User login
Impact of Return to Office on VA Telehealth Remains Unclear
Nearly 96,000 US Department of Veterans Affairs (VA) employees—about 20% of the workforce—will be required to return to in-office work by the end of February. The announcement follows a Jan. 20 presidential memorandum, which states agency heads must “take all necessary steps to terminate remote work arrangements and require employees to return to work in-person at their respective duty stations on a full-time basis.” According to a Jan. 28 email sent from the Office of Personnel Management but without a signature, federal employees who refuse will be offered “dignified, fair departure from the federal government utilizing a deferred resignation program.”
The revised VA work policy states “eligible employees must work full-time at their respective duty stations (agency worksites) unless excused due to a disability, qualifying medical condition or other compelling reason.” All nonbargaining unit employees and supervisors who are within 50 miles of their office have until Feb. 24 to return. The VA stated that further guidance is coming for those who live > 50 miles from a facility.
“VA’s policy allows exceptions for arrangements approved for employees as a reasonable accommodation due to a disability or a qualifying medical condition. Exceptions may also be allowed for military spouses with permanent change of station orders,” according to a VA press release.
“This is a commonsense step toward treating all VA employees equally,” acting VA Secretary Todd Hunter said. “Most VA clinical staff don’t have the luxury of working remotely, and we believe the performance, collaboration and productivity of the department will improve if all VA employees are held to the same standard.”
The impact on Veterans Health Administration operations remains difficult to determine. The order appears to include personnel providing telehealth care from remote locations, including those at the Clinical Resource Hub (CRH) program. CRH uses a hub and spoke model for limited time primary care and mental health care staffing to cover local clinician vacancies. CRH clinicians have provided > 500,000 veterans with care, averaging > 25,000 encounters in the program’s first year. Started during fiscal year 2020 amid the COVID-19 pandemic, CRH employed 636 clinicians, but more recent data are not available. The VA provided > 28 million telehealth sessions to veterans across all of its telehealth modalities in 2023. Details on how many CRH clinicians and other telehealth practitioners work remotely are also not available.
On Feb. 3, the Office of Personnel Management issued a memo to federal agency heads arguing that any collective bargaining agreements that include teleworking may “conflict with management rights” and therefore may be “unlawful and cannot be enforced.”
The American Federation of Government Employees (AFGE), which represents 800,000 federal employees, disputed the memo. “Federal employees should know that approved union contracts are enforceable by law, and the President does not have the authority to make unilateral changes to those agreements,” AFGE President Everett Kelley said. “AFGE members will not be intimidated. If our contracts are violated, we will aggressively defend them.”
The VA must decide where to put the more than 47,000 workers who may be coming back. According to the Government Accountability Office, “Federal agencies have long struggled to determine how much office space they needed to fulfill their missions efficiently.” The VA has reduced its office space by > 290,000 ft2 over the last few years in the National Capital Region alone.
In his confirmation hearing last month, VA Secretary nominee Doug Collins told lawmakers that, if confirmed, he would “encourage employees to come back to work,” but he also said he would ensure the department was following the White House’s remote work limits. “We’re going to make sure that we get people in there,” he said, “because at the end of the day, it’s about veterans.”
Nearly 96,000 US Department of Veterans Affairs (VA) employees—about 20% of the workforce—will be required to return to in-office work by the end of February. The announcement follows a Jan. 20 presidential memorandum, which states agency heads must “take all necessary steps to terminate remote work arrangements and require employees to return to work in-person at their respective duty stations on a full-time basis.” According to a Jan. 28 email sent from the Office of Personnel Management but without a signature, federal employees who refuse will be offered “dignified, fair departure from the federal government utilizing a deferred resignation program.”
The revised VA work policy states “eligible employees must work full-time at their respective duty stations (agency worksites) unless excused due to a disability, qualifying medical condition or other compelling reason.” All nonbargaining unit employees and supervisors who are within 50 miles of their office have until Feb. 24 to return. The VA stated that further guidance is coming for those who live > 50 miles from a facility.
“VA’s policy allows exceptions for arrangements approved for employees as a reasonable accommodation due to a disability or a qualifying medical condition. Exceptions may also be allowed for military spouses with permanent change of station orders,” according to a VA press release.
“This is a commonsense step toward treating all VA employees equally,” acting VA Secretary Todd Hunter said. “Most VA clinical staff don’t have the luxury of working remotely, and we believe the performance, collaboration and productivity of the department will improve if all VA employees are held to the same standard.”
The impact on Veterans Health Administration operations remains difficult to determine. The order appears to include personnel providing telehealth care from remote locations, including those at the Clinical Resource Hub (CRH) program. CRH uses a hub and spoke model for limited time primary care and mental health care staffing to cover local clinician vacancies. CRH clinicians have provided > 500,000 veterans with care, averaging > 25,000 encounters in the program’s first year. Started during fiscal year 2020 amid the COVID-19 pandemic, CRH employed 636 clinicians, but more recent data are not available. The VA provided > 28 million telehealth sessions to veterans across all of its telehealth modalities in 2023. Details on how many CRH clinicians and other telehealth practitioners work remotely are also not available.
On Feb. 3, the Office of Personnel Management issued a memo to federal agency heads arguing that any collective bargaining agreements that include teleworking may “conflict with management rights” and therefore may be “unlawful and cannot be enforced.”
The American Federation of Government Employees (AFGE), which represents 800,000 federal employees, disputed the memo. “Federal employees should know that approved union contracts are enforceable by law, and the President does not have the authority to make unilateral changes to those agreements,” AFGE President Everett Kelley said. “AFGE members will not be intimidated. If our contracts are violated, we will aggressively defend them.”
The VA must decide where to put the more than 47,000 workers who may be coming back. According to the Government Accountability Office, “Federal agencies have long struggled to determine how much office space they needed to fulfill their missions efficiently.” The VA has reduced its office space by > 290,000 ft2 over the last few years in the National Capital Region alone.
In his confirmation hearing last month, VA Secretary nominee Doug Collins told lawmakers that, if confirmed, he would “encourage employees to come back to work,” but he also said he would ensure the department was following the White House’s remote work limits. “We’re going to make sure that we get people in there,” he said, “because at the end of the day, it’s about veterans.”
Nearly 96,000 US Department of Veterans Affairs (VA) employees—about 20% of the workforce—will be required to return to in-office work by the end of February. The announcement follows a Jan. 20 presidential memorandum, which states agency heads must “take all necessary steps to terminate remote work arrangements and require employees to return to work in-person at their respective duty stations on a full-time basis.” According to a Jan. 28 email sent from the Office of Personnel Management but without a signature, federal employees who refuse will be offered “dignified, fair departure from the federal government utilizing a deferred resignation program.”
The revised VA work policy states “eligible employees must work full-time at their respective duty stations (agency worksites) unless excused due to a disability, qualifying medical condition or other compelling reason.” All nonbargaining unit employees and supervisors who are within 50 miles of their office have until Feb. 24 to return. The VA stated that further guidance is coming for those who live > 50 miles from a facility.
“VA’s policy allows exceptions for arrangements approved for employees as a reasonable accommodation due to a disability or a qualifying medical condition. Exceptions may also be allowed for military spouses with permanent change of station orders,” according to a VA press release.
“This is a commonsense step toward treating all VA employees equally,” acting VA Secretary Todd Hunter said. “Most VA clinical staff don’t have the luxury of working remotely, and we believe the performance, collaboration and productivity of the department will improve if all VA employees are held to the same standard.”
The impact on Veterans Health Administration operations remains difficult to determine. The order appears to include personnel providing telehealth care from remote locations, including those at the Clinical Resource Hub (CRH) program. CRH uses a hub and spoke model for limited time primary care and mental health care staffing to cover local clinician vacancies. CRH clinicians have provided > 500,000 veterans with care, averaging > 25,000 encounters in the program’s first year. Started during fiscal year 2020 amid the COVID-19 pandemic, CRH employed 636 clinicians, but more recent data are not available. The VA provided > 28 million telehealth sessions to veterans across all of its telehealth modalities in 2023. Details on how many CRH clinicians and other telehealth practitioners work remotely are also not available.
On Feb. 3, the Office of Personnel Management issued a memo to federal agency heads arguing that any collective bargaining agreements that include teleworking may “conflict with management rights” and therefore may be “unlawful and cannot be enforced.”
The American Federation of Government Employees (AFGE), which represents 800,000 federal employees, disputed the memo. “Federal employees should know that approved union contracts are enforceable by law, and the President does not have the authority to make unilateral changes to those agreements,” AFGE President Everett Kelley said. “AFGE members will not be intimidated. If our contracts are violated, we will aggressively defend them.”
The VA must decide where to put the more than 47,000 workers who may be coming back. According to the Government Accountability Office, “Federal agencies have long struggled to determine how much office space they needed to fulfill their missions efficiently.” The VA has reduced its office space by > 290,000 ft2 over the last few years in the National Capital Region alone.
In his confirmation hearing last month, VA Secretary nominee Doug Collins told lawmakers that, if confirmed, he would “encourage employees to come back to work,” but he also said he would ensure the department was following the White House’s remote work limits. “We’re going to make sure that we get people in there,” he said, “because at the end of the day, it’s about veterans.”
PharmDs, Not MDs, RNs in VA Hiring Freeze Exemption List
The US Department of Veterans Affairs (VA) has outlined > 300,000 exemptions to the federal hiring freeze to fill essential benefits and health positions. The exempted positions are primarily medical support staff. While the exemptions include pharmacists, physicians and nurses were not included. The day after taking office for the second time, President Trump signed an Executive Order implementing a “freeze on the hiring of Federal civilian employees, to be applied throughout the executive branch” but left many of the details to individual agencies.
Set to last 90 days, the hiring freeze forced Federal agencies to develop plans to reduce the size of their workforces through efficiencies and attrition, Trump said. These agencies would also not be able to hire contractors.
Three days later, however, the VA responded “Following successful implementation of President Trump’s federal hiring freeze, the Department of Veterans Affairs announced several exemptions to the policy. These exemptions clarify the department’s ability to continue filling essential positions that provide health care and other vital services to Veterans and VA beneficiaries.”
This allowed > 304,000 jobs to be exempt from the freeze. Almost 92% of the VA’s 450,000 employees work in health care and health administration and support services. Most of the exemptions involve support staff. No physicians, mental health professionals or nursing positions are on the list. However, it does include 12,622 pharmacists and 5,975 pharmacy technicians.
The VA worked in accordance with the White House and Office of Personnel Management to develop the updated guidance, Acting Veterans Affairs Secretary Todd Hunter said. In a Jan. 21 memo, Hunter wrote: "Positions critical to delivering care to veterans in the Veteran[s] Health Administration ... are exempted under the category of public safety.”
According to Hunter's memo, no other vacancies that existed as of midday Monday will be filled. Candidates who received job offers before noon on Jan. 20 and have a start date on or before Feb. 8 will be onboarded, while those with a start date after Feb. 8—or one that is undetermined—will have their offers rescinded.
The first Trump Administration began the same way in 2017, initiating a freeze on Federal hiring and receiving a similar response from the VA. In 2017, the hiring of doctors and nurses continued while that freeze was in effect, but onboarding of new support and administrative staff was not. Then-Secretary of Veterans Affairs Dr. David J. Shulkin said, “VA is committed to serving veterans, but at the same time improving efficiency and reducing bureaucracy.”
The current Executive Order states it “shall not adversely impact veterans’ benefits and does not apply to positions related to public safety” (or military personnel, immigration enforcement, and national security). It also says it does not adversely impact the provision of Social Security, Medicare, or Veterans’ benefits.
“Under President Trump’s leadership, VA will always do what is necessary to provide America’s Veterans with the benefits and services they have earned. The targeted hiring-freeze exemptions announced today underscore that fact,” said VA Director of Media Affairs Morgan Ackley.
Some in Congress feel the VA should be doing more, though, and are pushing for an exemption of all VA employees. On Friday, Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) released a statement on the exemptions. “The latest Administration hiring freeze announcement still falls short. While I’m encouraged the President responded to our concerns by exempting certain VA personnel, only a clear, unequivocal statement to exempt all VA employees from the hiring freeze will reassure me—and veterans—they will receive the care and benefits they need and deserve. The exemptions listed yesterday provide more questions than answers and fail to include key personnel, including Veterans Benefits Administration employees. The Trump Administration is going to try to confuse the issue with a lot of vague assurances. We need a clear commitment every VA employee is exempt—effective immediately. Moreover, the Trump Administration must address the offers it has already rescinded that are now exempt.”
Blumenthal and 24 Democratic Senators also signed a letter to that effect, stressing concerns about the negative impact the hiring freeze will have on the delivery of veterans’ health care and benefits nationwide “if not quickly reversed.” Blumenthal also pressed Doug Collins (R-GA), Trump’s nominee for VA Secretary, to push back against a hiring freeze at VA, if his nomination is confirmed: “This is going to be a first test of your leadership.”
“We’ll take a look at the current levels of employees that we have and where they’re properly located,” Collins said, adding that he was “still examining” the freeze’s impact on the VA. “We will work under the Executive Order [Trump] has given us.”
Blumenthal argued that the new exemptions exclude a number of critical positions at VA. Among them include all positions at the Veterans Benefits Administration and National Cemetery Administration, which provide veterans’ claims processing, survivor benefits, GI Bill education benefits, and burial scheduling and operations; many nonclinical positions critical to VA hospital functioning, including patient advocates, food service workers, and chaplains; and positions relating to construction project management for new hospitals and clinics, new nursing homes, new cemetery construction, leases, and repairs to existing VA facilities.
The US Department of Veterans Affairs (VA) has outlined > 300,000 exemptions to the federal hiring freeze to fill essential benefits and health positions. The exempted positions are primarily medical support staff. While the exemptions include pharmacists, physicians and nurses were not included. The day after taking office for the second time, President Trump signed an Executive Order implementing a “freeze on the hiring of Federal civilian employees, to be applied throughout the executive branch” but left many of the details to individual agencies.
Set to last 90 days, the hiring freeze forced Federal agencies to develop plans to reduce the size of their workforces through efficiencies and attrition, Trump said. These agencies would also not be able to hire contractors.
Three days later, however, the VA responded “Following successful implementation of President Trump’s federal hiring freeze, the Department of Veterans Affairs announced several exemptions to the policy. These exemptions clarify the department’s ability to continue filling essential positions that provide health care and other vital services to Veterans and VA beneficiaries.”
This allowed > 304,000 jobs to be exempt from the freeze. Almost 92% of the VA’s 450,000 employees work in health care and health administration and support services. Most of the exemptions involve support staff. No physicians, mental health professionals or nursing positions are on the list. However, it does include 12,622 pharmacists and 5,975 pharmacy technicians.
The VA worked in accordance with the White House and Office of Personnel Management to develop the updated guidance, Acting Veterans Affairs Secretary Todd Hunter said. In a Jan. 21 memo, Hunter wrote: "Positions critical to delivering care to veterans in the Veteran[s] Health Administration ... are exempted under the category of public safety.”
According to Hunter's memo, no other vacancies that existed as of midday Monday will be filled. Candidates who received job offers before noon on Jan. 20 and have a start date on or before Feb. 8 will be onboarded, while those with a start date after Feb. 8—or one that is undetermined—will have their offers rescinded.
The first Trump Administration began the same way in 2017, initiating a freeze on Federal hiring and receiving a similar response from the VA. In 2017, the hiring of doctors and nurses continued while that freeze was in effect, but onboarding of new support and administrative staff was not. Then-Secretary of Veterans Affairs Dr. David J. Shulkin said, “VA is committed to serving veterans, but at the same time improving efficiency and reducing bureaucracy.”
The current Executive Order states it “shall not adversely impact veterans’ benefits and does not apply to positions related to public safety” (or military personnel, immigration enforcement, and national security). It also says it does not adversely impact the provision of Social Security, Medicare, or Veterans’ benefits.
“Under President Trump’s leadership, VA will always do what is necessary to provide America’s Veterans with the benefits and services they have earned. The targeted hiring-freeze exemptions announced today underscore that fact,” said VA Director of Media Affairs Morgan Ackley.
Some in Congress feel the VA should be doing more, though, and are pushing for an exemption of all VA employees. On Friday, Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) released a statement on the exemptions. “The latest Administration hiring freeze announcement still falls short. While I’m encouraged the President responded to our concerns by exempting certain VA personnel, only a clear, unequivocal statement to exempt all VA employees from the hiring freeze will reassure me—and veterans—they will receive the care and benefits they need and deserve. The exemptions listed yesterday provide more questions than answers and fail to include key personnel, including Veterans Benefits Administration employees. The Trump Administration is going to try to confuse the issue with a lot of vague assurances. We need a clear commitment every VA employee is exempt—effective immediately. Moreover, the Trump Administration must address the offers it has already rescinded that are now exempt.”
Blumenthal and 24 Democratic Senators also signed a letter to that effect, stressing concerns about the negative impact the hiring freeze will have on the delivery of veterans’ health care and benefits nationwide “if not quickly reversed.” Blumenthal also pressed Doug Collins (R-GA), Trump’s nominee for VA Secretary, to push back against a hiring freeze at VA, if his nomination is confirmed: “This is going to be a first test of your leadership.”
“We’ll take a look at the current levels of employees that we have and where they’re properly located,” Collins said, adding that he was “still examining” the freeze’s impact on the VA. “We will work under the Executive Order [Trump] has given us.”
Blumenthal argued that the new exemptions exclude a number of critical positions at VA. Among them include all positions at the Veterans Benefits Administration and National Cemetery Administration, which provide veterans’ claims processing, survivor benefits, GI Bill education benefits, and burial scheduling and operations; many nonclinical positions critical to VA hospital functioning, including patient advocates, food service workers, and chaplains; and positions relating to construction project management for new hospitals and clinics, new nursing homes, new cemetery construction, leases, and repairs to existing VA facilities.
The US Department of Veterans Affairs (VA) has outlined > 300,000 exemptions to the federal hiring freeze to fill essential benefits and health positions. The exempted positions are primarily medical support staff. While the exemptions include pharmacists, physicians and nurses were not included. The day after taking office for the second time, President Trump signed an Executive Order implementing a “freeze on the hiring of Federal civilian employees, to be applied throughout the executive branch” but left many of the details to individual agencies.
Set to last 90 days, the hiring freeze forced Federal agencies to develop plans to reduce the size of their workforces through efficiencies and attrition, Trump said. These agencies would also not be able to hire contractors.
Three days later, however, the VA responded “Following successful implementation of President Trump’s federal hiring freeze, the Department of Veterans Affairs announced several exemptions to the policy. These exemptions clarify the department’s ability to continue filling essential positions that provide health care and other vital services to Veterans and VA beneficiaries.”
This allowed > 304,000 jobs to be exempt from the freeze. Almost 92% of the VA’s 450,000 employees work in health care and health administration and support services. Most of the exemptions involve support staff. No physicians, mental health professionals or nursing positions are on the list. However, it does include 12,622 pharmacists and 5,975 pharmacy technicians.
The VA worked in accordance with the White House and Office of Personnel Management to develop the updated guidance, Acting Veterans Affairs Secretary Todd Hunter said. In a Jan. 21 memo, Hunter wrote: "Positions critical to delivering care to veterans in the Veteran[s] Health Administration ... are exempted under the category of public safety.”
According to Hunter's memo, no other vacancies that existed as of midday Monday will be filled. Candidates who received job offers before noon on Jan. 20 and have a start date on or before Feb. 8 will be onboarded, while those with a start date after Feb. 8—or one that is undetermined—will have their offers rescinded.
The first Trump Administration began the same way in 2017, initiating a freeze on Federal hiring and receiving a similar response from the VA. In 2017, the hiring of doctors and nurses continued while that freeze was in effect, but onboarding of new support and administrative staff was not. Then-Secretary of Veterans Affairs Dr. David J. Shulkin said, “VA is committed to serving veterans, but at the same time improving efficiency and reducing bureaucracy.”
The current Executive Order states it “shall not adversely impact veterans’ benefits and does not apply to positions related to public safety” (or military personnel, immigration enforcement, and national security). It also says it does not adversely impact the provision of Social Security, Medicare, or Veterans’ benefits.
“Under President Trump’s leadership, VA will always do what is necessary to provide America’s Veterans with the benefits and services they have earned. The targeted hiring-freeze exemptions announced today underscore that fact,” said VA Director of Media Affairs Morgan Ackley.
Some in Congress feel the VA should be doing more, though, and are pushing for an exemption of all VA employees. On Friday, Senate Veterans’ Affairs Committee Ranking Member Richard Blumenthal (D-CT) released a statement on the exemptions. “The latest Administration hiring freeze announcement still falls short. While I’m encouraged the President responded to our concerns by exempting certain VA personnel, only a clear, unequivocal statement to exempt all VA employees from the hiring freeze will reassure me—and veterans—they will receive the care and benefits they need and deserve. The exemptions listed yesterday provide more questions than answers and fail to include key personnel, including Veterans Benefits Administration employees. The Trump Administration is going to try to confuse the issue with a lot of vague assurances. We need a clear commitment every VA employee is exempt—effective immediately. Moreover, the Trump Administration must address the offers it has already rescinded that are now exempt.”
Blumenthal and 24 Democratic Senators also signed a letter to that effect, stressing concerns about the negative impact the hiring freeze will have on the delivery of veterans’ health care and benefits nationwide “if not quickly reversed.” Blumenthal also pressed Doug Collins (R-GA), Trump’s nominee for VA Secretary, to push back against a hiring freeze at VA, if his nomination is confirmed: “This is going to be a first test of your leadership.”
“We’ll take a look at the current levels of employees that we have and where they’re properly located,” Collins said, adding that he was “still examining” the freeze’s impact on the VA. “We will work under the Executive Order [Trump] has given us.”
Blumenthal argued that the new exemptions exclude a number of critical positions at VA. Among them include all positions at the Veterans Benefits Administration and National Cemetery Administration, which provide veterans’ claims processing, survivor benefits, GI Bill education benefits, and burial scheduling and operations; many nonclinical positions critical to VA hospital functioning, including patient advocates, food service workers, and chaplains; and positions relating to construction project management for new hospitals and clinics, new nursing homes, new cemetery construction, leases, and repairs to existing VA facilities.
VA Pays Billions for Costs Shifted From Medicare
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
Areas of Hope Offered in 2024 VA Suicide Report
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
Suicide was the 12th-leading cause of death for veterans in 2022. However, fewer veterans died by suicide in 2022 than in 12 of the previous 14 years, according to the 2024 National Veteran Suicide Prevention Annual Report released by the US Department of Veterans Affairs (VA).
The review is the most comprehensive national report on veteran suicide and is based on verified data from the Centers for Disease Control and US Department of Defense from 2001-2022, or the most recent years the VA has data.
The report states that 6407 veterans died by suicide in 2022, 3 more than the year before. For comparison, 41,484 nonveteran US adults died by suicide in 2022, 1476 more than 2021. It is important to assess suicide mortality rates in the context of population changes, the report cautions. From 2001-2022, the veteran population dropped from 25.8 million to 18.5 million, a 28.4% decrease. During that same period, the nonveteran US adult population increased from 186.5 million to 242.4 million, a 30.0% jump.
On average, 131 US adults died by suicide each day in 2022: 18 veterans and 114 nonveterans. Among all US adults, including veterans, the average number of suicides per day rose from 81 per day in 2001 to 131 per day in 2022. The average number of veteran suicides per day rose from 16.5 in 2001 to 17.6 in 2022.
“Hope serves an important role within suicide prevention efforts,” the VA said. “Within the challenges faced in 2022, key areas of hope emerged.”
Among those key findings are a 24.1% decrease in age-adjusted suicide rates, a 37% suicide rate reduction among individuals who received VA homeless program services, 3.8% suicide rate decrease in veterans aged 18 to 34 years, and considerable drops in suicide rates for veterans with Veterans Health Administration mental health diagnoses of anxiety (36.1%), depression (34.5%), posttraumatic stress disorder (31.6%), and alcohol use disorder (13.7%).
Eliminating veteran suicide is VA’s top clinical priority and a critical aspect of the strategy for reducing military and veteran suicide. Since 2022, VA has worked aggressively to expand support, including offering no-cost health care to veterans in suicidal crisis; launching the 988 (then press 1) hotline, qualified responders through the Veterans Crisis Line; expanding firearm suicide prevention efforts; and encouraging veterans to reach out for help through a national veteran suicide prevention awareness campaign.
“There is nothing more important to VA than ending veteran suicide,“ said Secretary of Veterans Affairs Denis McDonough. “We will learn from this report to better serve veterans and save lives.”
Congress and VA Aim to Improve Health Care Access for Rural Veterans
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
Veterans living in rural areas are often too far away from health care institutions to easily travel to their appointments. Even if they can drive, the cost of gas and other related travel expenses may be too much for some. Telehealth was meant to help relieve that problem, but poor internet access can mitigate its convenience and accessibility for those patients. Two proposals offer solutions.
In February, Sens. Jon Ossoff (D-GA), Susan Collins (R-ME), and John Thune (R-SD) introduced the Rural Veterans Transportation to Care Act, a bill that would expand eligibility to the US Department of Veterans Affairs (VA) Highly Rural Transportation Grants, a program currently only available to counties with < 7 people per square mile.
“As I’ve sat down with veterans in rural areas across Georgia, one of their key concerns is lack of transportation,” Sen. Ossoff said. “That’s why I’m introducing this bipartisan bill to ensure veterans have more access to transportation services that can bring them to VA clinics and medical centers to get the care they need.”
Amanda Flener and her husband, John, a veteran wounded while serving in Iraq, were driving as long as 3 hours from Fitzgerald, Georgia (population 8900) to attend his medical appointments. In the last 2 years, Flener told the Daily Yonder she had put nearly 72,000 miles on her vehicle. Following hurricane Helene, she said, "We had been driving 30 miles just to get gas to power our generator … and we were fortunate to be able to do that.”
Telehealth appointments can help fill coverage gaps, Flener said. But even while paying for the most expensive internet plan available in her county, the signal isn't always strong enough. Telehealth care is "progress, for sure," Flener said. "So, we pay for the best Wi-Fi we can get in our area, but it isn't always reliable enough to take the video calls from the VA."
As a result, veterans and their caregivers could benefit not only from the bipartisan transportation proposal, but also from a decision announced in November. The VA is proposing to eliminate copayments for all VA telehealth services and establish a grant program to fund designated VA telehealth access points in non-VA facilities, with a focus on rural and medically underserved communities.
The program, called Accessing Telehealth through Local Area Stations (ATLAS), would provide funding to organizations — including nonprofits and private businesses — to offer veterans comfortable, private spaces equipped with high-speed internet access and the technology to remotely meet with VA clinicians. Grants would also provide designated funding to train on-site personnel to support the program.
These proposed changes would advance the VA’s and the Biden-Harris Administration’s ongoing efforts to lower costs and expand access to care for veterans. They also could make a life-changing difference for the 2.7 million rural veterans enrolled in VA health care.
According to a 2024 RAND study, just under half of military and veteran caregivers live in a county without a VA facility, and nearly half live in a primary care physician shortage area. For military/veteran caregivers in particular, the survey found, reduced access to support related to the more complicated care some patients require, greater distances to reach opportunities (eg, retail, economic, or social), and even differences in Wi-Fi/broadband internet access may create “unique needs.” The survey found that 24% of rural military/veteran caregivers did not have reliable broadband internet.
“Waiving copays for telehealth services and launching this grant program are both major steps forward in ensuring veterans can access health care where and when they need it,” said VA Secretary Denis McDonough. “VA is the best and most affordable care in America for veterans — with these steps, we can make it easier for veterans to access their earned VA health care.”
The rulemaking can be viewed in the Federal Register under public inspection, and is open for comment. The VA anticipates a notice of funding opportunity for this grant program following publication of the final rule.
The Year of AI: Learning With Machines to Improve Veteran Health Care
The Year of AI: Learning With Machines to Improve Veteran Health Care
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
The Year of AI: Learning With Machines to Improve Veteran Health Care
The Year of AI: Learning With Machines to Improve Veteran Health Care
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
As Veterans Day approaches, stores and restaurants will offer discounts and free meals to veterans. Children will write thank you letters, and citizens nationwide will raise flags to honor and thank veterans. We can never repay those who lost their life, health, or livelihood in defense of the nation. Since the American Revolution, and in gratitude for that incalculable debt, the US government, on behalf of the American public, has seen fit to grant a host of benefits and services to those who wore the uniform.2,3 Among the best known are health care, burial services, compensation and pensions, home loans, and the GI Bill.
Less recognized yet arguably essential for the fair and consistent provision of these entitlements is a legal principle: the veteran’s canon. A canon is a system of rules or maxims used to interpret legal instruments, such as statutes. They are not rules but serve as a “principle that guides the interpretation of the text.”4 Since I am not a lawyer, I will undoubtedly oversimplify this legal principle, but I hope to get enough right to explain why the veteran’s canon should matter to federal health care professionals.
At its core, the veteran’s canon means that when the US Department of Veterans Affairs (VA) and a veteran have a legal dispute about VA benefits, the courts will give deference to the veteran. Underscoring that any ambiguity in the statute is resolved in the veteran’s favor, the canon is known in legal circles as the Gardner deference. This is a reference to a 1994 case in which a Korean War veteran underwent surgery in a VA facility for a herniated disc he alleged caused pain and weakness in his left lower extremity.5 Gardner argued that federal statutes 38 USC § 1151 underlying corresponding VA regulation 38 CFR § 3.358(c)(3) granted disability benefits to veterans injured during VA treatment. The VA denied the disability claim, contending the regulation restricted compensation to veterans whose injury was the fault of the VA; thus, the disability had to have been the result of negligent treatment or an unforeseen therapeutic accident.5
The case wound its way through various appeals boards and courts until the Supreme Court of the United States (SCOTUS) ruled that the statute’s context left no ambiguity, and that any care provided under VA auspices was covered under the statute. What is important for this column is that the justices opined that had ambiguity been present, it would have legally necessitated, “applying the rule that interpretive doubt is to be resolved in the veteran’s favor.”5 In Gardner’s case, the courts reaffirmed nearly 80 years of judicial precedent upholding the veteran’s canon.
Thirty years later, Rudisill v McDonough again questioned the veteran’s canon.6 Educational benefits, namely the GI Bill, were the issue in this case. Rudisill served during 3 different periods in the US Army, totaling 8 years. Two educational programs overlapped during Rudisill’s tenure in the military: the Montgomery GI Bill and Post-9/11 Veterans Educational Assistance Act. Rudisill had used a portion of his Montgomery benefits to fund his undergraduate education and now wished to use the more extensive Post-9/11 assistance to finance his graduate degree. Rudisill and the VA disagreed about when his combined benefits would be capped, either at 36 or 48 months. After working its way through appeals courts, SCOTUS was again called upon for judgment.
The justices found that Rudisill qualified under both programs and could use them in any order he wished up to the cap. The majority found no ambiguity in the statute; however, if interpretation was required, the majority of justices indicated that the veteran’s canon would have supported Rudisill. While this sounds like good news for veterans, 2 justices authored a dissenting opinion that questioned the constitutional grounding of the veteran’s canon, noting that the “canon appears to have developed almost by accident.”6 The minority opinion suggested that when the veteran’s canon allocates resources to pay for specific veteran benefits, other interests and groups are deprived of those same resources, resulting in potential inequity.7
The potential ethical import and clinical impact of striking down the veteran’s canon is serious. It is especially concerning given that in a recent case, the SCOTUS ruling struck down another legal interpretation that also benefited the VA and ultimately veterans: the Chevron deference.8 This precedent held that when a legal dispute arises about the meaning of a specific federal agency regulation or policy, the courts should defer to the federal agency’s presumably superior understanding of the matter. The principle places the locus of decision-making with the subject-matter experts of the respective agency rather than the courts.
Ironically, given the legislative purposes of both interpretive principles, their overturning would likely introduce much more uncertainty, variation, and unpredictability in cases involving veteran benefits. This is bad news for both veterans and the VA. Veterans might not prevail as often in court when they have a reasonable claim, leading to more aggressive challenges. In response, the VA would have a heavier and more costly burden of administrative proof to defend sound decisions.9 Recently, the VA has tried to reduce the backlog of claims. The inability to have legal recourse to Chevron or Gardener could result in even more delay in adjudicating veterans’ claims that enable them to access benefits and services, already an object of congressional pressure.10
Courts will continue to debate the issue with another judicial test of the canon on the current SCOTUS docket (Bufkin v McDonough).11 The veteran’s canon was put in place to equalize the power differential between the VA and the veteran: in administrative language, to make it more likely than not that the veteran would prevail when regulations were ambiguous. There are many legal and political rationales for veteran’s canon, including enabling veterans to file claims for service-connected illnesses. The veteran’s cannon helped Vietnam War-era veterans receive VA care while researchers were still studying the sequela of Agent Orange exposure. 12 The legislative purpose of the veteran’s canon is the same as that of all VA benefits and services commemorated on Veterans Day. As expressed by SCOTUS justices in the wake of World War II, the benefit statutes should be “liberally construed for the benefit of those who left private life to serve their country in its hour of greatest need.”13
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
- Henderson v Shinseki, 562 US. 428, 440-441 (2011).
- US Department of Veterans Affairs, National Veteran Outreach Office. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed October 21, 2024. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- US Department of Veterans Affairs. VA history summary. Updated August 6, 2024. Accessed October 21, 2024. https://department.va.gov/history/history-overview
- Cornell Law School, Legal Information Institute. Canons of construction. Updated March 2022. Accessed October 21, 2024. https://www.law.cornell.edu/wex/canons_of_construction
- Brown v Gardner, 513 US 115 (1994).
- Rudisill v McDonough, 601 US __ (2024).
- Hoover J. Justices will decide if vets are getting the ‘benefit of the doubt’. National Law Journal. April 30, 2024. Accessed October 21, 2024. https://www.law.com/nationallawjournal/2024/04/30/justices-will-decide-if-vets-are-getting-the-benefit-of-the-doubt/
- Relentless, Inc. v Department of Commerce Docket # 22-219, January 17, 2024.
- Kime P. Two veterans will argue to Supreme Court that VA disability claims aren’t getting, ‘benefit of the doubt’. Military. com. October 15, 2024. Accessed October 21, 2024. https:// www.military.com/daily-news/2024/10/15/supreme-court-hears-case-questioning-vas-commitment-favoring-veterans-benefits-decisions.html
- Rehagen J. SCOTUS’s chevron deference ruling: how it could hurt veterans and the VA. Veteran.com. Updated July 9, 2024. Accessed October 21, 2024. https://veteran.com/scotus-chevron-deference-impact-va-veteran/
- Hersey LF. Lawmakers urge VA to reduce backlog, wait times on veterans claims for benefits. Stars & Stripes. June 27, 2024. Accessed October 21, 2024. https://www.stripes.com/veterans/2024-06-27/veterans-benefits-claims-backlog-pact-act-14315042.html
- Harper CJ. Give veterans the benefit of the doubt: Chevron, Auer, and the veteran’s canon. Harvard J Law Public Policy. 2019; 42(3):931-969. https://journals.law.harvard.edu/jlpp/wp-content/uploads/sites/90/2019/06/42_3-Full-Issue.pdf
- Fishgold v Sullivan Drydock & Repair Corp, 328 US 275, 285 (1946).
The Veteran’s Canon Under Fire
The Veteran’s Canon Under Fire
How is VA Doing? Report Card Grades Are In
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
VA Awards Grants to Support Adaptive Sports
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
VHA Support for Home Health Agency Staff and Patients During Natural Disasters
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).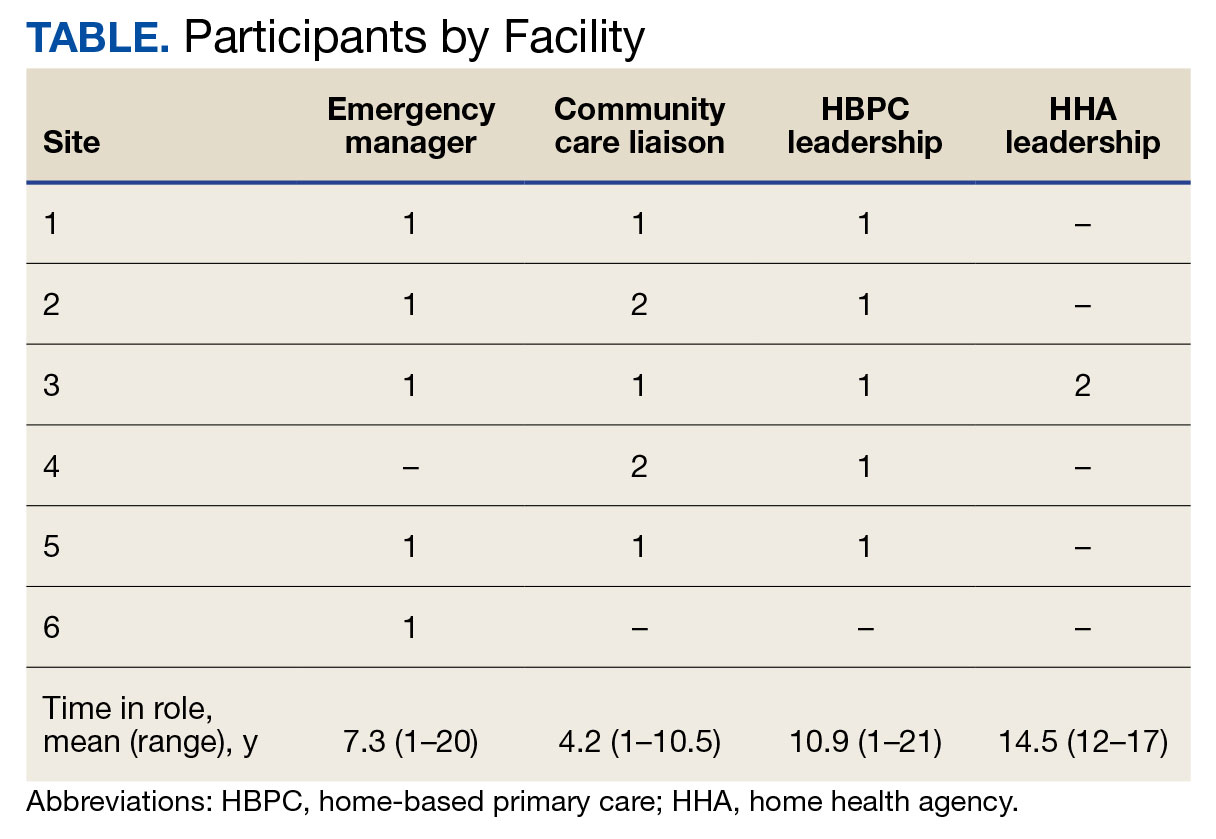
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690




