, are we promoting ketogenic diets for those with obesity and type 2 diabetes? Ketogenic or low-carbohydrate diets are easy to teach and can rapidly help patients lose weight and return their blood glucose, blood pressure, and other metabolic parameters to normal.
The patient will be instructed to avoid all highly processed foods. Studies have shown that highly processed foods, created to maximize flavor, “coerce” people to eat more calories than when presented with the same number of calories in unprocessed foods, a way to fool the brain.
Why are we fooling the brain?
We circumvent the natural satiety mechanisms that start with the gut. When we eat, our gastric fundus and intestinal stretch receptors start the process that informs the hypothalamus about food intake. Highly processed foods are usually devoid of fiber and volume, and pack in the calories in small volumes so that the stretch receptors are not activated until more calories are ingested. The study mentioned above developed two ad lib diets with the same number of calories, sugar, fat, and carbohydrate content – one ultraprocessed and the other unprocessed.
That explanation is just the tip of the iceberg, because a lot more than primitive stretch receptors is informing the brain. There are gut hormones that are secreted before and after meals, such as ghrelin, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and cholecystokinin (CCK), among a slew of others. These peptide hormones are all secreted from gut cells into the blood or vagus nerve, or both, and alert the brain that there is or is not enough food to maintain body weight at its set point.
It’s a highly regulated and precise system that regulates body weight for survival of the species in this environment. However, the environment has changed over the past 100 years but our genetic makeup for survival of the fittest has not. The mechanism of action for defense of a higher body weight set point in this new environment has not been elucidated as yet. Most likely, there are many players or instigators involved, such as food-supply changes, sedentary lifestyle, ambient temperature, fetal programming, air quality, and global warming and climate change, to name a few.
The goal of obesity researchers is to investigate the underlying mechanisms of the increased prevalence of obesity over the past 100 years. The goal of obesity medicine specialists is to treat obesity in adults and children, and to prevent obesity as much as possible with lifestyle change and medications that have been shown to help “reverse” the metabolic adaptation to this environment. Our newest GLP-1/GIP receptor agonists have been shown in animal models to hit several pathways that lead to obesity. They are not just appetite suppressants. Yes, they do modulate appetite and satiety, but they also affect energy expenditure. The body’s normal reaction to a lack of calorie intake is to reduce resting energy expenditure until body weight increases back to “set point levels.” These agonists prevent that metabolic adaptation. That is why they are true agents that can treat obesity – the disease.
Back to the ketogenic diet. The ketogenic diet can fool the brain temporarily by using protein and fat to elicit satiety with less food intake in calories. After a while, however, gut hormones and other factors begin to counteract the weight loss with a reduction in resting energy and total energy expenditure, and other metabolic measures, to get the body back to a certain body weight set point.
The ketogenic diet also can help dieters avoid ultra- and highly processed foods. In the end, any type of diet that lowers caloric intake will work for weight loss, but it’s the maintenance of that weight loss that makes a long-term difference, and that involves closing the metabolic gap that the body generates to defend fat mass. Understanding this pathophysiology will allow obesity medicine specialists to assist patients with obesity to lose weight and keep it off.
Dr. Apovian is in the department of medicine, division of endocrinology, diabetes, and hypertension, and codirector, Center for Weight Management and Wellness, Harvard Medical School, Boston. She disclosed ties with Altimmune, Cowen and Company, Currax Pharmaceuticals, EPG Communication Holdings, Gelesis Srl, L-Nutra, NeuroBo Pharmaceuticals, National Institutes of Health, Patient-Centered Outcomes Research Institute, GI Dynamics, and Novo Nordisk. A version of this article first appeared on Medscape.com.
Is the ketogenic diet the only way to lose weight? Of course not! Keep track of calories in vs. calories out and almost anyone can lose weight. The problem is keeping it off. To understand that, we need to look at metabolic adaptation and the biology of obesity.
Our bodies have a “set point” that is epigenetically latched onto the environment the brain senses, just as the fetal environment responds to the maternal environment.
If food is plentiful, our hormones force us to eat until our bodies feel that there are enough fat stores to survive. Because of environmental influences such as highly processed food, preservatives, climate change, and regulation of temperature, our brains have decided that we need more adipose tissue than we did 50-100 years ago. It could be that an element in food has caused a dysfunction of the pathways that regulate our body weight, and most of us “defend” a higher body weight in this environment.
How to counteract that? Not easily. The ketogenic diet works temporarily just like any other diet where calorie intake is lower than usual. It seems to be agreeable to many people because they say they feel full after eating protein, fat, and perhaps some vegetables. Protein and fat are certainly more satiating than simple carbohydrates.
If strictly followed, a ketogenic diet will force the body to burn fat and go into ketosis. Without a source for glucose, the brain will burn ketones from fat stores. Owen and colleagues discovered this in 1969 when they did their now-famous studies of fasting in inpatients at Brigham and Women’s hospital, using IV amino acids to protect muscle mass.
Keto for life?
Is the ketogenic diet a healthy diet for the long term? That is a different question.
Of course not – we need high-fiber carbohydrate sources such as whole grains, fruits, and vegetables to keep the colon healthy and obtain the vitamins and minerals needed to make the Krebs cycle, or citric acid cycle, work at its best.
Why, then, are we promoting ketogenic diets for those with obesity and type 2 diabetes? Ketogenic or low-carbohydrate diets are easy to teach and can rapidly help patients lose weight and return their blood glucose, blood pressure, and other metabolic parameters to normal.
The patient will be instructed to avoid all highly processed foods. Studies have shown that highly processed foods, created to maximize flavor, “coerce” people to eat more calories than when presented with the same number of calories in unprocessed foods, a way to fool the brain.
Why are we fooling the brain?
We circumvent the natural satiety mechanisms that start with the gut. When we eat, our gastric fundus and intestinal stretch receptors start the process that informs the hypothalamus about food intake. Highly processed foods are usually devoid of fiber and volume, and pack in the calories in small volumes so that the stretch receptors are not activated until more calories are ingested. The study mentioned above developed two ad lib diets with the same number of calories, sugar, fat, and carbohydrate content – one ultraprocessed and the other unprocessed.
That explanation is just the tip of the iceberg, because a lot more than primitive stretch receptors is informing the brain. There are gut hormones that are secreted before and after meals, such as ghrelin, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and cholecystokinin (CCK), among a slew of others. These peptide hormones are all secreted from gut cells into the blood or vagus nerve, or both, and alert the brain that there is or is not enough food to maintain body weight at its set point.
It’s a highly regulated and precise system that regulates body weight for survival of the species in this environment. However, the environment has changed over the past 100 years but our genetic makeup for survival of the fittest has not. The mechanism of action for defense of a higher body weight set point in this new environment has not been elucidated as yet. Most likely, there are many players or instigators involved, such as food-supply changes, sedentary lifestyle, ambient temperature, fetal programming, air quality, and global warming and climate change, to name a few.
The goal of obesity researchers is to investigate the underlying mechanisms of the increased prevalence of obesity over the past 100 years. The goal of obesity medicine specialists is to treat obesity in adults and children, and to prevent obesity as much as possible with lifestyle change and medications that have been shown to help “reverse” the metabolic adaptation to this environment. Our newest GLP-1/GIP receptor agonists have been shown in animal models to hit several pathways that lead to obesity. They are not just appetite suppressants. Yes, they do modulate appetite and satiety, but they also affect energy expenditure. The body’s normal reaction to a lack of calorie intake is to reduce resting energy expenditure until body weight increases back to “set point levels.” These agonists prevent that metabolic adaptation. That is why they are true agents that can treat obesity – the disease.
Back to the ketogenic diet. The ketogenic diet can fool the brain temporarily by using protein and fat to elicit satiety with less food intake in calories. After a while, however, gut hormones and other factors begin to counteract the weight loss with a reduction in resting energy and total energy expenditure, and other metabolic measures, to get the body back to a certain body weight set point.
The ketogenic diet also can help dieters avoid ultra- and highly processed foods. In the end, any type of diet that lowers caloric intake will work for weight loss, but it’s the maintenance of that weight loss that makes a long-term difference, and that involves closing the metabolic gap that the body generates to defend fat mass. Understanding this pathophysiology will allow obesity medicine specialists to assist patients with obesity to lose weight and keep it off.
Dr. Apovian is in the department of medicine, division of endocrinology, diabetes, and hypertension, and codirector, Center for Weight Management and Wellness, Harvard Medical School, Boston. She disclosed ties with Altimmune, Cowen and Company, Currax Pharmaceuticals, EPG Communication Holdings, Gelesis Srl, L-Nutra, NeuroBo Pharmaceuticals, National Institutes of Health, Patient-Centered Outcomes Research Institute, GI Dynamics, and Novo Nordisk. A version of this article first appeared on Medscape.com.
Persistent gaps in drug use by patients with type 2 diabetes
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Adults with mainly type 2 diabetes had gaps in the use of medications for managing blood glucose, hypertension, and lipids, in an analysis of nationally representative U.S. survey data.
A mean of 19.5%, 17.1%, and 43.3% of survey participants had inconsistent use of glucose-, BP-, or lipid-lowering medications, respectively, over 2 years in a series of successive 2-year surveys in 2005-2019.
A new group of participants was enrolled for each successive 2-year survey.
“We found persistent and sometimes increasing gaps in continuity of use of these [glycemia, hypertension, and lipid] treatments at the national level,” the researchers wrote.
Moreover, “this outcome was found despite long-lasting guidelines that generally recommend medications as an ongoing part of therapy for adults with type 2 diabetes to reduce macrovascular and microvascular disease risk,” they stressed.
The data did not distinguish between type 1 and type 2 diabetes, but more than 90% of diabetes diagnoses in the United States are type 2 diabetes, the researchers noted.
Therefore, it is “correct, our findings primarily reflect type 2 diabetes,” lead author Puneet Kaur Chehal, PhD, assistant professor, Emory University, Atlanta, clarified in an email.
“The clinical guidelines for treatment of type 1 diabetes are distinct,” she added, so “it is difficult to draw any conclusions from our study for this population.”
“To observe national trends in continuous use decrease at the same time that diabetes complications are increasing and physicians are guided to shift away from treat-to-target and towards individual patient needs certainly caught our attention,” she said.
“Our findings highlight the need for additional research to understand what is going on here,” according to Dr. Chehal.
“We did not observe levels of glucose (or blood pressure and lipids) to explore if the decrease in glucose-lowering drugs was warranted,” she added. “Our evidence of differences in continuity in use across subgroups (by race/ethnicity, payer, and age) does warrant further analysis of whether the decreasing trends we observe are lapses in access or deliberate changes in treatment.”
The study was published online in JAMA Network Open.
Investigating trends in medication adherence
Type 2 diabetes is a chronic condition and medications to control blood glucose, BP, and lipids lower the risk of diabetes-associated complications, Dr. Chehal and colleagues wrote.
After years of improvement, these cardiometabolic parameters plateaued and even decreased in 2013-2021, in parallel with increasing rates of diabetes complications, especially in younger adults, certain ethnic minority groups, and people with increased risks.
Suboptimal medication adherence among people with type 2 diabetes is associated with preventable complications and onset of heart disease, kidney disease, or diabetic neuropathy, which can lead to amputation.
However, previous studies of medication adherence were typically limited to patients covered by Medicare or commercial insurance, or studies only had 1-year follow-up.
Therefore, the researchers performed a cross-sectional analysis of a series of 2-year data from the Medical Expenditure Panel Survey (MEPS), in which participants reply to five interviews in 2 years and new participants are selected each year.
The researchers analyzed data from 15,237 adults aged 18 and older with type 2 diabetes who participated in 1 of 14 2-year MEPS survey panels in 2005-2019.
About half of participants (47.4%) were age 45-64 and about half (54.2%) were women. They were also racially diverse (43% non-Latino White, 25% Latino, and 24% non-Latino Black).
Participants were classified as having “inconsistent use” of glucose-lowering medication, for example, if they did not fill at least one prescription for a glucose-lowering drug in each of the 2 years.
“As long as [the medication] was some type of glucose-, blood pressure–, or lipid-lowering medication and was filled, it counted as continued use for that category,” Dr. Chehal explained.
They are preparing another paper that explores changes in medication regimens.
The current study showed continued use of glucose-lowering medication in both years decreased from 84.5% in 2005-2006 to 77.4% in 2018-2019, no use of glucose-lowering medication in either of the 2 years increased from 8.1% in 2005-2006 to 12.9% in 2018-2019, inconsistent use of glucose-lowering medication increased from 3.3% in 2005-2006 to 7.1% in 2018-2019, and new use of glucose-lowering medications in year 2 fluctuated between 2% and 4% across panels.
It also showed inconsistent use of BP-lowering medication increased from 3.9% in 2005-2006 to 9.0% in 2016-2017 and inconsistent use of lipid-lowering medication increased to a high of 9.9% in 2017-2018.
Younger and Black participants were less likely to consistently use glucose-lowering medication, Latino patients were less likely to consistently use BP-lowering medications, and Black and Latino patients were less likely to continuously use lipid-lowering medications. Uninsured adults were more likely to use no medications or use medications inconsistently.
“Changes and inconsistencies in payer formularies and out-of-pocket cost burden, especially among adults with no or insufficient insurance (i.e., Medicare Part D), remain prominent issues,” according to Dr. Chehal and colleagues.
“Decreases in continuity in use of glucose-lowering medications in recent panels may explain worsening diabetes complications,” they wrote.
This may be partly caused by recommended decreases in sulfonylurea and thiazolidinedione use and increased prescribing of new and more cost-prohibitive medications, they suggested.
Or this may be caused by the shift away from treating aggressively until a target is achieved toward individualizing treatment based on a patient’s age, phenotype, or comorbidities (for example, kidney disease).
The study was supported by a grant from MSD, a subsidiary of Merck, to Emory University. Some of the researchers received grants from Merck for the submitted work or were partially supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to the Georgia Center for Diabetes Translation Research. Dr. Chehal reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
‘Ozempic face’: Accepting wrinkles for improved health
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Last week, a number of patients emailed me regarding their concerns about this phenomenon known as Ozempic face. I went on to read about what this meant. I live in Los Angeles, where most people appear to be on semaglutide (Ozempic). It’s the phenomenon where people lose weight relatively rapidly, making their faces thin out. Then what happens, apparently, is they look older because their face is more wrinkled and baggier. They might have to have further plastic surgery. I say that with slight sarcasm because of where I live.
I want to talk about what I think about this, living here where there’s a great pressure to prescribe semaglutide off label, and what I think about it for my patients with diabetes.
Historically, we haven’t had much in terms of effective medication for treating obesity, and frankly, now we do. We now have agents that are effective, that have relatively few side effects, and that have become part of what’s out there. People now want to use these agents, semaglutide, and there’s been a great need for these agents.
The problem, however, is twofold. One, as we all know, is that it has basically caused a shortage of medication for treating our patients who actually have type 2 diabetes and really need these medications to manage their disease. Then we have people who want these medications who can’t pay for them. Insurance doesn’t cover obesity medications, which is problematic and actually quite frustrating for people who, I think, really would benefit from using these medications.
What I tell people, frankly, is that until I have enough supply for my patients with type 2 diabetes, who need these agents to control their blood sugars, I want to keep this class of drugs available to them. I also hope we’re able to expand it more and more with improving insurance coverage – and that’s a big if, if you ask me – both for people who have prediabetes and for patients who are overweight and obese, because I think it’s really hard for people to lose weight.
It’s frustrating, and for many people, being overweight and obese causes all sorts of other health issues, not only diabetes. I believe that these drugs are both safe and effective and should be more available. I do think we need to be careful in terms of who we prescribe them to, at least at the moment. Hopefully, we’ll be able to expand their use.
Anything that can encourage our population to lose weight and maintain that weight loss is very important. We need to couple weight loss medications with lifestyle interventions. I think people can out-eat any medication; therefore, it’s very important to encourage our patients to eat better, to exercise more, and to do all the other things they need to do to reduce their risks for other comorbidities.
I am incredibly happy to have these newer agents on the market. I tell my patients – at least those who have diabetes – that they have to accept looking a little bit too thin for the benefits that we can see in using these medications.
Thank you.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She has ties with Abbott Diabetes Care, AstraZeneca Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article originally appeared on Medscape.com.