User login
Recurrent Cutaneous Exophiala Phaeohyphomycosis in an Immunosuppressed Patient
To the Editor:
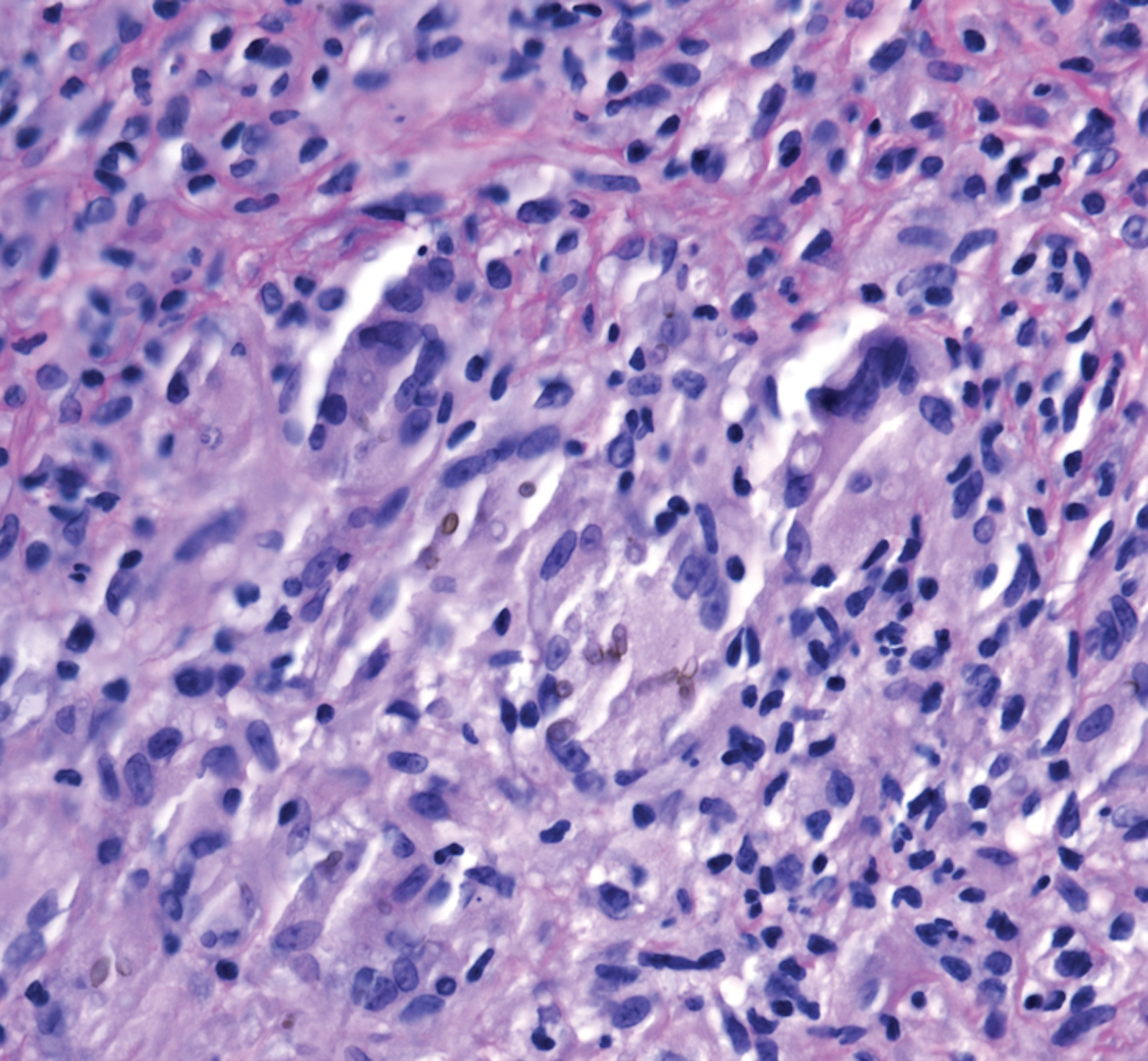
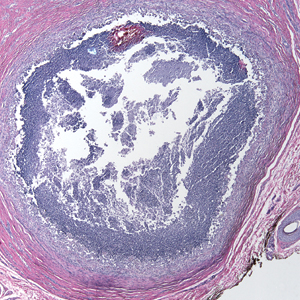
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.
The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
To the Editor:
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.
The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
To the Editor:
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.
The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
Practice Points
- Phaeohyphomycosis is an infection with dematiaceous fungi that most commonly affects immunosuppressed patients.
- Subcutaneous phaeohyphomycosis may present with nodulocystic lesions that recur over the course of years.
- Tissue fungal culture should be obtained when the diagnosis is suspected, as the risk for dissemination is related to the culprit organism.
- Surgical excision with close follow-up may be an appropriate management strategy for patients on immunosuppressive medications to avoid interactions with azole therapy.
Pronounced racial differences in HBsAg loss after stopping nucleos(t)ide
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Vanquishing hepatitis C: A remarkable success story
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
Harnessing the HIV care continuum model to improve HCV treatment success
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Abnormal anal paps in people with HIV can go more than a year without follow-up
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
That delay “revealed missed opportunities for a better experience on the patient, clinic, and provider level,” Jessica Wells, PhD, research assistant professor at the Nell Hodgson Woodruff School of Nursing at Emory University, Atlanta, said in an interview. After all, “a lot can happen in that 1 year,” including early development of human papillomavirus (HPV)–associated anal cancer.
Although it’s too soon to say how significant that delay is with respect to the natural history of anal cancer, Dr. Wells said the data are a potential signal of disparities.
“The findings from my study may foreshadow potential disparities if we don’t have the necessary resources in place to promote follow-up care after an abnormal Pap test, similar to the disparities that we see in cervical cancer,” she said during the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
Single-center study
In the United States, people living with HIV are 19 times more likely to develop anal cancer than the general population, according to a 2018 article in the Journal of Clinical Oncology. Another single-center study from Yale University found that, in minority communities, anal cancer rates were 75% higher than in White communities. Anal cancer rates were 72% higher in communities with greater poverty. As a result, many clinics are beginning to administer Pap tests to determine early signs of HPV infection and associated changes.
In Dr. Wells’ study, which was conducted from 2012 to 2015, 150 adults with HIV who were aged 21 and older were recruited from Grady Ponce De Leon Center in Atlanta. According to a 2018 study from that center, a large minority of participants had late-stage HIV and suppressed immune systems.
All participants had been referred for HRA after a recent abnormal anal Pap test. Participants filled out questionnaires on sociodemographics, internalized HIV-related stigma, depression, risk behaviors, social support, and knowledge about HPV and anal cancer.
Participants were disproportionately older (mean age, 50.9 years); cisgender (86.7%), Black (78%); and gay, lesbian, or bisexual (84.3%). Slightly more than 1 in 10 participants (11.3%) were transgender women.
Although for 6% of participants, Pap test results indicated high-grade squamous intraepithelial lesions (HSIL), an additional 8% had atypical Pap findings that couldn’t exclude HSIL – the kinds of results that are one step away from a cancer diagnosis. More than 80% of participants had low-grade or inconclusive results. Nearly half (44%) of participants’ Pap tests revealed low-grade squamous cell intraepithelial cell lesions (LSIL); 42% indicated atypical squamous cells of undetermined significance.
When Dr. Wells looked at how long participants had waited to undergo HRA, she found something that surprised her: although some participants underwent follow-up assessment in 17 days, for many, it took much longer. The longest wait was 2,350 days – more than 6 years.
“There were quite a few patients who had follow-up beyond 1,000-plus days,” Dr. Wells said in an interview. “I didn›t think the delays were that long — at most, I would say that patients will get scheduled and come back within a few weeks or months.”
What’s more, she discovered through the HPV knowledge questionnaire that many participants did not understand why they were having a follow-up appointment. Anecdotally, some confused HPV with HIV.
“There’s education to be done to inform this target population that those living with HIV are more prone or at increased risk of this virus causing cancer later,” she said. “There are a lot of campaigns around women living with HIV, that they need to do cervical cancer screening. I think we need to really expand this campaign to include that HPV can also cause anal cancer.”
Dr. Wells had planned to primarily investigate the impact of psychosocial factors on wait time to follow-up, but none of those factors were associated with longer wait times.
Systems-level factors
That led Ann Gakumo, PhD, chair of nursing at the College of Nursing and Health Sciences of the University of Massachusetts, Boston, to ask what other factors could account for the delay.
There were several, Dr. Wells said. Precarious housing, for example, could have influenced this lag in follow-up. About one in four participants were in transient housing, and one participant reported having been incarcerated. She gathered street addresses and plans to analyze that data to see whether the cases occurred in clusters in specific neighborhoods, as the Yale data indicated.
In addition, the anoscopy clinic was only available to receive patients one day a week and was staffed with only one clinician who was trained to perform HRA. Wait times could stretch for hours. Sometimes, participants had to leave the clinic to attend to other business, and their appointments needed to be rescheduled, Wells said.
In addition to the sometimes poor understanding of the importance of the follow-up test, Dr. Wells said, “we start to see a layering of these barriers. That’s where we start seeing breakdowns. So I’m hoping in a larger study I can address some of these barriers on a multilevel approach.”
This resonated with Dr. Gakumo.
“Oftentimes, we put so much of the responsibility for this on the part of the client and not enough on the part of the provider or on the systems level,” she said.
Guiding guidelines
Guidelines on follow-up for abnormal anal Pap test results are scarce, mostly because, unlike cervical cancer, the natural history of HPV-related anal cancers hasn’t been established. The HIV Medical Association does recommend anal Pap tests, but only in cases in which “access to appropriate referral for follow-up, including high-resolution anoscopy, is available.”
In an interview, Cecile Lahiri, MD, assistant professor of infectious disease at Emory University, said that, at Ponce De Leon Center, they recommend an anal Pap for women with HIV who have a history of cervical dysplasia.
There is a reliable association between high-grade abnormal Pap tests and cervical cancer, although low-grade changes can resolve on their own. In the case of anal cancer, especially in patients with HIV, low-grade cell changes are predictive; moreover, for such patients, anal cancer is more likely to recur and is harder to treat, Dr. Lahiri said.
“The cervical environment and the anal environment are very different,” said Dr. Lahiri, who works at the Grady Ponce De Leon Center but was not involved in Dr. Wells’ study. Dr. Lahiri is also a coinvestigator of the multisite, randomized, controlled Anal Cancer HSIL Outcomes Research (ANCHOR) study, which seeks to establish whether early treatment of high-grade anal Pap changes is better than a watch-and-wait approach.
Dr. Lahiri said that when the results of that trial become available, they are more likely to know how important early anoscopy and treatment are. The findings should inform guidelines and insurance coverage of anal Pap tests and anoscopy.
In the meantime, she said, she suspected that, with the ANCHOR trial in 2015, many sites’ capacity for anoscopy may have increased, and the wait times may have gone down.
“One of the most important pieces of the study is actually the time period in which it was conducted,” said Dr. Lahiri, who in 2015 became the clinic’s second physician trained in anoscopy. Currently, more than 200 people at the Ponce De Leon Center are enrolled in the ANCHOR trial. In addition, the general capacity for performing anoscopies has gone up nationwide as a result of the trial, which required that more providers learn how to properly perform an HRA. Many clinicians are not routinely trained in performing HRA, including gastroenterologists and surgeons, Dr. Lahiri said.
“It would be interesting to look at the differences, with the start of ANCHOR being the time point for before and after,” she said.
This article first appeared on Medscape.com.
‘Uptake is only the first step’ for effective HIV PrEP protection
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
To Amanda Allmacher, DNP, RN, nurse practitioner at the Detroit Public Health STD Clinic, that means that same-day PrEP prescribing works and is acceptable. But there’s more work to do on the clinic and pharmacy side to make HIV protection a reality for most of her patients. Allmacher presented her data at the Association of Nurses in AIDS Care 2020 virtual annual meeting.
Dawn K. Smith, MD, epidemiologist and medical officer in the Division of HIV/AIDS Prevention at the Centers for Disease Control and Prevention’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said this adds to other data to show that we’re now entering the next phase of PrEP implementation.
“Our original focus was on uptake — informing folks what PrEP is, why they might benefit from its use, and then prescribing it if accepted,” Smith told Medscape Medical News via email. “Whether standard or same-day [PrEP prescribing], it is clear that uptake is only the first step.”
Nurses help navigate
Patients who attended the Detroit Public Health STD Clinic are more likely to be younger, have no insurance, and otherwise “have little to no contact with the healthcare system,” Allmacher said in her presentation. They also tend to come from communities that bear the greatest burden of HIV in the US — in other words, they are often the people most missed in PrEP rollouts thus far.
In response, the clinic implemented a same-day PrEP protocol, in which registered nurses trained in HIV risk assessment identify clients who might most benefit from PrEP. Criteria often include the presence of other STIs. Once the nurse explains what PrEP is and how it works, if the patient is interested, clients meet with a nurse practitioner right then to get the prescription for PrEP. The clinic also does labs to rule out current HIV infection, hepatitis B, metabolic issues, and other STI screening.
But it doesn’t stop there. The clinic used grant funding to offer PrEP navigation and financial counseling services, which help clients navigate the sometimes-thorny process of paying for PrEP. Payment comes either through Medicaid, which in Michigan charges $3 a month for a PrEP prescription, through patient assistance programs, or through private insurance. With clients under age 18 who are interested in PrEP, the clinic works to find a way to access PrEP without having to inform their parents. These same navigators schedule follow-up appointments, offer appointment reminders, and contact clients when they miss an appointment.
“Our navigators and financial counselors are a huge support for our same-day PrEP starts, helping with financial assistance, prior authorization, navigating different plans, and helping patients apply for Medicaid when appropriate,” she said.
The clinic also offers community outreach and incentives, which can include gift cards, bus passes, and pill containers, among other things.
This was a key lesson in setting up the program, Allmacher told Medscape Medical News.
“Starting PrEP at that initial visit allows for clinicians to meet patients where they are and administer care in a more equitable manner,” Allmacher said via email. “Use all available resources and funding sources. We have a versatile team working together to increase access for patients and promote HIV prevention and risk reduction.”
Script vs. follow-up
This approach is common, used in places like New York City and San Francisco. So once it was set up Allmacher sat back and waited to see how the program helped clients protect themselves from HIV.
Of the 451 clients eligible for PrEP in 2019, 336 were gay and bisexual men, 6 were transgender women, 61 were heterosexual, cisgender men, and 48 were cisgender women. One transgender man also screened as eligible. Allmacher did not break down data by race.
Uptake was high: 70% of all eligible clients did receive a prescription for PrEP, either generic tenofovir disoproxil fumarate/emtricitabine (Truvada) or tenofovir alafenamide/emtricitabine (Descovy). And uptake was high among people most at risk: 80% of gay and bisexual men who were eligible got a prescription, 60% of eligible cisgender women, 50% of the small number of transgender women, and 32.7% of heterosexual cisgender men did as well. The 1 transgender man also received a prescription.
This is a higher rate than found in a recent PrEP demonstration project, which found that despite gay and bisexual men, transgender adults, and Black people having the highest risk for HIV in the US, state health departments were more likely to refer heterosexual adults for PrEP.
That high uptake rate is encouraging, but follow-up? Not so much. After initial intake, clients are meant to return in a month to double-check their labs, ask about side effects, and start their 90-day supply of the medication. But just 40% showed up for their 30-day appointment, Allmacher said. And only one third of those showed up for the follow-up in 90 days.
By the end of 2019, just 73 of the original 451 clients screened were still taking PrEP.
“It was surprising to see just how significant the follow-up dropped off after that first visit, when the patient initially accepted the prescription,” Allmacher said.
And while it’s possible that some clients get their follow-up care from their primary care providers, “our clinic serves individuals regardless of insurance status and many do not identify having access to primary care for any type of service, PrEP or otherwise,” she said.
5 HIV acquisitions
In addition, the program review identified five clients who had been offered PrEP or had taken PrEP briefly who later acquired HIV. Those clients were offered same-day antiretroviral treatment, Allmacher said.
“So we’re finding people who are at high risk for HIV and we can prevent them, but we’re still not quite doing enough,” Allmacher said of those acquisitions. “Clearly we have a lot of work to do to focus on HIV prevention, and we are looking to create a more formal follow-up process” from the clinic’s side.
For instance, clinic staff call clients 1 week after their initial visit to share lab results. “This was identified as a missed opportunity for us to ask about their status, whether they filled their prescription, or if they need further assistance,” she said. “This is an area where our registered nurses are going to be taking on a greater role moving forward.”
Allmacher and team also discovered that, despite PrEP navigators arranging insurance coverage for clients on the day they receive their prescription, sometimes there were still barriers when the client showed up at the pharmacy to pick up their meds. The clinic does not have an in-house pharmacy and does not currently have the funding that would allow them to hand patients a bottle of the appropriate medication when they leave the clinic.
“Navigating the copays and the insurance coverage and using financial assistance through the drug manufacturer — even though we have the support in the clinic, it seems like there’s a disconnect between our clinic and getting to the pharmacy. Not every pharmacy is super familiar with navigating those,” she said. “So we have started to identify some area pharmacies near our clinic that are great at navigating these, and we really try to get our patients to go to places we know can give them assistance.”
A version of this story originally appeared on Medscape.com.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
Metapneumovirus infections clinically indistinguishable from flu, RSV
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
FROM IDWEEK 2020
'Tragic' milestone: 1 million children with COVID-19
The number of new cases soared in the past week as the United States exceeded 1 million children infected with the coronavirus, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the first time, the number of cases in children for the week ending Nov. 12 passed 100,000, and it didn’t stop until it reached 111,946, bringing the total for the pandemic to 1,039,464 reported cases in 49 states (New York is not reporting ages), the District of Columbia, New York City, and Guam, the AAP and the CHA said in their weekly COVID-19 update.
“As a pediatrician who has practiced medicine for over 3 decades, I find this number staggering and tragic. We haven’t seen a virus flash through our communities in this way since before we had vaccines for measles and polio,” AAP President Sally Goza, MD, said in a written statement.
The previous 1-week high of almost 74,000 cases came just last week, and that number had surpassed the previous week’s new high of 61,000. The number of cumulative child cases, meanwhile, has doubled since Sept. 3, when it was just over 513,000. Children now represent 11.5% of all COVID-19 cases since the start of the pandemic in the jurisdictions reporting age distribution, the AAP and CHA said.
For the week ending Nov. 12, COVID-19 cases children made up 14% of cases nationally, rising from 13% the week before and reversing a decline that started in mid-October, the AAP/CHA data show.
The two groups continue to note the rarity of severe illness in children, but the number of deaths nationally had its biggest 1-week increase since late July, as the total rose from 123 to 133 in the 42 states reporting such data by age, as well as New York City. The cumulative hospitalization rate for children decreased slightly in the past week and is now down to 1.6% in the 23 states (and NYC) with available data, the AAP and CHA said.
The AAP called on elected leaders to enact a national strategy to combat the spread of the virus and urged health authorities to do more to collect data on longer-term impacts on children.
We’re very concerned about how this will impact all children, including toddlers who are missing key educational opportunities, as well as adolescents who may be at higher risk for anxiety and depression,” Dr. Goza said.
The number of new cases soared in the past week as the United States exceeded 1 million children infected with the coronavirus, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the first time, the number of cases in children for the week ending Nov. 12 passed 100,000, and it didn’t stop until it reached 111,946, bringing the total for the pandemic to 1,039,464 reported cases in 49 states (New York is not reporting ages), the District of Columbia, New York City, and Guam, the AAP and the CHA said in their weekly COVID-19 update.
“As a pediatrician who has practiced medicine for over 3 decades, I find this number staggering and tragic. We haven’t seen a virus flash through our communities in this way since before we had vaccines for measles and polio,” AAP President Sally Goza, MD, said in a written statement.
The previous 1-week high of almost 74,000 cases came just last week, and that number had surpassed the previous week’s new high of 61,000. The number of cumulative child cases, meanwhile, has doubled since Sept. 3, when it was just over 513,000. Children now represent 11.5% of all COVID-19 cases since the start of the pandemic in the jurisdictions reporting age distribution, the AAP and CHA said.
For the week ending Nov. 12, COVID-19 cases children made up 14% of cases nationally, rising from 13% the week before and reversing a decline that started in mid-October, the AAP/CHA data show.
The two groups continue to note the rarity of severe illness in children, but the number of deaths nationally had its biggest 1-week increase since late July, as the total rose from 123 to 133 in the 42 states reporting such data by age, as well as New York City. The cumulative hospitalization rate for children decreased slightly in the past week and is now down to 1.6% in the 23 states (and NYC) with available data, the AAP and CHA said.
The AAP called on elected leaders to enact a national strategy to combat the spread of the virus and urged health authorities to do more to collect data on longer-term impacts on children.
We’re very concerned about how this will impact all children, including toddlers who are missing key educational opportunities, as well as adolescents who may be at higher risk for anxiety and depression,” Dr. Goza said.
The number of new cases soared in the past week as the United States exceeded 1 million children infected with the coronavirus, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the first time, the number of cases in children for the week ending Nov. 12 passed 100,000, and it didn’t stop until it reached 111,946, bringing the total for the pandemic to 1,039,464 reported cases in 49 states (New York is not reporting ages), the District of Columbia, New York City, and Guam, the AAP and the CHA said in their weekly COVID-19 update.
“As a pediatrician who has practiced medicine for over 3 decades, I find this number staggering and tragic. We haven’t seen a virus flash through our communities in this way since before we had vaccines for measles and polio,” AAP President Sally Goza, MD, said in a written statement.
The previous 1-week high of almost 74,000 cases came just last week, and that number had surpassed the previous week’s new high of 61,000. The number of cumulative child cases, meanwhile, has doubled since Sept. 3, when it was just over 513,000. Children now represent 11.5% of all COVID-19 cases since the start of the pandemic in the jurisdictions reporting age distribution, the AAP and CHA said.
For the week ending Nov. 12, COVID-19 cases children made up 14% of cases nationally, rising from 13% the week before and reversing a decline that started in mid-October, the AAP/CHA data show.
The two groups continue to note the rarity of severe illness in children, but the number of deaths nationally had its biggest 1-week increase since late July, as the total rose from 123 to 133 in the 42 states reporting such data by age, as well as New York City. The cumulative hospitalization rate for children decreased slightly in the past week and is now down to 1.6% in the 23 states (and NYC) with available data, the AAP and CHA said.
The AAP called on elected leaders to enact a national strategy to combat the spread of the virus and urged health authorities to do more to collect data on longer-term impacts on children.
We’re very concerned about how this will impact all children, including toddlers who are missing key educational opportunities, as well as adolescents who may be at higher risk for anxiety and depression,” Dr. Goza said.
Opt-out policy at a syringe service program increased HIV/HCV testing
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
FROM INTERNATIONAL JOURNAL OF DRUG POLICY