User login
COAPT 5-year results ‘remarkable,’ but patient selection issues remain
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.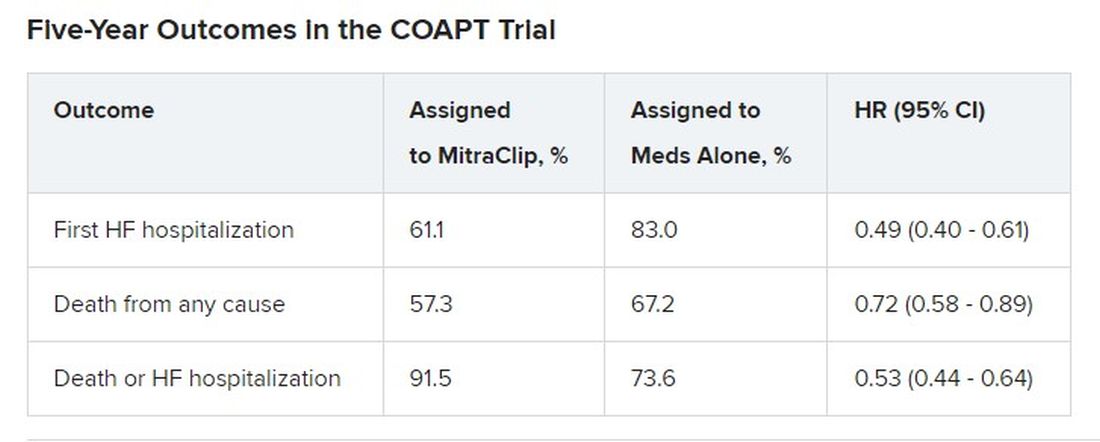
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
It remained an open question in 2018, on the unveiling of the COAPT trial’s 2-year primary results, whether the striking reductions in mortality and heart-failure (HF) hospitalization observed for transcatheter edge-to-edge repair (TEER) with the MitraClip (Abbott) would be durable with longer follow-up.
The trial had enrolled an especially sick population of symptomatic patients with mitral regurgitation (MR) secondary to HF.
As it turns out, the therapy’s benefits at 2 years were indeed durable, at least out to 5 years, investigators reported March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The results were simultaneously published in the New England Journal of Medicine.
Patients who received the MitraClip on top of intensive medical therapy, compared with a group assigned to medical management alone, benefited significantly at 5 years with risk reductions of 51% for HF hospitalization, 28% for death from any cause, and 47% for the composite of the two events.
Still, mortality at 5 years among the 614 randomized patients was steep at 57.3% in the MitraClip group and 67.2% for those assigned to meds only, underscoring the need for early identification of patients appropriate for the device therapy, Gregg W. Stone, MD, said during his presentation.
Dr. Stone, of the Icahn School of Medicine at Mount Sinai, New York, is a COAPT co-principal investigator and lead author of the 5-year outcomes publication.
Outcomes were consistent across all prespecified patient subgroups, including by age, sex, MR, left ventricular (LV) function and volume, cardiomyopathy etiology, and degree of surgical risk, the researchers reported.
Symptom status, as measured by New York Heart Association (NYHA) functional class, improved throughout the 5-year follow-up for patients assigned to the MitraClip group, compared with the control group, and the intervention group was significantly more likely to be in NYHA class 1 or 2, the authors noted.
The relative benefits in terms of clinical outcomes of MitraClip therapy narrowed after 2-3 years, Dr. Stone said, primarily because at 2 years, patients who had been assigned to meds only were eligible to undergo TEER. Indeed, he noted, 45% of the 138 patients in the control group who were eligible for TEER at 2 years “crossed over” to receive a MitraClip. Those patients benefited despite their delay in undergoing the procedure, he observed.
However, nearly half of the control patients died before becoming eligible for crossover at 2 years. “We have to identify the appropriate patients for treatment and treat them early because the mortality is very high in this population,” Dr. Stone said.
“We need to do more because the MitraClip doesn’t do anything directly to the underlying left ventricular dysfunction, which is the cause of the patient’s disease,” he said. “We need advanced therapies to address the underlying left ventricular dysfunction” in this high-risk population.
Exclusions based on LV dimension
The COAPT trial included 614 patients with HF and symptomatic MR despite guideline-directed medical therapy. They were required to have moderate to severe (3+) or severe (4+) MR confirmed by an echocardiographic core laboratory and a left ventricular ejection fraction (LVEF) of 20%-50%.
Among the exclusion criteria were an LV end-systolic diameter greater than 70 mm, severe pulmonary hypertension, and moderate to severe symptomatic right ventricular failure.
The systolic LV dimension exclusion helped address the persistent question of whether “severe mitral regurgitation is a marker of a bad left ventricle or ... contributes to the pathophysiology” of MR and its poor outcomes, Dr. Stone said.
The 51% reduction in risk for time-to-first HF hospitalization among patients assigned to TEER “accrued very early,” Dr. Stone pointed out. “You can see the curves start to separate almost immediately after you reduce left atrial pressure and volume overload with the MitraClip.”
The curves stopped diverging after about 3 years because of crossover from the control group, he said. Still, “we had shown a substantial absolute 17% reduction in mortality at 2 years” with MitraClip. “That has continued out to 5 years, with a statistically significant 28% relative reduction,” he continued, and the absolute risk reduction reaching 10%.
Patients in the control group who crossed over “basically assumed the death and heart failure hospitalization rate of the MitraClip group,” Dr. Stone said. That wasn’t surprising “because most of the patients enrolled in the trial originally had chronic heart failure.” It’s “confirmation of the principal results of the trial.”
Comparison With MITRA-FR
“We know that MITRA-FR was a negative trial,” observed Wayne B. Batchelor, MD, an invited discussant following Dr. Stone’s presentation, referring to an earlier similar trial that showed no advantage for MitraClip. Compared with MITRA-FR, COAPT “has created an entirely different story.”
The marked reductions in mortality and risk for adverse events and low number-needed-to-treat with MitraClip are “really remarkable,” said Dr. Batchelor, who is with the Inova Heart and Vascular Institute, Falls Church, Va.
But the high absolute mortality for patients in the COAPT control group “speaks volumes to me and tells us that we’ve got to identify our patients well early,” he agreed, and to “implement transcatheter edge-to-edge therapy in properly selected patients on guideline-directed medical therapy in order to avoid that.”
The trial findings “suggest that we’re reducing HF hospitalization,” he said, “so this is an extremely potent therapy, potentially.
“The dramatic difference between the treated arm and the medical therapy arm in this trial makes me feel that this therapy is here to stay,” Dr. Batchelor concluded. “We just have to figure out how to deploy it properly in the right patients.”
The COAPT trial presents “a practice-changing paradigm,” said Suzanne J. Baron, MD, of Lahey Hospital & Medical Center, Burlington, Mass., another invited discussant.
The crossover data “really jumped out,” she added. “Waiting to treat patients with TEER may be harmful, so if we’re going to consider treating earlier, how do we identify the right patient?” Dr. Baron asked, especially given the negative MITRA-FR results.
MITRA-FR didn’t follow patients beyond 2 years, Dr. Stone noted. Still, “we do think that the main difference was that COAPT enrolled a patient population with more severe MR and slightly less LV dysfunction, at least in terms of the LV not being as dilated, so they didn’t have end-stage LV disease. Whereas in MITRA-FR, more of the patients had only moderate mitral regurgitation.” And big dilated left ventricles “are less likely to benefit.”
There were also differences between the studies in technique and background medical therapies, he added.
The Food and Drug Administration has approved – and payers are paying – for the treatment of patients who meet the COAPT criteria, “in whom we can be very confident they have a benefit,” Dr. Stone said.
“The real question is: Where are the edges where we should consider this? LVEF slightly less than 20% or slightly greater than 50%? Or primary atrial functional mitral regurgitation? There are registry data to suggest that they would benefit,” he said, but “we need more data.”
COAPT was supported by Abbott. Dr. Stone disclosed receiving speaker honoraria from Abbott and consulting fees or equity from Neovasc, Ancora, Valfix, and Cardiac Success; and that Mount Sinai receives research funding from Abbott. Disclosures for the other authors are available at nejm.org. Dr. Batchelor has disclosed receiving consultant fees or honoraria from Abbott, Boston Scientific, Idorsia, and V-Wave Medical, and having other ties with Medtronic. Dr. Baron has disclosed receiving consultant fees or honoraria from Abiomed, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Shockwave, and Zoll Medical, and conducting research or receiving research grants from Abiomed and Boston Scientific.
A version of this article originally appeared on Medscape.com.
FROM ACC 2023
Dapagliflozin’s HFpEF benefit tied to lower filling pressure
NEW ORLEANS – Treatment of patients with heart failure with preserved ejection fraction (HFpEF) with the SGLT2 inhibitor dapagliflozin (Farxiga) for 24 weeks produced significant and beneficial reductions in left-heart filling pressures in a mechanistic, randomized clinical study.
The findings “provide new insight into the mechanisms underlying the favorable clinical effects of dapagliflozin in patients with HFpEF,” Barry A. Borlaug, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. “Elevations in left heart filling pressures at rest and during exercise are fundamental pathophysiologic features of HFpEF,” he noted.
Results from prior studies documented the benefit of dapagliflozin for improving clinical outcomes in patients with HFpEF in the DELIVER trial, and for the related sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in the EMPEROR-Preserved trial. The new findings presented by Dr. Borlaug provide evidence from a placebo-controlled, prospective study for one way by which these SGLT2 inhibitors exert this benefit in patients with HFpEF.
The results of his single-center study showed that, in patients with HFpEF who also exhibited “severe” elevations in pulmonary capillary wedge pressure (PCWP) during exercise, 24 weeks of treatment with dapagliflozin led to a significant reduction in PCWP during exercise. The treatment produced an average 6.1–mm Hg drop from baseline compared with control patients who received placebo. A similar pattern occurred when these patients were at rest, when dapagliflozin treatment linked with a significant average reduction in PCWP from baseline of 3.5 mm Hg compared with controls.
Improving a ‘specific and fundamental’ feature of HFpEF
“This fantastic study looked at one of the fundamental aspects of HFpEF,” said John R. Teerlink, MD, designated discussant for the study. “You’ve shown that dapagliflozin targets a specific and fundamental” manifestation of HFpEF by lowering PCWP, said Dr. Teerlink, director of Heart Failure at the San Francisco Veterans Affairs Medical Center.
However, Dr. Teerlink added, the study did not directly address the related question of what physiologic action of dapagliflozin produces this notable drop in PCWP.
“We’re just starting to look at that,” replied Dr. Borlaug, a cardiologist and professor at the Mayo Clinic in Rochester, Minn.
He reported finding an intriguing correlate in the current study linked to the cut in PCWP with dapagliflozin treatment. The SGLT2 inhibitor at a standard daily 10-mg dose produced an average 3.5-kg drop in body weight in the dapagliflozin-treated patients that significantly linked with the changes in PCWP both at rest and during exercise. Dapagliflozin-treated patients also showed a significant reduction from their baseline plasma volume compared with placebo-treated patients, but this “poorly correlated” with the dapagliflozin-linked cuts in PCWP, Dr. Borlaug said.
“I don’t think this means weight loss is the cause of the hemodynamic benefit, but maybe it’s an indicator. When patients [with HFpEF] lose weight, they are in a metabolic state that leads to good changes in hemodynamics,” he suggested. “My guess is that there is probably a combination of many different little things [caused by dapagliflozin treatment of patients with HFpEF] that together result in the 20%-25% relative improvement we see in filling pressure.”
An ‘obese, cardiometabolic’ HFpEF phenotype
The study enrolled patients with HFpEF and a left ventricular ejection fraction of at least 50%, a New York Heart Association functional class of 2 or 3, and a PCWP during exercise of at least 25 mm Hg. Of the 37 evaluable patients, about two-thirds of the patients were women, more than two-thirds were in functional class 3, about 70% were obese, and their average ejection fraction was about 62%. The study excluded patients with HFpEF who also had type 1 diabetes, cardiomyopathy, pericardial disease, or other causes of dyspnea or heart failure.
Dr. Teerlink asked about the generalizability of the findings, as the study cohort seemed to differ in certain respects from the patients enrolled in the DELIVER trial, and because of the many apparently distinct patient phenotypes that exist within the scope of HFpEF.
An “obese, cardiometabolic phenotype” predominated the study cohort, Dr. Borlaug said. “The patients we enrolled look like the HFpEF patients seen in U.S. clinics.” However, he added that “in reality, many [HFpEF phenotypes] coexist in one patient. It’s not that simple,” that every patient with HFpEF can be categorized into a single HFpEF phenotype.
The researchers monitored PCWP invasively with high-fidelity micromanometer catheters.
The study was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Borlaug has received research funding from AstraZeneca, as well as from Corvia, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax. Dr. Teerlink has had financial relationships with AstraZeneca, as well as with Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics.
NEW ORLEANS – Treatment of patients with heart failure with preserved ejection fraction (HFpEF) with the SGLT2 inhibitor dapagliflozin (Farxiga) for 24 weeks produced significant and beneficial reductions in left-heart filling pressures in a mechanistic, randomized clinical study.
The findings “provide new insight into the mechanisms underlying the favorable clinical effects of dapagliflozin in patients with HFpEF,” Barry A. Borlaug, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. “Elevations in left heart filling pressures at rest and during exercise are fundamental pathophysiologic features of HFpEF,” he noted.
Results from prior studies documented the benefit of dapagliflozin for improving clinical outcomes in patients with HFpEF in the DELIVER trial, and for the related sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in the EMPEROR-Preserved trial. The new findings presented by Dr. Borlaug provide evidence from a placebo-controlled, prospective study for one way by which these SGLT2 inhibitors exert this benefit in patients with HFpEF.
The results of his single-center study showed that, in patients with HFpEF who also exhibited “severe” elevations in pulmonary capillary wedge pressure (PCWP) during exercise, 24 weeks of treatment with dapagliflozin led to a significant reduction in PCWP during exercise. The treatment produced an average 6.1–mm Hg drop from baseline compared with control patients who received placebo. A similar pattern occurred when these patients were at rest, when dapagliflozin treatment linked with a significant average reduction in PCWP from baseline of 3.5 mm Hg compared with controls.
Improving a ‘specific and fundamental’ feature of HFpEF
“This fantastic study looked at one of the fundamental aspects of HFpEF,” said John R. Teerlink, MD, designated discussant for the study. “You’ve shown that dapagliflozin targets a specific and fundamental” manifestation of HFpEF by lowering PCWP, said Dr. Teerlink, director of Heart Failure at the San Francisco Veterans Affairs Medical Center.
However, Dr. Teerlink added, the study did not directly address the related question of what physiologic action of dapagliflozin produces this notable drop in PCWP.
“We’re just starting to look at that,” replied Dr. Borlaug, a cardiologist and professor at the Mayo Clinic in Rochester, Minn.
He reported finding an intriguing correlate in the current study linked to the cut in PCWP with dapagliflozin treatment. The SGLT2 inhibitor at a standard daily 10-mg dose produced an average 3.5-kg drop in body weight in the dapagliflozin-treated patients that significantly linked with the changes in PCWP both at rest and during exercise. Dapagliflozin-treated patients also showed a significant reduction from their baseline plasma volume compared with placebo-treated patients, but this “poorly correlated” with the dapagliflozin-linked cuts in PCWP, Dr. Borlaug said.
“I don’t think this means weight loss is the cause of the hemodynamic benefit, but maybe it’s an indicator. When patients [with HFpEF] lose weight, they are in a metabolic state that leads to good changes in hemodynamics,” he suggested. “My guess is that there is probably a combination of many different little things [caused by dapagliflozin treatment of patients with HFpEF] that together result in the 20%-25% relative improvement we see in filling pressure.”
An ‘obese, cardiometabolic’ HFpEF phenotype
The study enrolled patients with HFpEF and a left ventricular ejection fraction of at least 50%, a New York Heart Association functional class of 2 or 3, and a PCWP during exercise of at least 25 mm Hg. Of the 37 evaluable patients, about two-thirds of the patients were women, more than two-thirds were in functional class 3, about 70% were obese, and their average ejection fraction was about 62%. The study excluded patients with HFpEF who also had type 1 diabetes, cardiomyopathy, pericardial disease, or other causes of dyspnea or heart failure.
Dr. Teerlink asked about the generalizability of the findings, as the study cohort seemed to differ in certain respects from the patients enrolled in the DELIVER trial, and because of the many apparently distinct patient phenotypes that exist within the scope of HFpEF.
An “obese, cardiometabolic phenotype” predominated the study cohort, Dr. Borlaug said. “The patients we enrolled look like the HFpEF patients seen in U.S. clinics.” However, he added that “in reality, many [HFpEF phenotypes] coexist in one patient. It’s not that simple,” that every patient with HFpEF can be categorized into a single HFpEF phenotype.
The researchers monitored PCWP invasively with high-fidelity micromanometer catheters.
The study was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Borlaug has received research funding from AstraZeneca, as well as from Corvia, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax. Dr. Teerlink has had financial relationships with AstraZeneca, as well as with Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics.
NEW ORLEANS – Treatment of patients with heart failure with preserved ejection fraction (HFpEF) with the SGLT2 inhibitor dapagliflozin (Farxiga) for 24 weeks produced significant and beneficial reductions in left-heart filling pressures in a mechanistic, randomized clinical study.
The findings “provide new insight into the mechanisms underlying the favorable clinical effects of dapagliflozin in patients with HFpEF,” Barry A. Borlaug, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. “Elevations in left heart filling pressures at rest and during exercise are fundamental pathophysiologic features of HFpEF,” he noted.
Results from prior studies documented the benefit of dapagliflozin for improving clinical outcomes in patients with HFpEF in the DELIVER trial, and for the related sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in the EMPEROR-Preserved trial. The new findings presented by Dr. Borlaug provide evidence from a placebo-controlled, prospective study for one way by which these SGLT2 inhibitors exert this benefit in patients with HFpEF.
The results of his single-center study showed that, in patients with HFpEF who also exhibited “severe” elevations in pulmonary capillary wedge pressure (PCWP) during exercise, 24 weeks of treatment with dapagliflozin led to a significant reduction in PCWP during exercise. The treatment produced an average 6.1–mm Hg drop from baseline compared with control patients who received placebo. A similar pattern occurred when these patients were at rest, when dapagliflozin treatment linked with a significant average reduction in PCWP from baseline of 3.5 mm Hg compared with controls.
Improving a ‘specific and fundamental’ feature of HFpEF
“This fantastic study looked at one of the fundamental aspects of HFpEF,” said John R. Teerlink, MD, designated discussant for the study. “You’ve shown that dapagliflozin targets a specific and fundamental” manifestation of HFpEF by lowering PCWP, said Dr. Teerlink, director of Heart Failure at the San Francisco Veterans Affairs Medical Center.
However, Dr. Teerlink added, the study did not directly address the related question of what physiologic action of dapagliflozin produces this notable drop in PCWP.
“We’re just starting to look at that,” replied Dr. Borlaug, a cardiologist and professor at the Mayo Clinic in Rochester, Minn.
He reported finding an intriguing correlate in the current study linked to the cut in PCWP with dapagliflozin treatment. The SGLT2 inhibitor at a standard daily 10-mg dose produced an average 3.5-kg drop in body weight in the dapagliflozin-treated patients that significantly linked with the changes in PCWP both at rest and during exercise. Dapagliflozin-treated patients also showed a significant reduction from their baseline plasma volume compared with placebo-treated patients, but this “poorly correlated” with the dapagliflozin-linked cuts in PCWP, Dr. Borlaug said.
“I don’t think this means weight loss is the cause of the hemodynamic benefit, but maybe it’s an indicator. When patients [with HFpEF] lose weight, they are in a metabolic state that leads to good changes in hemodynamics,” he suggested. “My guess is that there is probably a combination of many different little things [caused by dapagliflozin treatment of patients with HFpEF] that together result in the 20%-25% relative improvement we see in filling pressure.”
An ‘obese, cardiometabolic’ HFpEF phenotype
The study enrolled patients with HFpEF and a left ventricular ejection fraction of at least 50%, a New York Heart Association functional class of 2 or 3, and a PCWP during exercise of at least 25 mm Hg. Of the 37 evaluable patients, about two-thirds of the patients were women, more than two-thirds were in functional class 3, about 70% were obese, and their average ejection fraction was about 62%. The study excluded patients with HFpEF who also had type 1 diabetes, cardiomyopathy, pericardial disease, or other causes of dyspnea or heart failure.
Dr. Teerlink asked about the generalizability of the findings, as the study cohort seemed to differ in certain respects from the patients enrolled in the DELIVER trial, and because of the many apparently distinct patient phenotypes that exist within the scope of HFpEF.
An “obese, cardiometabolic phenotype” predominated the study cohort, Dr. Borlaug said. “The patients we enrolled look like the HFpEF patients seen in U.S. clinics.” However, he added that “in reality, many [HFpEF phenotypes] coexist in one patient. It’s not that simple,” that every patient with HFpEF can be categorized into a single HFpEF phenotype.
The researchers monitored PCWP invasively with high-fidelity micromanometer catheters.
The study was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Borlaug has received research funding from AstraZeneca, as well as from Corvia, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax. Dr. Teerlink has had financial relationships with AstraZeneca, as well as with Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics.
AT ACC 2023
Oral PCSK9 inhibitor shows encouraging LDL lowering
A new oral formulation of a PCSK9-inhibiting, cholesterol-lowering drug in development by Merck has shown encouraging results in a phase 2 study.
The study was presented by Christie Ballantyne, MD, Baylor College of Medicine, Houston, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“In this diverse population of hypercholesterolemic patients, all doses of MK-0616 showed superior reduction of LDL vs. placebo up to a 60.9% placebo-adjusted reduction from baseline to week 8, which was consistent across subgroups,” Dr. Ballantyne reported.
“Reduction in ApoB and non-HDL cholesterol were consistent with that of LDL cholesterol, with up to a 51.8% reduction in ApoB and a 55.8% reduction in non-HDL,” he noted.
He added that the drug was well tolerated with no difference in adverse events across the treatment groups, compared with placebo.
“These data support the further development of MK-0616, an oral PCSK9 inhibitor that may improve access to effective LDL-cholesterol lowering therapies and improve attainment of guideline-recommended LDL goals aimed at reducing cardiovascular risk,” Dr. Ballantyne concluded. “The results are encouraging for a phase 3 program that is now being designed.”
He explained that elevated LDL is a primary causative factor for atherosclerotic cardiovascular disease (ASCVD), and despite effective treatments (statins), a large proportion of patients fail to achieve guideline-recommended LDL levels. Injectable treatments targeting PCSK9 have demonstrated large reductions in LDL and decreased risk of ASCVD events, but access barriers and need for repeat injections have led to poor adoption. An oral PCSK9 inhibitor may widen access and improve attainment of guideline-recommended treatment goals.
Dr. Ballantyne described the new drug, MK-0616, as a “macrocyclic peptide that can bind PCSK9 with monoclonal antibody-like affinity at 1/100th of the molecular weight.”
The current phase 2 study included 381 adult patients (49% female; median age 62 years) with a wide range of ASCVD risk. Average LDL-C level was 119.5 mg/dL at baseline. Around 40% of patients were not taking statins, 35% were on low- to moderate-intensity statin therapy, and 26% were on high-intensity statin therapy.
They were randomly assigned to four different doses of MK-0616 (6, 12, 18, or 30 mg once daily) or matching placebo.
Results showed that all doses of MK-0616 demonstrated statistically significant differences in percentage change in LDL-C from baseline to week 8 vs. placebo: –41.2% (6 mg), –55.7% (12 mg), –59.1% (18 mg), and –60.9% (30 mg).
The mean percentage changes in ApoB from baseline vs. placebo were –32.8%, –45.8%, –48.7%, and 51.8% for the four escalating doses of the drug. And non-HDL cholesterol changes were –35.9%, –50.5%, –53.2%, and –55.8% respectively.
The proportion of participants at protocol-defined goals for LDL reduction was 80.5%, 85.5%, 90.8%, and 90.8% with MK-0616 at the 6-mg, 12-mg, 18-mg, and 30-mg doses, compared with 9.3% with placebo.
Dr. Ballantyne reported that the efficacy looked similar in all subgroups, and regardless of baseline therapy.
“This was a dose-finding study, which will help select a dose to be taken forward in larger studies, and it looks from these results as though you get most of the efficacy by 12 mg,” he added.
Adverse events occurred in a proportion of participants in the MK-0616 groups (39.5% to 43.4%) similar to that of placebo (44.0%), and discontinuations as a result of adverse events occurred in two or fewer participants in any treatment group.
‘Super exciting’
Putting the results of his study into perspective at an ACC press conference, Rhonda Cooper-DeHoff, PharmD, associate professor in the department of pharmacotherapy and translational research at the University of Florida in Gainesville, commented.
“For the last quarter of a century we have had statins available to treat elevated LDL and atherosclerosis and despite that we have many patients who refuse to take statins or are afraid to take statins,” she said. “This is not about cost as the statins are all available generically now. But many patients claim to be intolerant or unresponsive.”
She noted that in 2015/2016 the first injectable PCSK9 inhibitors became available “which really were very exciting molecules, but they have a high cost and access issues, and patients often do not like injections so there are still a lot of issues.”
Dr. Cooper-DeHoff pointed out that this oral PCSK9 inhibitor seems to be as effective at lowering LDL as the injectable products regardless of whether statins are on board or not, which she said was “super exciting.”
She added: “We are all going to be waiting excitedly for the outcome data with this oral PCSK9 inhibitor.”
She also noted that another study (CLEAR Outcomes) presented at the ACC meeting showed good lipid-lowering results and a reduction in cardiovascular outcomes in statin-intolerant patients with another oral lipid lowering drug, bempedoic acid (Nexletol).
She said the two oral drugs promised a “very bright for the future for LDL lowering and the treatment of atherosclerosis in our patients,” adding that “we are now really chipping away at the barriers to achieving the holy grail of guideline-directed LDL lowering to prevent hard outcomes.”
The results were published online in the Journal of the American College of Cardiology at the time of presentation.
This study was funded by Merck. Dr. Ballantyne has received grant/research support through his institution from Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, New Amsterdam, Novartis, Novo Nordisk, Regeneron, and Roche Diagnostics and has been a consultant for 89Bio, Abbott Diagnostics, Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Ionis, Matinas BioPharma, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
A new oral formulation of a PCSK9-inhibiting, cholesterol-lowering drug in development by Merck has shown encouraging results in a phase 2 study.
The study was presented by Christie Ballantyne, MD, Baylor College of Medicine, Houston, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“In this diverse population of hypercholesterolemic patients, all doses of MK-0616 showed superior reduction of LDL vs. placebo up to a 60.9% placebo-adjusted reduction from baseline to week 8, which was consistent across subgroups,” Dr. Ballantyne reported.
“Reduction in ApoB and non-HDL cholesterol were consistent with that of LDL cholesterol, with up to a 51.8% reduction in ApoB and a 55.8% reduction in non-HDL,” he noted.
He added that the drug was well tolerated with no difference in adverse events across the treatment groups, compared with placebo.
“These data support the further development of MK-0616, an oral PCSK9 inhibitor that may improve access to effective LDL-cholesterol lowering therapies and improve attainment of guideline-recommended LDL goals aimed at reducing cardiovascular risk,” Dr. Ballantyne concluded. “The results are encouraging for a phase 3 program that is now being designed.”
He explained that elevated LDL is a primary causative factor for atherosclerotic cardiovascular disease (ASCVD), and despite effective treatments (statins), a large proportion of patients fail to achieve guideline-recommended LDL levels. Injectable treatments targeting PCSK9 have demonstrated large reductions in LDL and decreased risk of ASCVD events, but access barriers and need for repeat injections have led to poor adoption. An oral PCSK9 inhibitor may widen access and improve attainment of guideline-recommended treatment goals.
Dr. Ballantyne described the new drug, MK-0616, as a “macrocyclic peptide that can bind PCSK9 with monoclonal antibody-like affinity at 1/100th of the molecular weight.”
The current phase 2 study included 381 adult patients (49% female; median age 62 years) with a wide range of ASCVD risk. Average LDL-C level was 119.5 mg/dL at baseline. Around 40% of patients were not taking statins, 35% were on low- to moderate-intensity statin therapy, and 26% were on high-intensity statin therapy.
They were randomly assigned to four different doses of MK-0616 (6, 12, 18, or 30 mg once daily) or matching placebo.
Results showed that all doses of MK-0616 demonstrated statistically significant differences in percentage change in LDL-C from baseline to week 8 vs. placebo: –41.2% (6 mg), –55.7% (12 mg), –59.1% (18 mg), and –60.9% (30 mg).
The mean percentage changes in ApoB from baseline vs. placebo were –32.8%, –45.8%, –48.7%, and 51.8% for the four escalating doses of the drug. And non-HDL cholesterol changes were –35.9%, –50.5%, –53.2%, and –55.8% respectively.
The proportion of participants at protocol-defined goals for LDL reduction was 80.5%, 85.5%, 90.8%, and 90.8% with MK-0616 at the 6-mg, 12-mg, 18-mg, and 30-mg doses, compared with 9.3% with placebo.
Dr. Ballantyne reported that the efficacy looked similar in all subgroups, and regardless of baseline therapy.
“This was a dose-finding study, which will help select a dose to be taken forward in larger studies, and it looks from these results as though you get most of the efficacy by 12 mg,” he added.
Adverse events occurred in a proportion of participants in the MK-0616 groups (39.5% to 43.4%) similar to that of placebo (44.0%), and discontinuations as a result of adverse events occurred in two or fewer participants in any treatment group.
‘Super exciting’
Putting the results of his study into perspective at an ACC press conference, Rhonda Cooper-DeHoff, PharmD, associate professor in the department of pharmacotherapy and translational research at the University of Florida in Gainesville, commented.
“For the last quarter of a century we have had statins available to treat elevated LDL and atherosclerosis and despite that we have many patients who refuse to take statins or are afraid to take statins,” she said. “This is not about cost as the statins are all available generically now. But many patients claim to be intolerant or unresponsive.”
She noted that in 2015/2016 the first injectable PCSK9 inhibitors became available “which really were very exciting molecules, but they have a high cost and access issues, and patients often do not like injections so there are still a lot of issues.”
Dr. Cooper-DeHoff pointed out that this oral PCSK9 inhibitor seems to be as effective at lowering LDL as the injectable products regardless of whether statins are on board or not, which she said was “super exciting.”
She added: “We are all going to be waiting excitedly for the outcome data with this oral PCSK9 inhibitor.”
She also noted that another study (CLEAR Outcomes) presented at the ACC meeting showed good lipid-lowering results and a reduction in cardiovascular outcomes in statin-intolerant patients with another oral lipid lowering drug, bempedoic acid (Nexletol).
She said the two oral drugs promised a “very bright for the future for LDL lowering and the treatment of atherosclerosis in our patients,” adding that “we are now really chipping away at the barriers to achieving the holy grail of guideline-directed LDL lowering to prevent hard outcomes.”
The results were published online in the Journal of the American College of Cardiology at the time of presentation.
This study was funded by Merck. Dr. Ballantyne has received grant/research support through his institution from Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, New Amsterdam, Novartis, Novo Nordisk, Regeneron, and Roche Diagnostics and has been a consultant for 89Bio, Abbott Diagnostics, Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Ionis, Matinas BioPharma, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
A new oral formulation of a PCSK9-inhibiting, cholesterol-lowering drug in development by Merck has shown encouraging results in a phase 2 study.
The study was presented by Christie Ballantyne, MD, Baylor College of Medicine, Houston, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“In this diverse population of hypercholesterolemic patients, all doses of MK-0616 showed superior reduction of LDL vs. placebo up to a 60.9% placebo-adjusted reduction from baseline to week 8, which was consistent across subgroups,” Dr. Ballantyne reported.
“Reduction in ApoB and non-HDL cholesterol were consistent with that of LDL cholesterol, with up to a 51.8% reduction in ApoB and a 55.8% reduction in non-HDL,” he noted.
He added that the drug was well tolerated with no difference in adverse events across the treatment groups, compared with placebo.
“These data support the further development of MK-0616, an oral PCSK9 inhibitor that may improve access to effective LDL-cholesterol lowering therapies and improve attainment of guideline-recommended LDL goals aimed at reducing cardiovascular risk,” Dr. Ballantyne concluded. “The results are encouraging for a phase 3 program that is now being designed.”
He explained that elevated LDL is a primary causative factor for atherosclerotic cardiovascular disease (ASCVD), and despite effective treatments (statins), a large proportion of patients fail to achieve guideline-recommended LDL levels. Injectable treatments targeting PCSK9 have demonstrated large reductions in LDL and decreased risk of ASCVD events, but access barriers and need for repeat injections have led to poor adoption. An oral PCSK9 inhibitor may widen access and improve attainment of guideline-recommended treatment goals.
Dr. Ballantyne described the new drug, MK-0616, as a “macrocyclic peptide that can bind PCSK9 with monoclonal antibody-like affinity at 1/100th of the molecular weight.”
The current phase 2 study included 381 adult patients (49% female; median age 62 years) with a wide range of ASCVD risk. Average LDL-C level was 119.5 mg/dL at baseline. Around 40% of patients were not taking statins, 35% were on low- to moderate-intensity statin therapy, and 26% were on high-intensity statin therapy.
They were randomly assigned to four different doses of MK-0616 (6, 12, 18, or 30 mg once daily) or matching placebo.
Results showed that all doses of MK-0616 demonstrated statistically significant differences in percentage change in LDL-C from baseline to week 8 vs. placebo: –41.2% (6 mg), –55.7% (12 mg), –59.1% (18 mg), and –60.9% (30 mg).
The mean percentage changes in ApoB from baseline vs. placebo were –32.8%, –45.8%, –48.7%, and 51.8% for the four escalating doses of the drug. And non-HDL cholesterol changes were –35.9%, –50.5%, –53.2%, and –55.8% respectively.
The proportion of participants at protocol-defined goals for LDL reduction was 80.5%, 85.5%, 90.8%, and 90.8% with MK-0616 at the 6-mg, 12-mg, 18-mg, and 30-mg doses, compared with 9.3% with placebo.
Dr. Ballantyne reported that the efficacy looked similar in all subgroups, and regardless of baseline therapy.
“This was a dose-finding study, which will help select a dose to be taken forward in larger studies, and it looks from these results as though you get most of the efficacy by 12 mg,” he added.
Adverse events occurred in a proportion of participants in the MK-0616 groups (39.5% to 43.4%) similar to that of placebo (44.0%), and discontinuations as a result of adverse events occurred in two or fewer participants in any treatment group.
‘Super exciting’
Putting the results of his study into perspective at an ACC press conference, Rhonda Cooper-DeHoff, PharmD, associate professor in the department of pharmacotherapy and translational research at the University of Florida in Gainesville, commented.
“For the last quarter of a century we have had statins available to treat elevated LDL and atherosclerosis and despite that we have many patients who refuse to take statins or are afraid to take statins,” she said. “This is not about cost as the statins are all available generically now. But many patients claim to be intolerant or unresponsive.”
She noted that in 2015/2016 the first injectable PCSK9 inhibitors became available “which really were very exciting molecules, but they have a high cost and access issues, and patients often do not like injections so there are still a lot of issues.”
Dr. Cooper-DeHoff pointed out that this oral PCSK9 inhibitor seems to be as effective at lowering LDL as the injectable products regardless of whether statins are on board or not, which she said was “super exciting.”
She added: “We are all going to be waiting excitedly for the outcome data with this oral PCSK9 inhibitor.”
She also noted that another study (CLEAR Outcomes) presented at the ACC meeting showed good lipid-lowering results and a reduction in cardiovascular outcomes in statin-intolerant patients with another oral lipid lowering drug, bempedoic acid (Nexletol).
She said the two oral drugs promised a “very bright for the future for LDL lowering and the treatment of atherosclerosis in our patients,” adding that “we are now really chipping away at the barriers to achieving the holy grail of guideline-directed LDL lowering to prevent hard outcomes.”
The results were published online in the Journal of the American College of Cardiology at the time of presentation.
This study was funded by Merck. Dr. Ballantyne has received grant/research support through his institution from Abbott Diagnostic, Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, New Amsterdam, Novartis, Novo Nordisk, Regeneron, and Roche Diagnostics and has been a consultant for 89Bio, Abbott Diagnostics, Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Denka Seiken, Esperion, Genentech, Gilead, Illumina, Ionis, Matinas BioPharma, Merck, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
‘Unheard of’ PAH improvement with novel drug: STELLAR
NEW ORLEANS – An investigational, first-in class agent that delivers a completely new type of intervention to patients with pulmonary arterial hypertension (PAH) scored a clear win in the STELLAR trial, the first to complete among three phase 3 trials that are testing this agent.
Sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved from baseline average 6-minute walk distance (6MWD) by a significant and clinically meaningful 40.8 meters, compared with placebo, for the trial’s primary efficacy endpoint (P < .001). The treatment also “delivered broad clinical benefit across multiple domains including hemodynamics, World Health Organization functional class, disease biomarkers, risk scores and patient-reported outcomes,” Marius M. Hoeper, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“These results establish the clinical utility of sotatercept, administered in combination with approved PAH therapies, as a new treatment for PAH,” added Dr. Hoeper, professor and deputy director of the department of respiratory medicine at Hannover (Germany) Medical School,
“The most important aspect was the hemodynamic improvement,” with sotatercept treatment, which led to an average 235 dyn/sec per cm−5 reduction in pulmonary vascular resistance from baseline and an average cut in pulmonary artery pressure of 13.9 mm Hg from baseline, compared with placebo, a result that’s “unheard of,” Dr. Hoeper said in a press conference during the meeting.
“With other tested agents we usually see very little improvement in pulmonary artery pressure. This is a signal that we achieved some reversing of the pathological changes in the pulmonary vessels that lead to” PAH, he added.
Simultaneously with his report the findings also appeared online in the New England Journal of Medicine.
‘A new hope’ for patients with PAH
Based on the reported findings, sotatercept is a “very exciting boutique molecule” that will “offer patients with PAH a very exciting new treatment,” commented Rhonda Cooper-DeHoff, PharmD, a designated discussant and a researcher at the University of Florida, Gainesville.
“This study is a new hope for patients with PAH. Until now, they’ve had really bad outcomes, but [in this study] we see significant differences in 6MWD, hemodynamics, and risk factors. Overall, I think the benefit is greater than the risk” it may pose to patients through potential adverse effects, commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, and another discussant at the meeting.
“The results are impressive” and “encouraging,” and “suggest that sotatercept may represent a new and clinically consequential addition to current medications for PAH,” wrote three clinicians from Canyons Region Intermountain Medical Center in Murray, Utah, in an editorial that accompanied the published report.
But the authors of the editorial also raised several cautions and concerns. They questioned the generalizability of the findings, noting that the patients with PAH enrolled in the study were all adults who were clinically stable and an average of more than 8 years out from their initial PAH diagnosis, and more than 90% were on stable treatment for PAH with two or three agents specific for treating the disorder. The study cohort also had a disproportionately high enrollment of patients with idiopathic (59%) or heritable (18%) forms of PAH, and the 15% of patients in the trial with connective tissue disease represented a disproportionately low prevalence of this PAH subtype.
The editorialists also called for “ongoing vigilance” for adverse effects from sotatercept treatment, although they acknowledged that the adverse effects reported to date from sotatercept are “largely reassuring.”
Death or clinical worsening cut by 84%
STELLAR randomized 323 patients at 91 sites in 21 countries with WHO Group 1 PAH and with WHO functional class II or III disease to receive either sotatercept or placebo for 24 weeks, with an option for treatment to continue beyond that until the last patient in the study reached 24 weeks on treatment, resulting in an overall median treatment duration of nearly 33 weeks.
In addition to the significant result for the primary endpoint, the 163 patients who received sotatercept had significant improvements, compared with 160 placebo-treated patients, for eight of nine secondary endpoints. The only secondary endpoint with a neutral result was for a measure of cognitive and emotional wellbeing, a parameter that was already at a normal level at baseline in most enrolled patients, Dr. Hoeper explained.
The incidence of either death or an event indicative of clinical worsening during the overall median follow-up of almost 33 weeks was 26.3% among the control patients and 5.5% among those who received sotatercept. This translated into a significant reduction for this endpoint of 84% with sotatercept treatment, compared with placebo.
The rates of treatment-emergent adverse events leading to discontinuation were roughly the same in the control and sotatercept arms, and the incidence of severe or serious treatment-emergent adverse events was higher among the control patients.
The most common adverse event on sotatercept was bleeding events, which occurred in 32% of those on sotatercept and in 16% of the control patients, but the events in the sotatercept arm were “mostly mild,” said Dr. Hoeper. The next most frequent adverse event during sotatercept treatment was appearance of telangiectasias, which occurred in 14% of those on sotatercept and in 4% of control patients.
“It’s an uncommon adverse event profile, but not unexpected for a drug with its mechanism of action,” he said.
Drug binds activin, a pathologic driver of PAH
Sotatercept is an engineered molecule that combines a section of a human immunoglobulin G molecule with a portion of the receptor for activin. This structure allows sotatercept to bind free activin molecules in a patient’s blood, thereby removing a key driver of the pulmonary vascular wall remodeling that is at the pathologic root of PAH.
“Hyperproliferation of blood vessel–wall cells” caused by activin signaling “is perhaps the most important driver of PAH,” Dr. Hoeper said. “Sotatercept allows us for the first time to target the underlying mechanism behind PAH.”
Still ongoing are the HYPERION and ZENITH phase 3 trials of sotatercept. HYPERION is enrolling patients with newly diagnosed or high-risk PAH and is expected to complete in 2028. ZENITH is enrolling patients with more advanced PAH and a higher mortality risk, with results expected in 2026.
Sotatercept has received “Breakthrough Therapy” designation and “Orphan Drug” designation by the Food and Drug Administration, and “Priority Medicines” designation and “Orphan Drug” designation by the European Medicines Agency for the treatment of PAH. One recent review estimated a worldwide PAH prevalence of about 3-4 cases/100,000, which for the United States translates into a total prevalence of perhaps 10,000-15,000 affected people.
STELLAR was funded by Acceleron Pharma, a subsidiary of Merck. Dr. Hoeper is a consultant to Acceleron. Dr. Cooper-DeHoff, Dr. Grapsa, and the authors of the editorial on STELLAR have no relevant disclosures.
NEW ORLEANS – An investigational, first-in class agent that delivers a completely new type of intervention to patients with pulmonary arterial hypertension (PAH) scored a clear win in the STELLAR trial, the first to complete among three phase 3 trials that are testing this agent.
Sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved from baseline average 6-minute walk distance (6MWD) by a significant and clinically meaningful 40.8 meters, compared with placebo, for the trial’s primary efficacy endpoint (P < .001). The treatment also “delivered broad clinical benefit across multiple domains including hemodynamics, World Health Organization functional class, disease biomarkers, risk scores and patient-reported outcomes,” Marius M. Hoeper, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“These results establish the clinical utility of sotatercept, administered in combination with approved PAH therapies, as a new treatment for PAH,” added Dr. Hoeper, professor and deputy director of the department of respiratory medicine at Hannover (Germany) Medical School,
“The most important aspect was the hemodynamic improvement,” with sotatercept treatment, which led to an average 235 dyn/sec per cm−5 reduction in pulmonary vascular resistance from baseline and an average cut in pulmonary artery pressure of 13.9 mm Hg from baseline, compared with placebo, a result that’s “unheard of,” Dr. Hoeper said in a press conference during the meeting.
“With other tested agents we usually see very little improvement in pulmonary artery pressure. This is a signal that we achieved some reversing of the pathological changes in the pulmonary vessels that lead to” PAH, he added.
Simultaneously with his report the findings also appeared online in the New England Journal of Medicine.
‘A new hope’ for patients with PAH
Based on the reported findings, sotatercept is a “very exciting boutique molecule” that will “offer patients with PAH a very exciting new treatment,” commented Rhonda Cooper-DeHoff, PharmD, a designated discussant and a researcher at the University of Florida, Gainesville.
“This study is a new hope for patients with PAH. Until now, they’ve had really bad outcomes, but [in this study] we see significant differences in 6MWD, hemodynamics, and risk factors. Overall, I think the benefit is greater than the risk” it may pose to patients through potential adverse effects, commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, and another discussant at the meeting.
“The results are impressive” and “encouraging,” and “suggest that sotatercept may represent a new and clinically consequential addition to current medications for PAH,” wrote three clinicians from Canyons Region Intermountain Medical Center in Murray, Utah, in an editorial that accompanied the published report.
But the authors of the editorial also raised several cautions and concerns. They questioned the generalizability of the findings, noting that the patients with PAH enrolled in the study were all adults who were clinically stable and an average of more than 8 years out from their initial PAH diagnosis, and more than 90% were on stable treatment for PAH with two or three agents specific for treating the disorder. The study cohort also had a disproportionately high enrollment of patients with idiopathic (59%) or heritable (18%) forms of PAH, and the 15% of patients in the trial with connective tissue disease represented a disproportionately low prevalence of this PAH subtype.
The editorialists also called for “ongoing vigilance” for adverse effects from sotatercept treatment, although they acknowledged that the adverse effects reported to date from sotatercept are “largely reassuring.”
Death or clinical worsening cut by 84%
STELLAR randomized 323 patients at 91 sites in 21 countries with WHO Group 1 PAH and with WHO functional class II or III disease to receive either sotatercept or placebo for 24 weeks, with an option for treatment to continue beyond that until the last patient in the study reached 24 weeks on treatment, resulting in an overall median treatment duration of nearly 33 weeks.
In addition to the significant result for the primary endpoint, the 163 patients who received sotatercept had significant improvements, compared with 160 placebo-treated patients, for eight of nine secondary endpoints. The only secondary endpoint with a neutral result was for a measure of cognitive and emotional wellbeing, a parameter that was already at a normal level at baseline in most enrolled patients, Dr. Hoeper explained.
The incidence of either death or an event indicative of clinical worsening during the overall median follow-up of almost 33 weeks was 26.3% among the control patients and 5.5% among those who received sotatercept. This translated into a significant reduction for this endpoint of 84% with sotatercept treatment, compared with placebo.
The rates of treatment-emergent adverse events leading to discontinuation were roughly the same in the control and sotatercept arms, and the incidence of severe or serious treatment-emergent adverse events was higher among the control patients.
The most common adverse event on sotatercept was bleeding events, which occurred in 32% of those on sotatercept and in 16% of the control patients, but the events in the sotatercept arm were “mostly mild,” said Dr. Hoeper. The next most frequent adverse event during sotatercept treatment was appearance of telangiectasias, which occurred in 14% of those on sotatercept and in 4% of control patients.
“It’s an uncommon adverse event profile, but not unexpected for a drug with its mechanism of action,” he said.
Drug binds activin, a pathologic driver of PAH
Sotatercept is an engineered molecule that combines a section of a human immunoglobulin G molecule with a portion of the receptor for activin. This structure allows sotatercept to bind free activin molecules in a patient’s blood, thereby removing a key driver of the pulmonary vascular wall remodeling that is at the pathologic root of PAH.
“Hyperproliferation of blood vessel–wall cells” caused by activin signaling “is perhaps the most important driver of PAH,” Dr. Hoeper said. “Sotatercept allows us for the first time to target the underlying mechanism behind PAH.”
Still ongoing are the HYPERION and ZENITH phase 3 trials of sotatercept. HYPERION is enrolling patients with newly diagnosed or high-risk PAH and is expected to complete in 2028. ZENITH is enrolling patients with more advanced PAH and a higher mortality risk, with results expected in 2026.
Sotatercept has received “Breakthrough Therapy” designation and “Orphan Drug” designation by the Food and Drug Administration, and “Priority Medicines” designation and “Orphan Drug” designation by the European Medicines Agency for the treatment of PAH. One recent review estimated a worldwide PAH prevalence of about 3-4 cases/100,000, which for the United States translates into a total prevalence of perhaps 10,000-15,000 affected people.
STELLAR was funded by Acceleron Pharma, a subsidiary of Merck. Dr. Hoeper is a consultant to Acceleron. Dr. Cooper-DeHoff, Dr. Grapsa, and the authors of the editorial on STELLAR have no relevant disclosures.
NEW ORLEANS – An investigational, first-in class agent that delivers a completely new type of intervention to patients with pulmonary arterial hypertension (PAH) scored a clear win in the STELLAR trial, the first to complete among three phase 3 trials that are testing this agent.
Sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved from baseline average 6-minute walk distance (6MWD) by a significant and clinically meaningful 40.8 meters, compared with placebo, for the trial’s primary efficacy endpoint (P < .001). The treatment also “delivered broad clinical benefit across multiple domains including hemodynamics, World Health Organization functional class, disease biomarkers, risk scores and patient-reported outcomes,” Marius M. Hoeper, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“These results establish the clinical utility of sotatercept, administered in combination with approved PAH therapies, as a new treatment for PAH,” added Dr. Hoeper, professor and deputy director of the department of respiratory medicine at Hannover (Germany) Medical School,
“The most important aspect was the hemodynamic improvement,” with sotatercept treatment, which led to an average 235 dyn/sec per cm−5 reduction in pulmonary vascular resistance from baseline and an average cut in pulmonary artery pressure of 13.9 mm Hg from baseline, compared with placebo, a result that’s “unheard of,” Dr. Hoeper said in a press conference during the meeting.
“With other tested agents we usually see very little improvement in pulmonary artery pressure. This is a signal that we achieved some reversing of the pathological changes in the pulmonary vessels that lead to” PAH, he added.
Simultaneously with his report the findings also appeared online in the New England Journal of Medicine.
‘A new hope’ for patients with PAH
Based on the reported findings, sotatercept is a “very exciting boutique molecule” that will “offer patients with PAH a very exciting new treatment,” commented Rhonda Cooper-DeHoff, PharmD, a designated discussant and a researcher at the University of Florida, Gainesville.
“This study is a new hope for patients with PAH. Until now, they’ve had really bad outcomes, but [in this study] we see significant differences in 6MWD, hemodynamics, and risk factors. Overall, I think the benefit is greater than the risk” it may pose to patients through potential adverse effects, commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, and another discussant at the meeting.
“The results are impressive” and “encouraging,” and “suggest that sotatercept may represent a new and clinically consequential addition to current medications for PAH,” wrote three clinicians from Canyons Region Intermountain Medical Center in Murray, Utah, in an editorial that accompanied the published report.
But the authors of the editorial also raised several cautions and concerns. They questioned the generalizability of the findings, noting that the patients with PAH enrolled in the study were all adults who were clinically stable and an average of more than 8 years out from their initial PAH diagnosis, and more than 90% were on stable treatment for PAH with two or three agents specific for treating the disorder. The study cohort also had a disproportionately high enrollment of patients with idiopathic (59%) or heritable (18%) forms of PAH, and the 15% of patients in the trial with connective tissue disease represented a disproportionately low prevalence of this PAH subtype.
The editorialists also called for “ongoing vigilance” for adverse effects from sotatercept treatment, although they acknowledged that the adverse effects reported to date from sotatercept are “largely reassuring.”
Death or clinical worsening cut by 84%
STELLAR randomized 323 patients at 91 sites in 21 countries with WHO Group 1 PAH and with WHO functional class II or III disease to receive either sotatercept or placebo for 24 weeks, with an option for treatment to continue beyond that until the last patient in the study reached 24 weeks on treatment, resulting in an overall median treatment duration of nearly 33 weeks.
In addition to the significant result for the primary endpoint, the 163 patients who received sotatercept had significant improvements, compared with 160 placebo-treated patients, for eight of nine secondary endpoints. The only secondary endpoint with a neutral result was for a measure of cognitive and emotional wellbeing, a parameter that was already at a normal level at baseline in most enrolled patients, Dr. Hoeper explained.
The incidence of either death or an event indicative of clinical worsening during the overall median follow-up of almost 33 weeks was 26.3% among the control patients and 5.5% among those who received sotatercept. This translated into a significant reduction for this endpoint of 84% with sotatercept treatment, compared with placebo.
The rates of treatment-emergent adverse events leading to discontinuation were roughly the same in the control and sotatercept arms, and the incidence of severe or serious treatment-emergent adverse events was higher among the control patients.
The most common adverse event on sotatercept was bleeding events, which occurred in 32% of those on sotatercept and in 16% of the control patients, but the events in the sotatercept arm were “mostly mild,” said Dr. Hoeper. The next most frequent adverse event during sotatercept treatment was appearance of telangiectasias, which occurred in 14% of those on sotatercept and in 4% of control patients.
“It’s an uncommon adverse event profile, but not unexpected for a drug with its mechanism of action,” he said.
Drug binds activin, a pathologic driver of PAH
Sotatercept is an engineered molecule that combines a section of a human immunoglobulin G molecule with a portion of the receptor for activin. This structure allows sotatercept to bind free activin molecules in a patient’s blood, thereby removing a key driver of the pulmonary vascular wall remodeling that is at the pathologic root of PAH.
“Hyperproliferation of blood vessel–wall cells” caused by activin signaling “is perhaps the most important driver of PAH,” Dr. Hoeper said. “Sotatercept allows us for the first time to target the underlying mechanism behind PAH.”
Still ongoing are the HYPERION and ZENITH phase 3 trials of sotatercept. HYPERION is enrolling patients with newly diagnosed or high-risk PAH and is expected to complete in 2028. ZENITH is enrolling patients with more advanced PAH and a higher mortality risk, with results expected in 2026.
Sotatercept has received “Breakthrough Therapy” designation and “Orphan Drug” designation by the Food and Drug Administration, and “Priority Medicines” designation and “Orphan Drug” designation by the European Medicines Agency for the treatment of PAH. One recent review estimated a worldwide PAH prevalence of about 3-4 cases/100,000, which for the United States translates into a total prevalence of perhaps 10,000-15,000 affected people.
STELLAR was funded by Acceleron Pharma, a subsidiary of Merck. Dr. Hoeper is a consultant to Acceleron. Dr. Cooper-DeHoff, Dr. Grapsa, and the authors of the editorial on STELLAR have no relevant disclosures.
At ACC 2023
Endurance exercise tied to more coronary atherosclerosis
In the Master@Heart study, lifelong endurance athletes had more coronary plaques, including more noncalcified plaques, than fit and healthy individuals with a similarly low cardiovascular risk profile.
The study was presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. It was also simultaneously published online in the European Heart Journal.
“We consistently see higher plaque burden in lifelong endurance athletes. This is regardless of the plaque type, whether it is calcified, mixed, noncalcified, in the proximal segment or causing more than 50% stenosis,” concluded Ruben De Bosscher, MD, Catholic University of Leuven (Belgium), during his presentation.
The researchers suggested that all the information to date suggests there may be a “reverse J-shaped” dose-response relationship between exercise and coronary atherosclerosis.
Dr. De Bosscher added that “the worst thing you can do is nothing at all. As soon as you do a little bit of exercise – just brisk walking or jogging up to 3 hours a week – it seems that’s where you get the most benefit. And after that, we tend to see an increase in coronary plaque burden.”
The discussant of the study at the ACC session, Michael Emery, MD, codirector of the Sports Cardiology Center at the Cleveland Clinic, asked how this information should be translated into advice for the general public, given that it is known that endurance athletes show much improved mortality.
“That is a very good question,” Dr. De Bosscher replied. “Yes, we do see less events and adverse outcomes in endurance athletes, but that is compared to the whole population, including those that are unhealthy and do not exercise.
“If we only look at healthy individuals who do exercise but at varying levels, the question is, do we then see the same relationship?” he asked. “There is increasing evidence that there may be a point of diminished returns – and at a certain point, an increased cardiovascular risk is seen in endurance athletes.”
On advice to the public, Dr. De Bosscher added, “one of the main findings here is that, despite having a very healthy lifestyle style and exercising a lot, no one is granted immunity to coronary atherosclerosis. It would seem that the most benefit occurs in individuals doing a moderate amount of exercise – up to about 3 hours a week.”
In a comment, Dr. Emery noted: “This continues to be a ‘hot topic,’ although I continue to be underwhelmed, given a lack of hard outcomes, and I worry about the wrong take-home message being sent, that too much exercise will do more harm than good.”
He added that fitness still matters regardless of calcium score, and he would not advise people to stop exercising, because “the better your fitness, the better the outcome.”
However, he acknowledged that “the study does nicely illustrate that exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear, honestly).”
Also commenting, Paul D. Thompson, MD, Hartford (Conn.) Hospital, who has studied the cardiac implications of exercise for many years, said: “The problem we have in the U.S. and in most developed countries is not too much exercise but rather that most people don’t exercise very much at all.”
He noted that the Master@Heart study as an “important contribution” to the field.
“We have seen in previous trials that lifelong endurance athletes appear to have more deposition of cholesterol in their coronary arteries than you would expect,” he said. “But, while prior studies suggested that most of the deposits in endurance athletes were the safer type of highly calcified plaques, this study shows that the plaques in endurance athletes are not quite as benign as we had previously thought.”
It’s not clear what this means though, he added, because “despite these findings, it’s pretty clear that endurance athletes live longer than most people. But do they live longer because of the amount of exercise they do or because they are just hardier than the rest of us?”
He does not believe the study should be interpreted to mean that endurance exercise is dangerous. “We don’t have great evidence for that. This is a finding in a coronary artery. We don’t have outcome data.”
However, he added, “it doesn’t seem like you have to do a lot of extreme sport to get the cardiovascular benefits of exercise. All the studies show that the greatest benefits happen in people who go from doing very little to doing a moderate amount of exercise. Then it seems to plateau.”
Dr. Thompson pointed out that the most recent physical activity guidelines in the United States recommend between 150 and 300 minutes of moderate exercise, such as brisk walking, or 75-150 minutes a week of vigorous activity, such as running.
But he does not believe this study should put people off participating in endurance exercise, noting that many individuals engage in high levels of vigorous exercise for other reasons, not necessarily for their cardiovascular health.
“If people want to do more – for competitive reasons or if it makes them feel good – I say go ahead and do it,” Dr. Thompson added. “You should enjoy your life. But if you’re doing high levels of endurance exercise for your health and you’re miserable doing it, you may be wasting your time, as it doesn’t look as these more extreme levels of exercise do you any good. Does it do you any harm? We don’t have evidence yet to conclude that.”
In his presentation, Dr. De Bosscher noted that previous studies have reported higher calcium scores in athletes as well as more coronary plaques, compared with control persons. But the atherosclerotic lesions observed in the athletes were predominantly calcified plaques that were considered more stable and less prone to rupture, whereas nonathletes had predominantly mixed plaques that were considered less stable and more prone to rupture.
He pointed out, however, that these studies had limitations in that they included some individuals with other cardiovascular risk factors, such as smoking and intake of statins or antihypertensive drugs; they did not always assess the association between exercise and coronary atherosclerosis in a dose-response relationship; and while they reported the relative difference in plaque types, they didn’t report the absolute prevalence in calcified, noncalcified, and mixed plaques.
The Master@Heart study aimed to look at this question in a more comprehensive way.
The observational cohort study evaluated coronary atherosclerosis in 191 lifelong master endurance athletes, 191 late-onset athletes (endurance sports initiation after age 30 years), and 176 healthy nonathletes who engaged in no more than 3 hours a week of exercise. All participants were male and had a low cardiovascular risk profile. The median age was 55 in the three groups.
Maximal oxygen uptake (VO2max) was used to quantify fitness. Lifelong and late-onset athletes had higher percentage predicted VO2max than nonathletes (159 vs. 155 vs. 122).
There was no significant difference between the three groups with regard to age, weight, blood pressure cholesterol levels, or hemoglobin A1c levels. While the control group had a healthy body mass index and body fat percentage (19%), both groups of athletes were significantly leaner (body fat percentage, 14%-15%).
The exercise performed by the lifelong and late-onset endurance athletes was similar – mainly cycling and running. The endurance athletes reported an average of 10-11 hours of exercise per week, compared with 1 hour per week for the control persons. Only 22% of the control group reported engaging in no exercise at all; the others reported jogging, cycling, or engaging in nonendurance exercise, such as tennis.
Results showed that the overall coronary plaque burden assessed by segment stenosis score and segment-involvement score was higher among lifelong athletes than control persons (between-group difference, 0.86 and 0.65, respectively).
In comparison to control persons, lifelong endurance sport participation was associated with having one or more of each of the following, compared with a healthy nonathletic lifestyle:
- More than one coronary plaque (odds ratio, 1.86; 95% confidence interval, 1.17-2.94)
- More than one proximal plaque (OR, 1.96; 95% CI, 1.24-3.11)
- More than one calcified plaque (OR, 1.58; 95% CI, 1.01-2.49)
- More than one calcified proximal plaque (OR, 2.07; 95% CI, 1.28-3.35)
- More than one noncalcified plaque (OR, 1.95; 95% CI, 1.12-3.40)
- More than one noncalcified proximal plaque (OR, 2.80; 95% CI, 1.39-5.65)
- More than one mixed plaque (OR, 1.78; 95% CI, 1.06-2.99)
In comparison with late-onset athletes, at least 50% stenosis in any coronary segment (OR, 2.79; 95% CI, 1.20-6.50) and at least 50% stenosis in a proximal segment (OR, 5.92; 95% CI, 1.22 – 28.80) were more prevalent among lifelong athletes.
Vulnerable plaques, as defined by the presence of at least two high-risk features, were uncommon in all groups, but a lifelong athletic lifestyle was associated with a lower prevalence (OR, 0.11; 95% CI, 0.01-0.98).
In their article in the European Heart Journal, the researchers noted that the Master@Heart study is the largest and most comprehensive study to assess the dose-response relationship between intensive endurance exercise and coronary atherosclerosis.
“The findings do not support the hypothesis that highly trained endurance athletes have a more benign plaque composition to explain their lower risk of cardiovascular events compared to nonathletes,” they wrote.
“As studies on the impact of physical activity in the upper range are lacking, our data open the question on whether coronary events are indeed less prevalent in this high-end exercise cohort, and if that is the case, on what explains the paradox,” they concluded. “More and longitudinal research at the higher end of the endurance exercise spectrum is definitely needed.”
A version of this article first appeared on Medscape.com.
In the Master@Heart study, lifelong endurance athletes had more coronary plaques, including more noncalcified plaques, than fit and healthy individuals with a similarly low cardiovascular risk profile.
The study was presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. It was also simultaneously published online in the European Heart Journal.
“We consistently see higher plaque burden in lifelong endurance athletes. This is regardless of the plaque type, whether it is calcified, mixed, noncalcified, in the proximal segment or causing more than 50% stenosis,” concluded Ruben De Bosscher, MD, Catholic University of Leuven (Belgium), during his presentation.
The researchers suggested that all the information to date suggests there may be a “reverse J-shaped” dose-response relationship between exercise and coronary atherosclerosis.
Dr. De Bosscher added that “the worst thing you can do is nothing at all. As soon as you do a little bit of exercise – just brisk walking or jogging up to 3 hours a week – it seems that’s where you get the most benefit. And after that, we tend to see an increase in coronary plaque burden.”
The discussant of the study at the ACC session, Michael Emery, MD, codirector of the Sports Cardiology Center at the Cleveland Clinic, asked how this information should be translated into advice for the general public, given that it is known that endurance athletes show much improved mortality.
“That is a very good question,” Dr. De Bosscher replied. “Yes, we do see less events and adverse outcomes in endurance athletes, but that is compared to the whole population, including those that are unhealthy and do not exercise.
“If we only look at healthy individuals who do exercise but at varying levels, the question is, do we then see the same relationship?” he asked. “There is increasing evidence that there may be a point of diminished returns – and at a certain point, an increased cardiovascular risk is seen in endurance athletes.”
On advice to the public, Dr. De Bosscher added, “one of the main findings here is that, despite having a very healthy lifestyle style and exercising a lot, no one is granted immunity to coronary atherosclerosis. It would seem that the most benefit occurs in individuals doing a moderate amount of exercise – up to about 3 hours a week.”
In a comment, Dr. Emery noted: “This continues to be a ‘hot topic,’ although I continue to be underwhelmed, given a lack of hard outcomes, and I worry about the wrong take-home message being sent, that too much exercise will do more harm than good.”
He added that fitness still matters regardless of calcium score, and he would not advise people to stop exercising, because “the better your fitness, the better the outcome.”
However, he acknowledged that “the study does nicely illustrate that exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear, honestly).”
Also commenting, Paul D. Thompson, MD, Hartford (Conn.) Hospital, who has studied the cardiac implications of exercise for many years, said: “The problem we have in the U.S. and in most developed countries is not too much exercise but rather that most people don’t exercise very much at all.”
He noted that the Master@Heart study as an “important contribution” to the field.
“We have seen in previous trials that lifelong endurance athletes appear to have more deposition of cholesterol in their coronary arteries than you would expect,” he said. “But, while prior studies suggested that most of the deposits in endurance athletes were the safer type of highly calcified plaques, this study shows that the plaques in endurance athletes are not quite as benign as we had previously thought.”
It’s not clear what this means though, he added, because “despite these findings, it’s pretty clear that endurance athletes live longer than most people. But do they live longer because of the amount of exercise they do or because they are just hardier than the rest of us?”
He does not believe the study should be interpreted to mean that endurance exercise is dangerous. “We don’t have great evidence for that. This is a finding in a coronary artery. We don’t have outcome data.”
However, he added, “it doesn’t seem like you have to do a lot of extreme sport to get the cardiovascular benefits of exercise. All the studies show that the greatest benefits happen in people who go from doing very little to doing a moderate amount of exercise. Then it seems to plateau.”
Dr. Thompson pointed out that the most recent physical activity guidelines in the United States recommend between 150 and 300 minutes of moderate exercise, such as brisk walking, or 75-150 minutes a week of vigorous activity, such as running.
But he does not believe this study should put people off participating in endurance exercise, noting that many individuals engage in high levels of vigorous exercise for other reasons, not necessarily for their cardiovascular health.
“If people want to do more – for competitive reasons or if it makes them feel good – I say go ahead and do it,” Dr. Thompson added. “You should enjoy your life. But if you’re doing high levels of endurance exercise for your health and you’re miserable doing it, you may be wasting your time, as it doesn’t look as these more extreme levels of exercise do you any good. Does it do you any harm? We don’t have evidence yet to conclude that.”
In his presentation, Dr. De Bosscher noted that previous studies have reported higher calcium scores in athletes as well as more coronary plaques, compared with control persons. But the atherosclerotic lesions observed in the athletes were predominantly calcified plaques that were considered more stable and less prone to rupture, whereas nonathletes had predominantly mixed plaques that were considered less stable and more prone to rupture.
He pointed out, however, that these studies had limitations in that they included some individuals with other cardiovascular risk factors, such as smoking and intake of statins or antihypertensive drugs; they did not always assess the association between exercise and coronary atherosclerosis in a dose-response relationship; and while they reported the relative difference in plaque types, they didn’t report the absolute prevalence in calcified, noncalcified, and mixed plaques.
The Master@Heart study aimed to look at this question in a more comprehensive way.
The observational cohort study evaluated coronary atherosclerosis in 191 lifelong master endurance athletes, 191 late-onset athletes (endurance sports initiation after age 30 years), and 176 healthy nonathletes who engaged in no more than 3 hours a week of exercise. All participants were male and had a low cardiovascular risk profile. The median age was 55 in the three groups.
Maximal oxygen uptake (VO2max) was used to quantify fitness. Lifelong and late-onset athletes had higher percentage predicted VO2max than nonathletes (159 vs. 155 vs. 122).
There was no significant difference between the three groups with regard to age, weight, blood pressure cholesterol levels, or hemoglobin A1c levels. While the control group had a healthy body mass index and body fat percentage (19%), both groups of athletes were significantly leaner (body fat percentage, 14%-15%).
The exercise performed by the lifelong and late-onset endurance athletes was similar – mainly cycling and running. The endurance athletes reported an average of 10-11 hours of exercise per week, compared with 1 hour per week for the control persons. Only 22% of the control group reported engaging in no exercise at all; the others reported jogging, cycling, or engaging in nonendurance exercise, such as tennis.
Results showed that the overall coronary plaque burden assessed by segment stenosis score and segment-involvement score was higher among lifelong athletes than control persons (between-group difference, 0.86 and 0.65, respectively).
In comparison to control persons, lifelong endurance sport participation was associated with having one or more of each of the following, compared with a healthy nonathletic lifestyle:
- More than one coronary plaque (odds ratio, 1.86; 95% confidence interval, 1.17-2.94)
- More than one proximal plaque (OR, 1.96; 95% CI, 1.24-3.11)
- More than one calcified plaque (OR, 1.58; 95% CI, 1.01-2.49)
- More than one calcified proximal plaque (OR, 2.07; 95% CI, 1.28-3.35)
- More than one noncalcified plaque (OR, 1.95; 95% CI, 1.12-3.40)
- More than one noncalcified proximal plaque (OR, 2.80; 95% CI, 1.39-5.65)
- More than one mixed plaque (OR, 1.78; 95% CI, 1.06-2.99)
In comparison with late-onset athletes, at least 50% stenosis in any coronary segment (OR, 2.79; 95% CI, 1.20-6.50) and at least 50% stenosis in a proximal segment (OR, 5.92; 95% CI, 1.22 – 28.80) were more prevalent among lifelong athletes.
Vulnerable plaques, as defined by the presence of at least two high-risk features, were uncommon in all groups, but a lifelong athletic lifestyle was associated with a lower prevalence (OR, 0.11; 95% CI, 0.01-0.98).
In their article in the European Heart Journal, the researchers noted that the Master@Heart study is the largest and most comprehensive study to assess the dose-response relationship between intensive endurance exercise and coronary atherosclerosis.
“The findings do not support the hypothesis that highly trained endurance athletes have a more benign plaque composition to explain their lower risk of cardiovascular events compared to nonathletes,” they wrote.
“As studies on the impact of physical activity in the upper range are lacking, our data open the question on whether coronary events are indeed less prevalent in this high-end exercise cohort, and if that is the case, on what explains the paradox,” they concluded. “More and longitudinal research at the higher end of the endurance exercise spectrum is definitely needed.”
A version of this article first appeared on Medscape.com.
In the Master@Heart study, lifelong endurance athletes had more coronary plaques, including more noncalcified plaques, than fit and healthy individuals with a similarly low cardiovascular risk profile.
The study was presented at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. It was also simultaneously published online in the European Heart Journal.
“We consistently see higher plaque burden in lifelong endurance athletes. This is regardless of the plaque type, whether it is calcified, mixed, noncalcified, in the proximal segment or causing more than 50% stenosis,” concluded Ruben De Bosscher, MD, Catholic University of Leuven (Belgium), during his presentation.
The researchers suggested that all the information to date suggests there may be a “reverse J-shaped” dose-response relationship between exercise and coronary atherosclerosis.
Dr. De Bosscher added that “the worst thing you can do is nothing at all. As soon as you do a little bit of exercise – just brisk walking or jogging up to 3 hours a week – it seems that’s where you get the most benefit. And after that, we tend to see an increase in coronary plaque burden.”
The discussant of the study at the ACC session, Michael Emery, MD, codirector of the Sports Cardiology Center at the Cleveland Clinic, asked how this information should be translated into advice for the general public, given that it is known that endurance athletes show much improved mortality.
“That is a very good question,” Dr. De Bosscher replied. “Yes, we do see less events and adverse outcomes in endurance athletes, but that is compared to the whole population, including those that are unhealthy and do not exercise.
“If we only look at healthy individuals who do exercise but at varying levels, the question is, do we then see the same relationship?” he asked. “There is increasing evidence that there may be a point of diminished returns – and at a certain point, an increased cardiovascular risk is seen in endurance athletes.”
On advice to the public, Dr. De Bosscher added, “one of the main findings here is that, despite having a very healthy lifestyle style and exercising a lot, no one is granted immunity to coronary atherosclerosis. It would seem that the most benefit occurs in individuals doing a moderate amount of exercise – up to about 3 hours a week.”
In a comment, Dr. Emery noted: “This continues to be a ‘hot topic,’ although I continue to be underwhelmed, given a lack of hard outcomes, and I worry about the wrong take-home message being sent, that too much exercise will do more harm than good.”
He added that fitness still matters regardless of calcium score, and he would not advise people to stop exercising, because “the better your fitness, the better the outcome.”
However, he acknowledged that “the study does nicely illustrate that exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear, honestly).”
Also commenting, Paul D. Thompson, MD, Hartford (Conn.) Hospital, who has studied the cardiac implications of exercise for many years, said: “The problem we have in the U.S. and in most developed countries is not too much exercise but rather that most people don’t exercise very much at all.”
He noted that the Master@Heart study as an “important contribution” to the field.
“We have seen in previous trials that lifelong endurance athletes appear to have more deposition of cholesterol in their coronary arteries than you would expect,” he said. “But, while prior studies suggested that most of the deposits in endurance athletes were the safer type of highly calcified plaques, this study shows that the plaques in endurance athletes are not quite as benign as we had previously thought.”
It’s not clear what this means though, he added, because “despite these findings, it’s pretty clear that endurance athletes live longer than most people. But do they live longer because of the amount of exercise they do or because they are just hardier than the rest of us?”
He does not believe the study should be interpreted to mean that endurance exercise is dangerous. “We don’t have great evidence for that. This is a finding in a coronary artery. We don’t have outcome data.”
However, he added, “it doesn’t seem like you have to do a lot of extreme sport to get the cardiovascular benefits of exercise. All the studies show that the greatest benefits happen in people who go from doing very little to doing a moderate amount of exercise. Then it seems to plateau.”
Dr. Thompson pointed out that the most recent physical activity guidelines in the United States recommend between 150 and 300 minutes of moderate exercise, such as brisk walking, or 75-150 minutes a week of vigorous activity, such as running.
But he does not believe this study should put people off participating in endurance exercise, noting that many individuals engage in high levels of vigorous exercise for other reasons, not necessarily for their cardiovascular health.
“If people want to do more – for competitive reasons or if it makes them feel good – I say go ahead and do it,” Dr. Thompson added. “You should enjoy your life. But if you’re doing high levels of endurance exercise for your health and you’re miserable doing it, you may be wasting your time, as it doesn’t look as these more extreme levels of exercise do you any good. Does it do you any harm? We don’t have evidence yet to conclude that.”
In his presentation, Dr. De Bosscher noted that previous studies have reported higher calcium scores in athletes as well as more coronary plaques, compared with control persons. But the atherosclerotic lesions observed in the athletes were predominantly calcified plaques that were considered more stable and less prone to rupture, whereas nonathletes had predominantly mixed plaques that were considered less stable and more prone to rupture.
He pointed out, however, that these studies had limitations in that they included some individuals with other cardiovascular risk factors, such as smoking and intake of statins or antihypertensive drugs; they did not always assess the association between exercise and coronary atherosclerosis in a dose-response relationship; and while they reported the relative difference in plaque types, they didn’t report the absolute prevalence in calcified, noncalcified, and mixed plaques.
The Master@Heart study aimed to look at this question in a more comprehensive way.
The observational cohort study evaluated coronary atherosclerosis in 191 lifelong master endurance athletes, 191 late-onset athletes (endurance sports initiation after age 30 years), and 176 healthy nonathletes who engaged in no more than 3 hours a week of exercise. All participants were male and had a low cardiovascular risk profile. The median age was 55 in the three groups.
Maximal oxygen uptake (VO2max) was used to quantify fitness. Lifelong and late-onset athletes had higher percentage predicted VO2max than nonathletes (159 vs. 155 vs. 122).
There was no significant difference between the three groups with regard to age, weight, blood pressure cholesterol levels, or hemoglobin A1c levels. While the control group had a healthy body mass index and body fat percentage (19%), both groups of athletes were significantly leaner (body fat percentage, 14%-15%).
The exercise performed by the lifelong and late-onset endurance athletes was similar – mainly cycling and running. The endurance athletes reported an average of 10-11 hours of exercise per week, compared with 1 hour per week for the control persons. Only 22% of the control group reported engaging in no exercise at all; the others reported jogging, cycling, or engaging in nonendurance exercise, such as tennis.
Results showed that the overall coronary plaque burden assessed by segment stenosis score and segment-involvement score was higher among lifelong athletes than control persons (between-group difference, 0.86 and 0.65, respectively).
In comparison to control persons, lifelong endurance sport participation was associated with having one or more of each of the following, compared with a healthy nonathletic lifestyle:
- More than one coronary plaque (odds ratio, 1.86; 95% confidence interval, 1.17-2.94)
- More than one proximal plaque (OR, 1.96; 95% CI, 1.24-3.11)
- More than one calcified plaque (OR, 1.58; 95% CI, 1.01-2.49)
- More than one calcified proximal plaque (OR, 2.07; 95% CI, 1.28-3.35)
- More than one noncalcified plaque (OR, 1.95; 95% CI, 1.12-3.40)
- More than one noncalcified proximal plaque (OR, 2.80; 95% CI, 1.39-5.65)
- More than one mixed plaque (OR, 1.78; 95% CI, 1.06-2.99)
In comparison with late-onset athletes, at least 50% stenosis in any coronary segment (OR, 2.79; 95% CI, 1.20-6.50) and at least 50% stenosis in a proximal segment (OR, 5.92; 95% CI, 1.22 – 28.80) were more prevalent among lifelong athletes.
Vulnerable plaques, as defined by the presence of at least two high-risk features, were uncommon in all groups, but a lifelong athletic lifestyle was associated with a lower prevalence (OR, 0.11; 95% CI, 0.01-0.98).
In their article in the European Heart Journal, the researchers noted that the Master@Heart study is the largest and most comprehensive study to assess the dose-response relationship between intensive endurance exercise and coronary atherosclerosis.
“The findings do not support the hypothesis that highly trained endurance athletes have a more benign plaque composition to explain their lower risk of cardiovascular events compared to nonathletes,” they wrote.
“As studies on the impact of physical activity in the upper range are lacking, our data open the question on whether coronary events are indeed less prevalent in this high-end exercise cohort, and if that is the case, on what explains the paradox,” they concluded. “More and longitudinal research at the higher end of the endurance exercise spectrum is definitely needed.”
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Wearable fluid sensor lowers risk of HF rehospitalizations: BMAD
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
AT ACC 2023
FREEDOM COVID: Full-dose anticoagulation cut mortality but missed primary endpoint
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
Study conducted in noncritically ill
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
AT ACC 2023
Fixed-dose combo pill for PAH promises accelerated benefit: A DUE
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
AT ACC 2023
Encouraging 3-year data for TAVR in low-risk patients: EVOLUT
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
Three-year results from the Evolut trial seem to provide more reassurance on the use of transcatheter aortic valve replacement (TAVR) in low-surgical-risk patients.
The 3-year results show that low-surgical-risk patients undergoing aortic valve replacement continue to show lower rates of all-cause mortality and disabling stroke with TAVR, compared with surgery.
The rates of all-cause mortality or disabling stroke (the primary endpoint) at 3 years were 7.4% with TAVR and 10.4% with surgery.
Rates of new pacemaker implantation continued to be higher after TAVR and the frequency of new onset atrial fibrillation was more common after surgery.
“At 3 years, the rate of all-cause mortality or disabling stroke after TAVR with the Evolut valve compared very favorably to surgery. The absolute difference between treatment arms remained consistent with a 30% relative reduction in the hazard of death or disabling stroke, with a P value that just missed statistical significance,” said Evolut investigator John Forrest, MD, Yale University School of Medicine, New Haven, Conn.
“The Kaplan-Meier curves show what we’ve come to expect – an early separation of the curves – but what’s unique here, and seen for the first time, is that the early separation is maintained at year 1 and year 2, and between years 2 and 3 the curve didn’t start to come together, but, if anything, separated a little,” Dr. Forrest commented.
“Both components of the primary endpoint – all cause mortality and disabling stroke – numerically favor TAVR. The separation of the curves for stroke are maintained, and if anything, we see a further slight separation of the curves as we go forward out to 3 years in terms of all-cause mortality,” he added.
Dr. Forrest presented the 3-year results from the Evolut trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. They were simultaneously published online in the Journal of the American College of Cardiology.
Dr. Forrest also reported that TAVR patients continued to have better valve hemodynamics at 3 years and very low rates of valve thrombosis; moreover, rates of moderate or greater paravalvular regurgitation and paravalvular leak (factors that can affect valve durability) were also low, although mild paravalvular regurgitation was higher with TAVR.
“In these low-risk patients, the durability of the valve is going to be critically important,” Dr. Forrest commented. “The excellent valve performance and durable outcomes out to 3 years in low-risk patients affirms the role of TAVR in this population,” he concluded.
On how these results may affect clinical practice, Dr. Forrest said: “I think in the U.S. these results reaffirm what we are doing. It gives us confidence to continue treating low-risk patients and being comfortable with that.”
He added: “Outside the U.S., the guidelines are a little different. Maybe we should reconsider some of these guidelines based on these data.”
David Moliterno, MD, Gill Heart and Vascular Institute, Lexington, Ky., who is not involved in the TAVR studies, said: “The results provide a little more reassurance ... that will go a little way further.”
“Uncertainty remains regarding long-term durability of the transcatheter valve in low-risk patients who are generally younger and likely more active than higher-risk cohorts,” he added. “The current 3-year results provide more confidence as the outcome curves for death and disabling stroke are trending in the right direction for TAVR versus surgery.”
Dr. Moliterno pointed out that while rates of paravalvular regurgitation and permanent pacemaker placement are decreasing with newer generation Evolut devices and implantation techniques, he noted that according to the U.S. Social Security Administration, patients aged 74 years as enrolled in this low-risk cohort have an additional life expectancy of approximately 12 years. “So, we have more device durability (and coronary access feasibility) to prove.”
In his presentation, Dr. Forrest explained that TAVR is now approved in the United States for all patients with aortic stenosis regardless of surgical risk and has become the dominant form of aortic valve replacement. Current ACC/AHA guidelines recommend that heart teams utilize a shared decision-making process when discussing aortic valve replacement with patients aged 65-80 years. In younger, lower-risk patients, the faster recovery and short-term benefits after TAVR must be balanced with long-term durability; however, only limited intermediate and long-term data exist to guide such discussions in this patient population.
The Evolut Low Risk trial randomly assigned 1,414 patients in need of aortic valve replacement to TAVR with a self-expanding, supra-annular valve or surgery. Results at 1 and 2 years have shown a similar benefit in the primary endpoint of all-cause mortality/disabling stroke for the less invasive TAVR procedure.
The current 3-year results suggest the benefit appears to be maintained out for another year.
The main results show that the rate of death or disabling stroke was 7.4% in the TAVR group versus 10.4% in the surgery group, giving a hazard ratio of 0.70 (P = .051).
In the JACC paper, the authors report that the absolute difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time: –1.8% at year 1; –2.0% at year 2; and –2.9% at year 3.
Other key results on valve durability show that mild paravalvular regurgitation was increased in the TAVR group (20.3%) versus 2.5% with surgery. However, rates of moderate or greater paravalvular regurgitation for both groups were below 1% and not significantly different between groups.
Patients who underwent TAVR had significantly improved valve hemodynamics (mean gradient 9.1 mm Hg TAVR vs. 12.1 mm Hg surgery; P < .001) at 3 years.
However, pacemaker placement was much higher in the TAVR group (23.2%), compared with 9.1% in the surgery group.
On the other hand, the surgery group had a greater incidence of atrial fibrillation (40%) versus 13% with TAVR.
Quality-of-life results looked good in both groups.
“As we’ve come to expect, patients recover more quickly after TAVR, so at 30 days their quality of life is better than those who have undergone surgery,” Dr. Forrest commented. “But by 1 year, both groups are doing exceptionally well and, remarkably, here by 3 years both groups have greater than a 20-point increase in their KCCQ score, showing a very large improvement in quality of life.”
Discussant of these latest results at the ACC late-breaking trials session, James Hermiller, MD, St. Vincent Ascension Heart Center, Indianapolis, said: “This 3-year data continues to demonstrate that the gift of TAVR keeps giving.”
Noting that the divergence in the effect curves was primarily driven by mortality rather than stroke, he asked whether this was cardiac or noncardiac mortality that was reduced.
Dr. Forrest responded: “It was a fairly equal contribution – a little bit more cardiac death. We have to remember that although the average age in this study was 74, there were some patients over 80 who were still low-surgical-risk included so we are going to see noncardiac death as well.”
Dr. Hermiller drew attention to the high pacemaker rate in the TAVR group and asked how these patients fared in comparison to those who didn’t need a pacemaker.
Dr. Forrest replied: “I think it’s fair to say that putting in a pacemaker is not a benign procedure. Patients who got a pacemaker did slightly worse than those who didn’t get a pacemaker, so we need to try to drive that rate down.”
He added that the number of patients needing a pacemaker after TAVR has come down with new implantation techniques and new generation valves.
“We realize that using a cusp overlap technique can significantly reduce the need for a pacemaker, and we see from registry data that with the use of this new technique the need for a pacemaker has dropped down to 8%-9%, significantly less than seen in this study,” Dr. Forrest commented.
Dr. Hermiller also asked about how TAVR affects future access for catheterization or percutaneous coronary intervention.
Dr. Forrest noted that 24 patients in the TAVR group required PCI in first 3 years, and all the PCI procedures had been successful. He noted that operators reported the procedure to be easy or moderately easy in about 75%-80% of cases and difficult in about 20% of patients. “So, it is slightly more challenging to engage the coronaries and have to go through the frame, but it is very feasible.”
Dr. Forrest concluded that: “These results provide patients and heart teams important data to aid in the shared decision-making process.”
But he acknowledged that longer term data are still needed. “And the potential impact that hemodynamics, valve design, new pacemakers, and other secondary endpoints have on long-term outcomes will be important to follow in this group of low-risk patients.”
The Evolut Low Risk trial was funded by Medtronic. Dr. Forrest has received grant support/research contracts and consultant fees/honoraria/speakers bureau fees from Edwards Lifesciences and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
‘Keto-like’ diet linked to doubling of heart disease risk
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ACC 2023

















