User login
Monthly needlestick rates suggest a steep learning curve
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Treatments for COVID-19: Update for hospitalists
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
United States adds nearly 74,000 more children with COVID-19
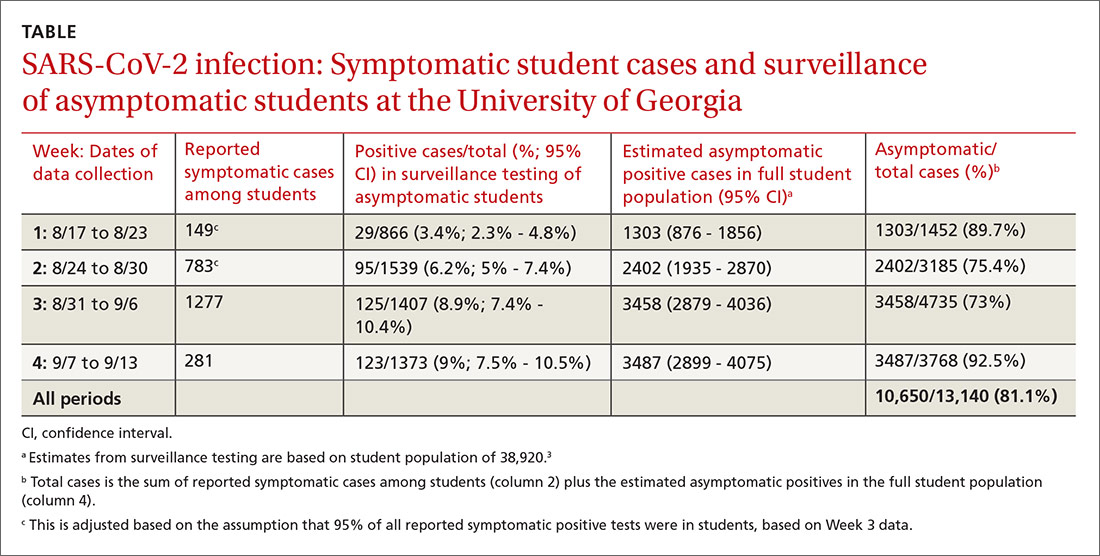
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.
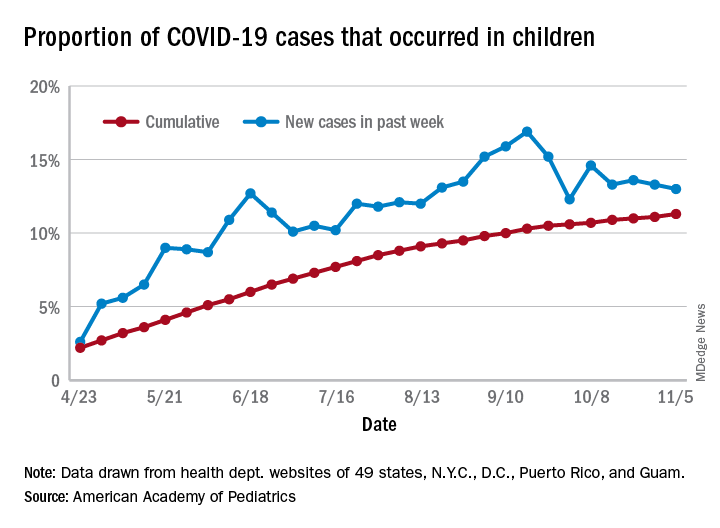
The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
Infectious disease is an increasing threat from climate change
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
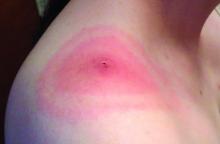
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
FROM AAP 2020
A high proportion of SARS-CoV-2–infected university students are asymptomatic
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.
Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
ebell@uga.edu
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.
Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
ebell@uga.edu
The authors reported no potential conflict of interest relevant to this article.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.
Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
ebell@uga.edu
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Pfizer vaccine data show 90% efficacy in early results
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
Methotrexate users need tuberculosis tests in high-TB areas
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.
Aging with HIV adds to comorbidity burden
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
FROM IDWEEK 2020