User login
Three pillars of a successful coronavirus vaccine program in minorities
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
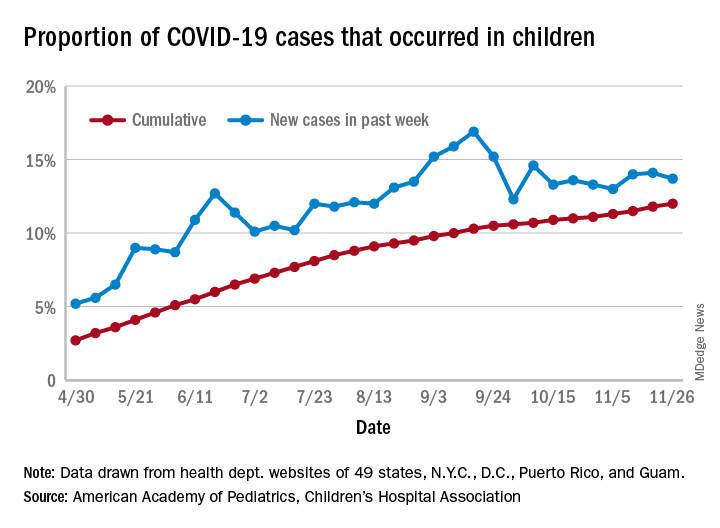
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
Prophylactic HIV treatment in female STI patients is rare
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
FROM OBSTETRICS & GYNECOLOGY
FDA expands Xofluza indication to include postexposure flu prophylaxis
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
Mortality rate of SARS-CoV-2 for similar patients is declining over time
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is the observed drop in COVID-19 mortality caused by changing demographics or improvements in patient care?
Background: At the start of the pandemic, COVID-19 had high mortality rates (6.9% in April according to the U.S. Centers for Disease Control and Prevention). More recently, the mortality rate had decreased to 1.9% of diagnosed cases at the end of September 2020. Concurrently, the median age of confirmed cases dropped as well, from 46 to 38 years, and availability of testing has expanded. It remains unclear whether the drop in mortality rate is because of affected patients with fewer comorbidities, less symptomatic patients, or improvements in clinical care.
Study design: Retrospective cohort study.
Setting: Large urban academic health system.
Synopsis: Researchers analyzed admissions from March 1 through Aug. 31, 2020, to NYU Langone Health System in New York of patients older than 18 years with laboratory-confirmed infection with SARS-CoV-2 during the hospitalization or in the preceding 2 weeks. In total, 5,118 patients qualified for analysis, of which 53% were hospitalized during March and April. Two separate multivariate logistic regression models for mortality were created based on patient demographics, comorbidities, and presenting vital signs and lab-result abnormalities. In the first model, the month of admission was not included, and the standardized mortality ratio (SMR) – the ratio of the sum of observed and expected deaths – for each month was obtained. In the second model, the month of admission was included as a covariate, and the average marginal effect (AME) – the difference in probability of death or discharge to hospice between March and a subsequent time period for equivalent patients – was calculated. The SMR declined progressively over time from 1.26 (95% confidence interval, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August. When accounting for demographic and clinical severity changes, the adjusted AME declined every subsequent month after March reaching a maximum of 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August.
Bottom line: Mortality from SARS-CoV-2 was significantly lower at the end of the 6-month period when adjusted for demographic and clinical factors for patients admitted to a single health care system in the United States.
Citation: Horwitz LI et al. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2020 Oct 23. doi: 10.12788/jhm.3552
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE
Rationale for baricitinib’s use in COVID-19 patients demonstrated
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
FROM SCIENCE ADVANCES
Combo DAA treatments may benefit patients with resistant HCV genotype 3
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
FROM ANNALS OF HEPATOLOGY
Antidepressant shows early promise for mild COVID-19
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.








