User login
A prescription for de-diagnosing
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
Youth with bipolar disorder at high risk of eating disorders
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.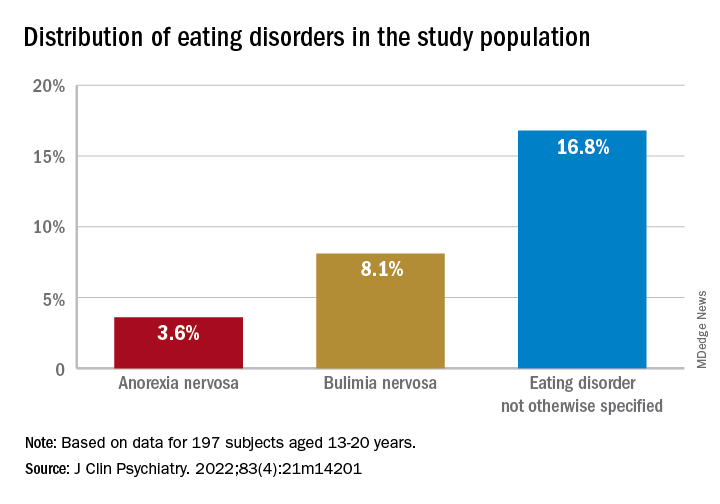
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.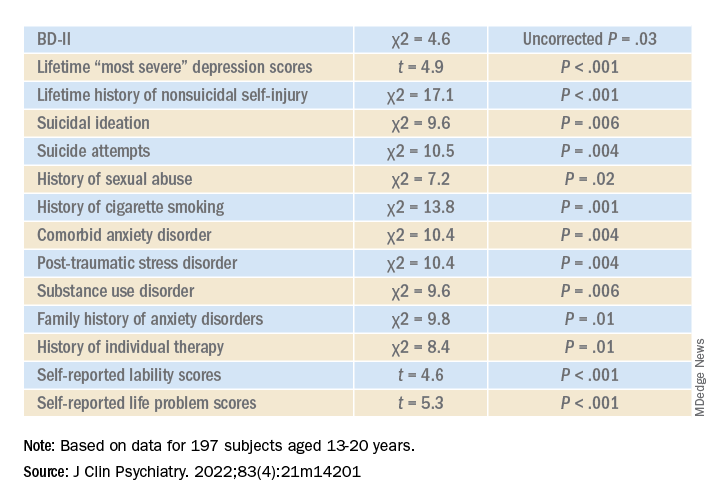
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”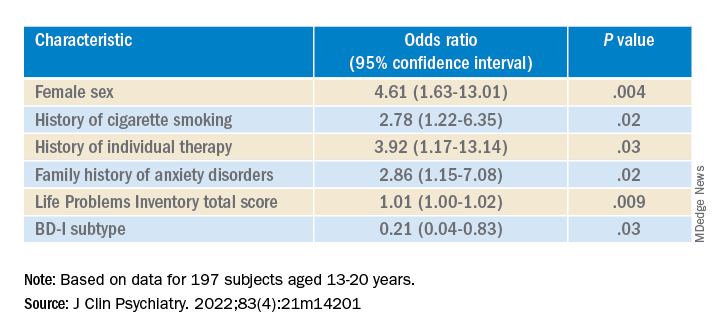
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Social activities may offset psychosis risk in poor communities
, new research suggests.
A study of more than 170 young participants showed reduced hippocampal volume in those living in poor neighborhoods who had low social engagement versus their peers with greater community engagement.
“These findings demonstrate the importance of considering broader environmental influences and indices of social engagement when conceptualizing adversity and potential interventions for individuals at clinical high risk for psychosis,” co-investigator Benson Ku, MD, a postdoctoral fellow and psychiatry resident at Emory University School of Medicine, Atlanta, told this news organization.
The results were presented at the virtual American Society of Clinical Psychopharmacology annual meeting.
A personal connection
It’s well known that growing up in low-income housing is associated with lower hippocampal volume and an increased risk for schizophrenia, said Dr. Ku.
“The inverse relationship between poverty and hippocampal gray matter volume has [also] been shown to be mediated by social stress, which can include things like lack of parental caregiving and stressful life events,” he added.
Dr. Ku himself grew up in a socioeconomically disadvantaged family in Queens, New York, and he said he had initially performed poorly in school. His early experiences have helped inform his clinical and research interests in the social determinants of mental health.
“I found community support in the Boys’ Club of New York and a local Magic Shop near where I lived, which helped me thrive and become the successful man I am today. I have also heard from my patients how their living conditions and neighborhood have significantly impacted their mental health,” Dr. Ku said.
“A more in-depth understanding of the social determinants of mental health has helped build rapport and empathy with my patients,” he added.
To explore the association between neighborhood poverty, social engagement, and hippocampal volume in youth at high risk for psychosis, the researchers analyzed data from the North American Prodrome Longitudinal Study Phase 2, a multisite consortium.
The researchers recruited and followed up with help-seeking adolescents and young adults from diverse neighborhoods. The analysis included 174 youth, ages 12-33 years, at high clinical risk for psychosis.
Hippocampal volume was assessed using structural MRI. Neighborhood poverty was defined as the percentage of residents with an annual income below the poverty level in the past year.
Social engagement was derived from the desirable events subscale items of the Life Events Scale. These activities included involvement in a church or synagogue; participation in a club, neighborhood, or other organization; taking a vacation; engaging in a hobby, sport, craft, or recreational activity; acquiring a pet; or making new friends.
Lower hippocampal volume
Results showed neighborhood poverty was associated with reduced hippocampal volume, even after controlling for several confounders, including race/ethnicity, family history of mental illnesses, household poverty, educational level, and stressful life events.
Among the 77 participants with lower social engagement, which was defined as three or fewer social activities, neighborhood poverty was associated with reduced hippocampal volume.
However, in the 97 participants who reported greater social engagement, which was defined as four or more social activities, neighborhood poverty was not significantly associated with hippocampal volume.
“It is possible that social engagement may mitigate the deleterious effects of neighborhood poverty on brain morphology, which may inform interventions offered to individuals from disadvantaged neighborhoods,” Dr. Ku said.
“If replication of the relationships between neighborhood poverty, hippocampal volume, and social engagement is established in other populations in longitudinal studies, then targeted interventions at the community level and increased social engagement may potentially play a major role in disease prevention among at-risk youth,” he said.
Dr. Ku noted social engagement might look different in urban versus rural settings.
“In urban areas, it might mean friends, clubs, neighborhood organizations, etc. In rural areas, it might mean family, pets, crafts, etc. The level of social engagement may also depend on neighborhood characteristics, and more research would be needed to better understand how geographic area characteristics – remote, rural, urban – affects social engagement,” he said.
Interesting, innovative
Nagy Youssef, MD, PhD, director of clinical research and professor of psychiatry, Ohio State University College of Medicine, Columbus, said the study suggests “social engagement may reduce the negative effect of poverty in this population, and if replicated in a larger study, could assist and be a part of the early intervention and prevention in psychosis.”
Overall, “this is an interesting and innovative study that has important medical and social implications and is a good step toward helping us understand these relationships and mitigate and prevent negative consequences, as best as possible, in this population,” said Dr. Youssef, who was not part of the research.
The analysis was supported by a grant from the National Institute of Mental Health to the North American Prodrome Longitudinal Study. Dr. Ku and Dr. Youssef report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
A study of more than 170 young participants showed reduced hippocampal volume in those living in poor neighborhoods who had low social engagement versus their peers with greater community engagement.
“These findings demonstrate the importance of considering broader environmental influences and indices of social engagement when conceptualizing adversity and potential interventions for individuals at clinical high risk for psychosis,” co-investigator Benson Ku, MD, a postdoctoral fellow and psychiatry resident at Emory University School of Medicine, Atlanta, told this news organization.
The results were presented at the virtual American Society of Clinical Psychopharmacology annual meeting.
A personal connection
It’s well known that growing up in low-income housing is associated with lower hippocampal volume and an increased risk for schizophrenia, said Dr. Ku.
“The inverse relationship between poverty and hippocampal gray matter volume has [also] been shown to be mediated by social stress, which can include things like lack of parental caregiving and stressful life events,” he added.
Dr. Ku himself grew up in a socioeconomically disadvantaged family in Queens, New York, and he said he had initially performed poorly in school. His early experiences have helped inform his clinical and research interests in the social determinants of mental health.
“I found community support in the Boys’ Club of New York and a local Magic Shop near where I lived, which helped me thrive and become the successful man I am today. I have also heard from my patients how their living conditions and neighborhood have significantly impacted their mental health,” Dr. Ku said.
“A more in-depth understanding of the social determinants of mental health has helped build rapport and empathy with my patients,” he added.
To explore the association between neighborhood poverty, social engagement, and hippocampal volume in youth at high risk for psychosis, the researchers analyzed data from the North American Prodrome Longitudinal Study Phase 2, a multisite consortium.
The researchers recruited and followed up with help-seeking adolescents and young adults from diverse neighborhoods. The analysis included 174 youth, ages 12-33 years, at high clinical risk for psychosis.
Hippocampal volume was assessed using structural MRI. Neighborhood poverty was defined as the percentage of residents with an annual income below the poverty level in the past year.
Social engagement was derived from the desirable events subscale items of the Life Events Scale. These activities included involvement in a church or synagogue; participation in a club, neighborhood, or other organization; taking a vacation; engaging in a hobby, sport, craft, or recreational activity; acquiring a pet; or making new friends.
Lower hippocampal volume
Results showed neighborhood poverty was associated with reduced hippocampal volume, even after controlling for several confounders, including race/ethnicity, family history of mental illnesses, household poverty, educational level, and stressful life events.
Among the 77 participants with lower social engagement, which was defined as three or fewer social activities, neighborhood poverty was associated with reduced hippocampal volume.
However, in the 97 participants who reported greater social engagement, which was defined as four or more social activities, neighborhood poverty was not significantly associated with hippocampal volume.
“It is possible that social engagement may mitigate the deleterious effects of neighborhood poverty on brain morphology, which may inform interventions offered to individuals from disadvantaged neighborhoods,” Dr. Ku said.
“If replication of the relationships between neighborhood poverty, hippocampal volume, and social engagement is established in other populations in longitudinal studies, then targeted interventions at the community level and increased social engagement may potentially play a major role in disease prevention among at-risk youth,” he said.
Dr. Ku noted social engagement might look different in urban versus rural settings.
“In urban areas, it might mean friends, clubs, neighborhood organizations, etc. In rural areas, it might mean family, pets, crafts, etc. The level of social engagement may also depend on neighborhood characteristics, and more research would be needed to better understand how geographic area characteristics – remote, rural, urban – affects social engagement,” he said.
Interesting, innovative
Nagy Youssef, MD, PhD, director of clinical research and professor of psychiatry, Ohio State University College of Medicine, Columbus, said the study suggests “social engagement may reduce the negative effect of poverty in this population, and if replicated in a larger study, could assist and be a part of the early intervention and prevention in psychosis.”
Overall, “this is an interesting and innovative study that has important medical and social implications and is a good step toward helping us understand these relationships and mitigate and prevent negative consequences, as best as possible, in this population,” said Dr. Youssef, who was not part of the research.
The analysis was supported by a grant from the National Institute of Mental Health to the North American Prodrome Longitudinal Study. Dr. Ku and Dr. Youssef report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
A study of more than 170 young participants showed reduced hippocampal volume in those living in poor neighborhoods who had low social engagement versus their peers with greater community engagement.
“These findings demonstrate the importance of considering broader environmental influences and indices of social engagement when conceptualizing adversity and potential interventions for individuals at clinical high risk for psychosis,” co-investigator Benson Ku, MD, a postdoctoral fellow and psychiatry resident at Emory University School of Medicine, Atlanta, told this news organization.
The results were presented at the virtual American Society of Clinical Psychopharmacology annual meeting.
A personal connection
It’s well known that growing up in low-income housing is associated with lower hippocampal volume and an increased risk for schizophrenia, said Dr. Ku.
“The inverse relationship between poverty and hippocampal gray matter volume has [also] been shown to be mediated by social stress, which can include things like lack of parental caregiving and stressful life events,” he added.
Dr. Ku himself grew up in a socioeconomically disadvantaged family in Queens, New York, and he said he had initially performed poorly in school. His early experiences have helped inform his clinical and research interests in the social determinants of mental health.
“I found community support in the Boys’ Club of New York and a local Magic Shop near where I lived, which helped me thrive and become the successful man I am today. I have also heard from my patients how their living conditions and neighborhood have significantly impacted their mental health,” Dr. Ku said.
“A more in-depth understanding of the social determinants of mental health has helped build rapport and empathy with my patients,” he added.
To explore the association between neighborhood poverty, social engagement, and hippocampal volume in youth at high risk for psychosis, the researchers analyzed data from the North American Prodrome Longitudinal Study Phase 2, a multisite consortium.
The researchers recruited and followed up with help-seeking adolescents and young adults from diverse neighborhoods. The analysis included 174 youth, ages 12-33 years, at high clinical risk for psychosis.
Hippocampal volume was assessed using structural MRI. Neighborhood poverty was defined as the percentage of residents with an annual income below the poverty level in the past year.
Social engagement was derived from the desirable events subscale items of the Life Events Scale. These activities included involvement in a church or synagogue; participation in a club, neighborhood, or other organization; taking a vacation; engaging in a hobby, sport, craft, or recreational activity; acquiring a pet; or making new friends.
Lower hippocampal volume
Results showed neighborhood poverty was associated with reduced hippocampal volume, even after controlling for several confounders, including race/ethnicity, family history of mental illnesses, household poverty, educational level, and stressful life events.
Among the 77 participants with lower social engagement, which was defined as three or fewer social activities, neighborhood poverty was associated with reduced hippocampal volume.
However, in the 97 participants who reported greater social engagement, which was defined as four or more social activities, neighborhood poverty was not significantly associated with hippocampal volume.
“It is possible that social engagement may mitigate the deleterious effects of neighborhood poverty on brain morphology, which may inform interventions offered to individuals from disadvantaged neighborhoods,” Dr. Ku said.
“If replication of the relationships between neighborhood poverty, hippocampal volume, and social engagement is established in other populations in longitudinal studies, then targeted interventions at the community level and increased social engagement may potentially play a major role in disease prevention among at-risk youth,” he said.
Dr. Ku noted social engagement might look different in urban versus rural settings.
“In urban areas, it might mean friends, clubs, neighborhood organizations, etc. In rural areas, it might mean family, pets, crafts, etc. The level of social engagement may also depend on neighborhood characteristics, and more research would be needed to better understand how geographic area characteristics – remote, rural, urban – affects social engagement,” he said.
Interesting, innovative
Nagy Youssef, MD, PhD, director of clinical research and professor of psychiatry, Ohio State University College of Medicine, Columbus, said the study suggests “social engagement may reduce the negative effect of poverty in this population, and if replicated in a larger study, could assist and be a part of the early intervention and prevention in psychosis.”
Overall, “this is an interesting and innovative study that has important medical and social implications and is a good step toward helping us understand these relationships and mitigate and prevent negative consequences, as best as possible, in this population,” said Dr. Youssef, who was not part of the research.
The analysis was supported by a grant from the National Institute of Mental Health to the North American Prodrome Longitudinal Study. Dr. Ku and Dr. Youssef report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASCP 2022
Antipsychotic tied to dose-related weight gain, higher cholesterol
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.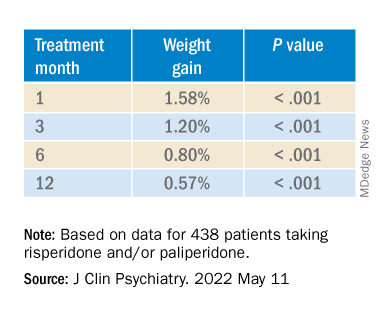
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.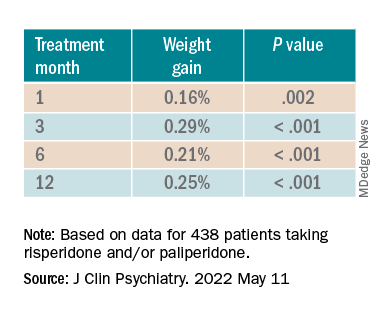
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.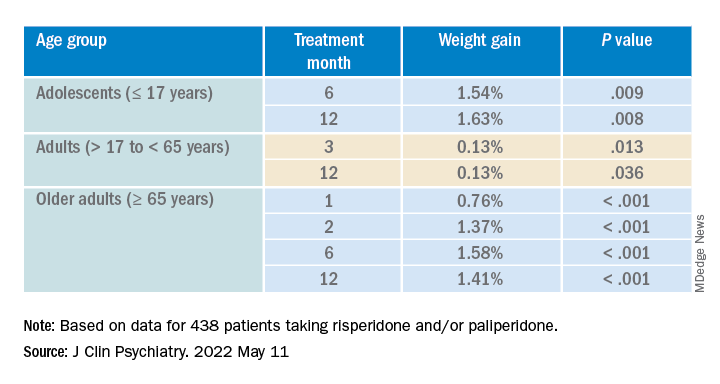
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Early metformin minimizes antipsychotic-induced weight gain
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MAR DEL PLATA, ARGENTINA – , according to a new evidence-based Irish guideline for the management of this common complication in adults with psychoses who are taking medications.
The document was discussed during one of the sessions of the XXXV Argentine Congress of Psychiatry of the Association of Argentine Psychiatrists. The document also was presented by one of its authors at the European Congress on Obesity 2022.
The guideline encourages psychiatrists not to underestimate the adverse metabolic effects of their treatments and encourages them to contemplate and carry out this prevention and management strategy, commented María Delia Michat, PhD, professor of clinical psychiatry and psychopharmacology at the APSA Postgraduate Training Institute, Buenos Aires.
“Although it is always good to work as a team, it is usually we psychiatrists who coordinate the pharmacological treatment of our patients, and we have to know how to manage drugs that can prevent cardiovascular disease,” Dr. Michat said in an interview.
“The new guideline is helpful because it protocolizes the use of metformin, which is the cheapest drug and has the most evidence for antipsychotic-induced weight gain,” she added.
Avoiding metabolic syndrome
In patients with schizophrenia, obesity rates are 40% higher than in the general population, and 80% of patients develop weight gain after their first treatment, noted Dr. Michat. “Right away, weight gain is seen in the first month. And it is a serious problem, because patients with schizophrenia, major depression, or bipolar disorder already have an increased risk of premature mortality, especially from cardiovascular diseases, and they have an increased risk of metabolic syndrome. And we sometimes give drugs that further increase that risk,” she said.
Being overweight is a major criterion for defining metabolic syndrome. Dr. Michat noted that, among the antipsychotic drugs that increase weight the most are clozapine, olanzapine, chlorpromazine, quetiapine, and risperidone, in addition to other psychoactive drugs, such as valproic acid, lithium, mirtazapine, and tricyclic antidepressants.
Several clinical trials, such as a pioneering Chinese study from 2008, have shown the potential of metformin to mitigate the weight gain induced by this type of drug.
However, Dr. Michat noted that so far the major guidelines (for example, the Canadian Network for Mood and Anxiety Treatments [CANMAT]/International Society for Bipolar Disorders [ISBD] for bipolar disorder and the American Psychiatric Association [APA] for schizophrenia) “say very little” on how to address this complication. They propose what she defined as a “problematic” order of action in which the initial emphasis is on promoting lifestyle changes, which are difficult for these patients to carry out, as well as general proposals for changing medication (which is not simple to implement when the patient’s condition is stabilized) and eventual consultation with a clinician to start therapy with metformin or other drugs, such as liraglutide, semaglutide, and topiramate.
The new clinical practice guideline, which was published in Evidence-Based Mental Health (of the BMJ journal group), was written by a multidisciplinary team of pharmacists, psychiatrists, and mental health nurses from Ireland. It aims to fill that gap. The investigators reviewed 1,270 scientific articles and analyzed 26 of them in depth, including seven randomized clinical trials and a 2016 systematic review and meta-analysis. The authors made a “strong” recommendation, for which there was moderate-quality evidence, that for patients for whom a lifestyle intervention is unacceptable or inappropriate the use of metformin is an “alternative first-line intervention” for antipsychotic drug–induced weight gain.
Likewise, as a strong recommendation with moderate-quality evidence, the guidance encourages the use of metformin when nonpharmacologic intervention does not seem to be effective.
The guideline also says it is preferable to start metformin early for patients who gain more than 7% of their baseline weight within the first month of antipsychotic treatment. It also endorses metformin when weight gain is established.
Other recommendations include evaluating baseline kidney function before starting metformin treatment and suggest a dose adjustment when the estimated glomerular filtration rate (eGFR) is < 60 mL/min/1.73 m2. The guidance says the use of metformin is contraindicated for patients in whom eGFR is <30 mL/min per 1.73 m2. The proposed starting dosage is 500 mg twice per day with meals, with increments of 500 mg every 1-2 weeks until reaching a target dose of 2,000 mg/day. The guidance recommends that consideration always be given to individual tolerability and efficacy.
Treatment goals should be personalized and agreed upon with patients. In the case of early intervention, the guideline proposes initially stabilizing the weight gained or, if possible, reverse excess weight. When weight gain is established, the goal would be to lose at least 5% of the weight within the next 6 months.
The authors also recommend monitoring kidney function annually, as well as vitamin B12 levels and individual tolerability and compliance. Gastrointestinal adverse effects can be managed by dose reduction or slower dose titration. The risk of lactic acidosis, which affects 4.3 per 100,000 person-years among those taking metformin, can be attenuated by adjusting the dose according to kidney function or avoiding prescribing it to patients who have a history of alcohol abuse or who are receiving treatment that may interact with the drug.
Validating pharmacologic management
The lead author of the new guideline, Ita Fitzgerald, a teacher in clinical pharmacy and senior pharmacist at St. Patrick’s Mental Health Services in Dublin, pointed out that there is a bias toward not using drugs for weight management and shifting the responsibility onto the patients themselves, something that is very often out of their control.
“The purpose of the guideline was to decide on a range of criteria to maximize the use of metformin, to recognize that for many people, pharmacological management is a valid and important option that could and should be more widely used and to provide precise and practical guidance to physicians to facilitate a more widespread use,” Ms. Fitzgerald said in an interview.
According to Fitzgerald, who is pursuing her doctorate at University College Cork (Ireland), one of the most outstanding results of the work is that it highlights that the main benefit of metformin is to flatten rather than reverse antipsychotic-induced weight gain and that indicating it late can nullify that effect.
“In all the recommendations, we try very hard to shift the focus from metformin’s role as a weight reversal agent to one as a weight management agent that should be used early in treatment, which is when most weight gain occurs. If metformin succeeds in flattening that increase, that’s a huge potential benefit for an inexpensive and easily accessible drug. When people have already established weight gain, metformin may not be enough and alternative treatments should be used,” she said.
In addition to its effects on weight, metformin has many other potential health benefits. Of particular importance is that it reduces hyperphagia-mediated antipsychotic-induced weight gain, Ms. Fitzgerald pointed out.
“This is subjectively very important for patients and provides a more positive experience when taking antipsychotics. Antipsychotic-induced weight gain is one of the main reasons for premature discontinuation or incomplete adherence to these drugs and therefore needs to be addressed proactively,” she concluded.
Ms. Fitzgerald and Dr. Michat have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Dexmedetomidine sublingual film for agitation
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Antipsychotic safe, effective for resistant depression in phase 3 trial
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new results from a phase 3 study show.
Already approved by the U.S. Food and Drug Administration to treat adults with schizophrenia and manic, mixed, or depressive episodes of bipolar I disorder, cariprazine is under investigation as an add-on therapy for MDD.
“Even patients who appear to be nonresponsive to standard antidepressant drugs have a very good chance of responding” to cariprazine, lead study author Gary Sachs, MD, associate clinical professor of psychiatry at Massachusetts General Hospital, Boston, told this news organization.
He noted that cariprazine, which is a partial agonist at D2 and D3, as well as 5-HT1A, “is an entirely different class” of drugs.
“It’s worth understanding how to use drugs like cariprazine and expanding our nomenclature; instead of referring to these drugs as atypical antipsychotics, perhaps referring to them as atypical antidepressants makes more sense,” Dr. Sachs said.
The findings were presented at the annual meeting of the American Psychiatric Association.
More options critical
MDD is among the most common psychiatric disorders in the United States. In 2020, an estimated 21 million adults had at least one major depressive episode.
Previous research has shown almost half of patients with MDD do not experience satisfactory results from their current treatment regimen. Therefore, research on more options for patients is critical, Dr. Sachs said.
Results from a previously published placebo-controlled study showed adjunctive treatment with cariprazine at 2-mg to 4.5-mg per day doses was more effective than placebo in improving depressive symptoms in adults with MDD.
The new analysis included patients with MDD and an inadequate response to antidepressant therapy, including selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants. They were recruited from 116 centers in the United States and Europe.
Dr. Sachs noted that a nonresponse to an adequate dose of an antidepressant typically means having less than a 50% improvement over 6 weeks or more.
Researchers randomly assigned the patients to oral cariprazine 1.5 mg/day, cariprazine 3 mg/day, or placebo. All continued to take their antidepressant monotherapy.
The analysis included 757 mostly White participants (mean age, 44.8 years; 73.4% women). All had experienced depression for a “huge” part of their life (average, about 14 years), “not to mention their adult life,” said Dr. Sachs.
In addition, at the start of the study, the participants had been depressed for almost 8 months on average.
The primary endpoint was change at week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score. The mean baseline MADRS total score was 32.5.
Less is sometimes more
Results showed a significantly greater mean reduction in MADRS total score for cariprazine 1.5 mg/day vs. placebo at week 6 (P = .005). Significant differences from placebo were observed as early as week 2 and were maintained at week 4, as well as week 6.
“I can say with great confidence that the 1.5-mg dose met all the standards for efficacy,” Dr. Sachs said.
However, this was not the case for the 3-mg/day dose. Although there was a numerically greater reduction in MADRS total score for this dosage of the drug vs. placebo at week 6, the difference was not statistically significant (P = .07).
At week 6, more patients taking the active drug at 1.5 mg/day than placebo responded to treatment, defined as 50% or greater reduction in MADRS total score (44% vs. 34.9%, respectively; P < .05).
Researchers also assessed scores on the Clinical Global Impressions, finding significantly greater score improvement for both the 1.5-mg/day (P = .0026) and 3-mg/day (P =.0076) groups vs. the placebo group.
Improvement at week 6 in mean total score on the Hamilton Depression Rating Scale (HAM-17) reached nominal significance for cariprazine 1.5 mg/day vs. placebo – but not for 3 mg/day.
The results of this “high-quality” double-blind, randomized, controlled, parallel group study provide “what I regard as proven efficacy,” Dr. Sachs said.
He added that the investigational drug was also relatively safe. “The vast majority of patients tolerated it quite well,” he stressed. In addition, the drop-out rate because of adverse events was “quite low overall.”
The only adverse events (AEs) that occurred with the active treatment at a frequency of 5% or more and double that of placebo were akathisia and nausea. Changes in weight were relatively small, at less than 1 kg, in all treatment groups.
There was one serious AE in each active drug group, one of which was a kidney infection. There were two serious AEs reported in the placebo group, including one patient with multiple sclerosis. There were no deaths.
Dr. Sachs noted an advantage of cariprazine is its long half-life, which makes it more user-friendly because “it forgives you if you miss a dose or two.”
Drug manufacturer AbbVie’s supplemental New Drug Application for cariprazine is currently under review by the FDA for expanded use as adjunctive treatment of MDD. A decision by the agency is expected by the end of this year.
Another potential treatment option
Commenting on the findings, James Murrough, MD, PhD, associate professor of psychiatry and of neuroscience and director of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai, New York, said he welcomes research into additional treatments for MDD.
“Each medicine in a particular class has a unique pharmacology, so a larger number of medication options may help the clinician find a good match for a particular patient,” said Dr. Murrough, who was not involved with the research.
He noted cariprazine is “somewhat unique” among the dopamine modulators in “preferring interactions with the D3 receptor, one of many types of dopamine receptors.”
Although the study results showed cariprazine was effective in MDD, it “does not entirely break new ground” because previous research has already established the drug’s efficacy as adjunctive therapy for patients with depression not responding to a standard antidepressant, said Dr. Murrough.
He also noted that the lower dose, but not the higher dose, of the drug was found to be significantly beneficial for patients, compared with placebo.
“This is a good reminder that higher doses of a medication are not always better,” Dr. Murrough said.
The study was funded by AbbVie. Dr. Sachs is a full-time employee of Signant Health, which conducted the training and quality control for this study. Dr. Murrough has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Lithium lowers osteoporosis risk in bipolar patients…and orthopedists take notice
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
NEW ORLEANS –
“Our findings emphasize that bone health should be a priority in the clinical management of bipolar disorder, and that the potential bone-protective effects of lithium should be subjected to further study – both in the context of osteoporosis and bipolar disorder,” said Soren D. Ostergaard, MD, PhD, the study’s first author and a professor in the psychosis research unit, Aarhus (Denmark) University Hospital – Psychiatry.
For the retrospective cohort study, presented at the annual meeting of the American Psychiatric Association, and also published recently in JAMA Psychiatry, the authors reviewed data on 22,912 patients treated for bipolar disorder in Denmark between 1996 and 2019, and compared each patient with 5 age- and sex-matched controls, amounting to 114,560 individuals in the general population.
Of the patients with bipolar disorder, 38.2% were treated with lithium, while 73.6% received an antipsychotic drug; 16.8% received valproate and 33.1% received lamotrigine.
With a median follow-up of 7.7 years, the incidence of osteoporosis per 1,000 person-years was 8.70 among patients with bipolar disorder, compared with an incidence of 7.84 among controls, (hazard rate ratio, 1.15).
The association of bipolar disorder with osteoporosis was notably more pronounced among males (HRR, 1.42) compared with females (HRR, 1.07).
Notably, those with bipolar disorder treated with lithium showed a significantly reduced risk of osteoporosis compared with patients not receiving lithium (HRR, 0.62), after adjustment for factors including age, sex, Charlson Comorbidity Index, use of systemic corticosteroids, use of sedative medication, and eating disorder diagnosis. No similar reductions in osteoporosis risk were observed among those treated with antipsychotics, valproate or lamotrigine.
Of note, the reduced risk of osteoporosis with lithium appeared after about year 2 of treatment (HR, 0.77) and remained steady at more than 4 years (HR, 0.76). A higher cumulative lithium dose was meanwhile associated with a greater decrease in the risk of osteoporosis (P < .001).
Results confirm prior research
The results are consistent with previous smaller studies indicating that people with bipolar disorders shown an increased risk of low bone density, osteopenia, and even fracture.
The higher risk of osteoporosis in bipolar disorder may be explained by lifestyle factors, Dr. Ostergaard noted in an interview.
“It could be the depressive and manic phases in bipolar disorder, but generally speaking, both phases can lead to an unhealthy lifestyle and that’s likely what drives the association between bipolar disorder and osteoporosis,” he said. “Increases in behaviors such as smoking and alcohol consumption may be factors as well. Similar findings are seen with depression.”
While more needs to be understood, Dr. Ostergaard speculated that higher rates of such behaviors in men with bipolar disorder may explain the higher osteoporosis risk observed in men.
In general, however, the increased risk underscores the importance of raising awareness of bone health among patients with bipolar disorder, the authors concluded.
“Specifically, guiding patients toward a lifestyle supporting bone health (no smoking, reduced alcohol consumption, healthy diet, and exercising) and monitoring bone density via dual-energy x-ray absorptiometry scans among those with additional risk factors seems warranted,” they wrote.
The implications of the lithium findings are trickier to determine, Dr. Ostergaard said.
“The evidence for lithium in bipolar disorder are well established, and our findings don’t really add to that,” he said. “The main thing is it suggests there might be some advantages of lithium that we’re not really aware of.”
Findings important for orthopedists
The unique properties observed with lithium have caught the attention of some in orthopedics, and researchers with the University of Toronto – having found intriguing bone healing with lithium in preclinical rodent studies – are currently conducting a first-of-its-kind multicenter, randomized, controlled clinical trial evaluating the potential effects of lithium in the healing of bone fractures.
Diane Nam, MD, of the division of orthopedic surgery, Sunnybrook Health Sciences Centre, Toronto, and lead investigator on the study, said in an interview that “I’m not surprised by [Dr. Ostergaard’s] paper because it’s consistent with what we have observed about the positive effects on bone healing.”
Dr. Nam and associates have already established administration parameters for their clinical study, determining that optimal effects in fracture healing appear to require that lithium treatment not begin at the time of fracture, but 2 weeks afterward, when new bone is ready to be laid down at the fracture site. In their trial, low daily doses of lithium (at 300 mg) are given only for a duration of 2 weeks.
“While our current trial is intended for a healthy, nonosteoporotic adult population, we have also demonstrated in our preclinical studies that lithium is just as effective in improving fracture healing in an osteoporotic model when the timing of administration is slightly delayed,” she said. “How this is relevant and translatable in patients with bipolar disorder requires further study.”
Dr. Nam said her research team thinks that “not only will the fracture heal faster, but it will heal reliably as delayed or impaired fracture healing remains a significant orthopedic problem.”
While details are not yet available, a preliminary analysis has shown results “going in a positive direction,” enough for the team to be granted funding for the multicenter trial.
Dr. Ostergaard and Dr. Nam reported no disclosures or conflicts.
AT APA 2022
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.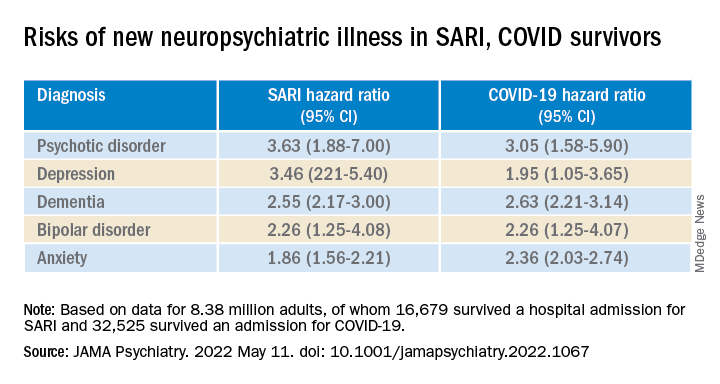
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.