User login
Do psychotropic meds raise or lower COVID risk in psych patients?
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Mood instability in childhood as a precursor to bipolar disorder
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at dmiklowitz@mednet.ucla.edu.
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Higher ‘chemical restraint’ rates in Black psych patients in the ED
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The woman who kept passing out
CASE An apparent code blue
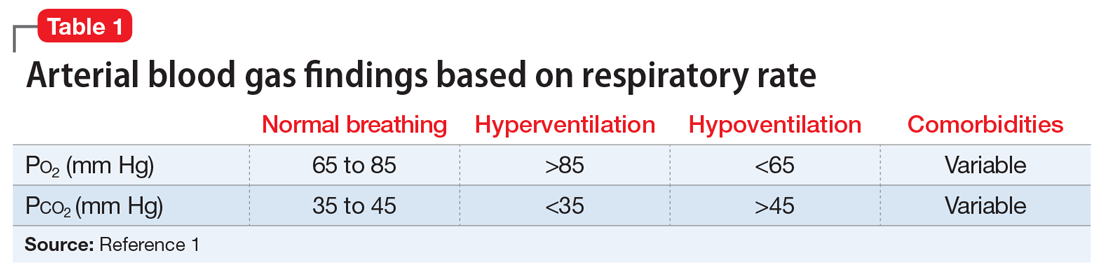
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
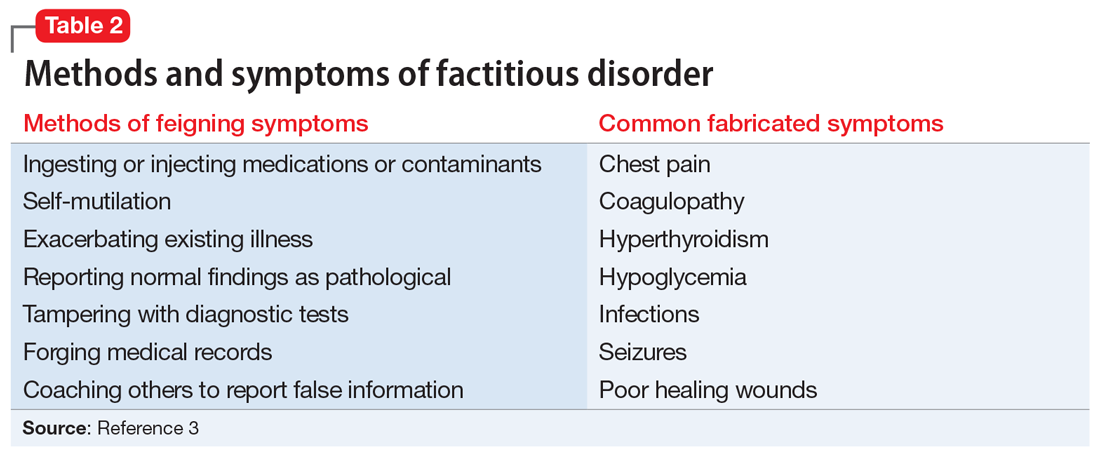
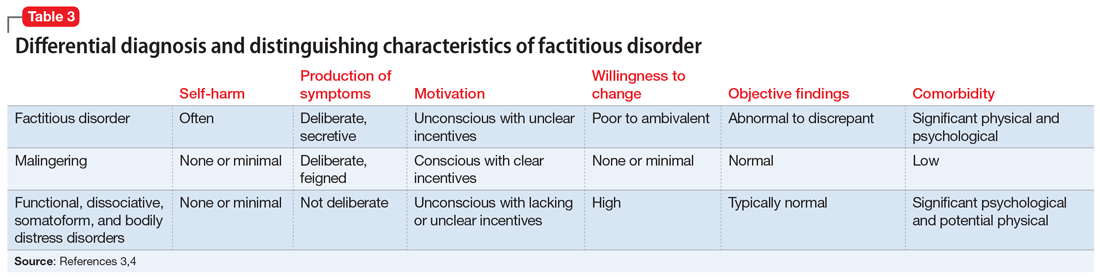
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
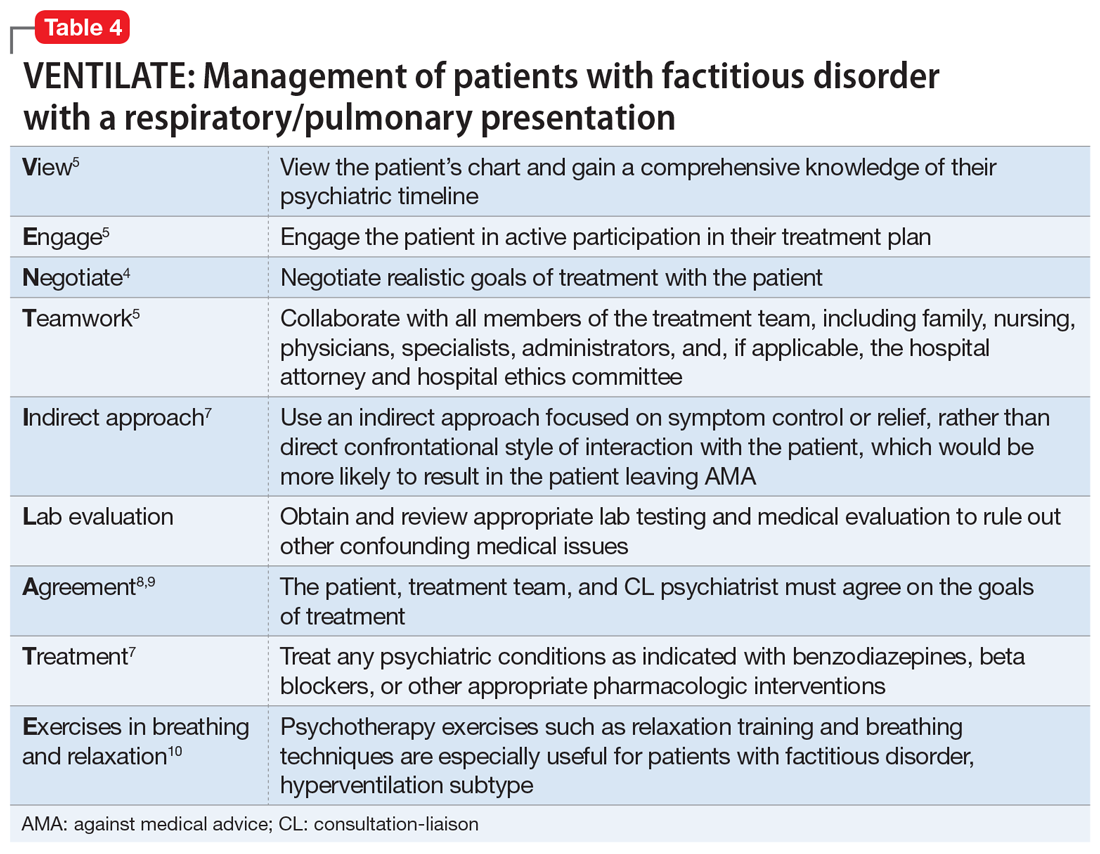
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
Managing bipolar disorder in women who are pregnant
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
High antipsychotic switch rates suggest ‘suboptimal’ prescribing for first-episode psychosis
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large-scale, real-world analysis of U.K. prescribing patterns, researchers found more than two-thirds of patients who received antipsychotics for FEP switched medication, and almost half switched drugs three times.
Although this is “one of the largest real-world studies examining antipsychotic treatment strategies,” it reflects findings from previous, smaller studies showing “antipsychotic switching in first episode psychosis is high,” said study investigator Aimee Brinn, Institute of Psychiatry, Psychology & Neuroscience at King’s College London.
This may reflect reports of poor efficacy and suggests that first-line prescribing is “suboptimal,” Ms. Brinn noted. In addition, olanzapine remains the most popular antipsychotic for prescribing despite recent guidelines indicating it is “not ideal ... due to its dangerous metabolic side effects,” she added.
The findings were presented at the Congress of the Schizophrenia International Research Society (SIRS) 2022.
Real-world data
The response to, and tolerability of, antipsychotics differs between patients with FEP; and prescribing patterns “reflect clinician and patient-led decisionmaking,” Ms. Brinn told meeting attendees.
Since randomized controlled trials “do not necessarily reflect prescribing practice in real-world clinical settings,” the researchers gathered data from a large mental health care electronic health record dataset.
The investigators examined records from the South London and Maudsley NHS Foundation Trust (SLaM), which has a catchment area of 1.2 million individuals across four boroughs of London. The group sees approximately 37,500 active patients per week.
The team used the Clinical Interactive Record Search tool to extract data on 2,309 adults with FEP who received care from a SLaM early intervention in psychosis service between April 1, 2008, and March 31, 2019.
They found that 12 different antipsychotics were prescribed as first-line treatment. The most common were olanzapine (43.9%), risperidone (24.7%), and aripiprazole (19.9%).
Results showed that over 81,969.5 person-years of follow-up, at a minimum of 24 months per patient, 68.8% had an antipsychotic switch. The most common first treatment switch, in 17.9% of patients, was from olanzapine to aripiprazole.
Of patients who switched to aripiprazole, 48.4% stayed on the drug, 26% switched back to olanzapine, and 25.6% received other treatment. Overall, 44.7% of patients switched medication at least three times.
Among patients with FEP who did not switch, 42.2% were prescribed olanzapine, 26.2% risperidone, 23.3% aripiprazole, 5.6% quetiapine, and 2.7% amisulpride.
During the post-presentation discussion, Ms. Brinn was asked whether the high rate of first-line olanzapine prescribing could be because patients started treatment as inpatients and were then switched once they were moved to community care.
“We found that a lot of patients would be prescribed olanzapine for around 7 days at the start of their prescription and then switch,” Ms. Brinn said, adding it is “likely” they started as inpatients. The investigators are currently examining the differences between inpatient and outpatient prescriptions to verify whether this is indeed the case, she added.
‘Pulling out the big guns too fast?’
Commenting on the findings, Thomas W. Sedlak, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, said the study raises a “number of questions.”
Both olanzapine and risperidone “tend to have higher treatment effect improvements than aripiprazole, so it’s curious that a switch to aripiprazole was common,” said Dr. Sedlak, who was not involved with the research.
“Are we pulling out the ‘big guns’ too fast, or inappropriately, especially as olanzapine and risperidone carry greater risk of weight gain?” he asked. In addition, “now that olanzapine is available with samidorphan to mitigate weight gain, will that shape future patterns, if it can be paid for?”
Dr. Sedlak noted it was unclear why olanzapine was chosen so often as first-line treatment in the study and agreed it is “possible that hospitalized patients had been prescribed a ‘stronger’ medication like olanzapine compared to never-hospitalized patients.”
He also underlined that it is “not clear if patients in this FEP program are representative of all FEP patients.”
“For instance, if the program is well known to inpatient hospital social workers, then the program might be disproportionately filled with patients who have had more severe symptoms,” Dr. Sedlak said.
The study was supported by Janssen-Cilag. The investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
New combination med for severe mental illness tied to less weight gain
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
, new research suggests. However, at least one expert says the weight difference between the two drugs is of “questionable clinical benefit.”
Last year, the Food and Drug Administration approved the drug for the treatment of adults with schizophrenia or bipolar I disorder, as a maintenance monotherapy or as either monotherapy or an adjunct to lithium or valproate for acute manic or mixed episodes.
In the ENLIGHTEN-Early trial, researchers examined weight-gain profiles of more than 400 patients with early schizophrenia, schizophreniform disorder, or bipolar I disorder.
Results showed those given combination treatment gained just over half the amount of weight as those given monotherapy. They were also 36% less likely to gain at least 10% of their body weight during the 12-week treatment period.
They indicate that the weight-mitigating effects shown with olanzapine plus samidorphan are “consistent, regardless of the stage of illness,” Dr. Kahn added.
He presented the findings at the annual congress of the Schizophrenia International Research Society.
Potential benefit
“Early intervention with antipsychotic treatment is critical in shaping the course of treatment and the disease trajectory,” coinvestigator Christine Graham, PhD, with Alkermes, which manufactures the drug, told this news organization.
Olanzapine is a “highly effective antipsychotic, but it’s really avoided a lot in this population,” Dr. Graham said. Therefore, patients “could really stand to benefit” from a combination that delivers the same amount of antipsychotic effect, but “reduces the propensity” for clinically significant weight gain, she added.
Dr. Kahn noted in his meeting presentation that antipsychotics are the “cornerstone” of the treatment of serious mental illness, but that “many are associated with concerning weight gain and cardiometabolic effects.”
While olanzapine is an effective medication, it has “one of the highest weight gain” profiles of the available antipsychotics and patients early on in their illness are “especially vulnerable,” Dr. Kahn said.
Previous studies have shown the combination of olanzapine plus samidorphan is similarly effective as olanzapine, but is associated with less weight gain.
To determine its impact in recent-onset illness, the current researchers screened patients with schizophrenia, schizophreniform disorder, or bipolar I disorder. The patients were aged 16-39 years and had an initial onset of active phase symptoms less than 4 years previously. They had less than 24 weeks’ cumulative lifetime exposure to antipsychotics.
Participants were randomly assigned to receive olanzapine plus samidorphan or olanzapine alone for 12 weeks, and then followed up for safety assessment for a further 4 weeks.
A total of 426 patients were recruited and 76.5% completed the study. The mean age was 25.8 years, 66.2% were men, 66.4% were White, and 28.2% were Black.
The mean body mass index at baseline was 23.69 kg/m2. The most common diagnosis among the participants was schizophrenia (62.9%) followed by bipolar I disorder (21.6%).
Less weight gain
Results of the 12-week study showed a significant difference in percent change in body weight from baseline between the two treatment groups, with a gain of 4.91% for the olanzapine plus samidorphan group vs. 6.77% for the olanzapine-alone group (between-group difference, 1.87%; P = .012).
Dr. Kahn noted this equates to an average weight gain of 2.8 kg (6.2 pounds) with olanzapine plus samidorphan and a gain of about 5 kg (11pounds) with olanzapine.
“It’s not a huge difference, but it’s certainly a significant one,” he said. “I also think it’s clinically important and significant.”
The reduction in weight gain compared with olanzapine was even maintained in patients assigned to olanzapine plus samidorphan who dropped out and did not complete the study, Dr. Kahn reported. “No one really had a weight gain,” he said.
In contrast, patients in the olanzapine groups who dropped out of the study had weight gain larger than their counterparts who stayed in it.
Further analysis showed the proportion of patients who gained 10% or more of their body weight by week 12 was 21.9% for those receiving olanzapine plus samidorphan vs. 30.4% for those receiving just olanzapine (odds ratio, 0.64; P = .075).
As expected, the improvement in Clinical Global Impression–Severity scale scores was almost identical between the olanzapine + samidorphan and olanzapine-only groups.
For safety, Dr. Kahn said the adverse event rates were “very, very similar” between the two treatment arms, which was a pattern that was repeated for serious AEs. This led him to note that “nothing out of the ordinary” was observed.
Clinical impact 'questionable'
Commenting on the study, Laura LaChance, MD, a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said the actual amount of weight loss shown in the study “is of questionable clinical significance.”
On the other hand, Dr. LaChance said she has achieved “better results with metformin, which has a great safety profile and is cheap and widely available.
“Cost is always a concern in patients with psychotic disorders,” she concluded.
The study was funded by Alkermes. Dr. Kahn reported having relationships with Alkermes, Angelini, Janssen, Sunovion, Otsuka, Merck, Minerva Neuroscience, Roche, and Teva. Dr. Graham is an employee of Alkermes.
A version of this article first appeared on Medscape.com.
FROM SIRS 2022
FDA okays first sublingual med for agitation in serious mental illness
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
This is the first FDA-approved, orally dissolving, self-administered sublingual treatment for this indication. With a demonstrated onset of action as early as 20 minutes, it shows a high response rate in patients at both 120-mcg and 180-mcg doses.
An estimated 7.3 million individuals in the United States are diagnosed with schizophrenia or bipolar disorders, and up to one-quarter of them experience episodes of agitation that can occur 10-17 times annually. These episodes represent a significant burden for patients, caregivers, and the health care system.
“There are large numbers of patients who experience agitation associated with schizophrenia and bipolar disorders, and this condition has been a long-standing challenge for health care professionals to treat,” said John Krystal, MD, the Robert L. McNeil Jr. Professor of Translational Research and chair of the department of psychiatry at Yale University, New Haven, Conn.
“The approval of Igalmi, a self-administered film with a desirable onset of action, represents a milestone moment. It provides health care teams with an innovative tool to help control agitation. As clinicians, we welcome this much-needed new oral treatment option,” he added.
“Igalmi is the first new acute treatment for schizophrenia or bipolar disorder–associated agitation in nearly a decade and represents a differentiated approach to helping patients manage this difficult and debilitating symptom,” said Vimal Mehta, PhD, CEO of BioXcel Therapeutics.
The FDA approval of Igalmi is based on data from two pivotal randomized, double-blinded, placebo-controlled, parallel-group, phase 3 trials that evaluated Igalmi for the acute treatment of agitation associated with schizophrenia (SERENITY I) or bipolar I or II disorder (SERENITY II).
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were somnolence, paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. All adverse drug reactions were mild to moderate in severity. While Igalmi was not associated with any treatment-related serious adverse effects in phase 3 studies, it may cause notable side effects, including hypotension, orthostatic hypotension, bradycardia, QT interval prolongation, and somnolence.
As previously reported by this news organization, data from the phase 3 SERENITY II trial that evaluated Igalmi in bipolar disorders were published in JAMA.
A version of this article first appeared on Medscape.com.
The importance of treating insomnia in psychiatric illness
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Data suggests this symptom, defined as chronic sleep onset and/or sleep continuity problems associated with impaired daytime functioning, is common in psychiatric illnesses, and can worsen their course.2
The incidence of psychiatric illness in patients with insomnia is estimated at near 50%, with the highest rates found in mood disorders such as depression and bipolar disorder, as well as anxiety disorders.3 In patients with diagnosed major depressive disorder, insomnia rates can approach 90%.4-6
Insomnia has been identified as a risk factor for development of mental illness, including doubling the risk of major depressive disorder and tripling the risk of any depressive or anxiety disorder.7,8 It can also significantly increase the risk of alcohol abuse and psychosis.8
Sleep disturbances can worsen symptoms of diagnosed mental illness, including substance abuse, mood and psychotic disorders.9-10 In one study, nearly 75% of patients with a diagnosis of schizophrenia or bipolar spectrum disorder had at least one type of sleep disturbance (insomnia, hypersomnia, or delayed sleep phase).10 This was almost twice the rate in healthy controls. Importantly, compared with well-rested subjects with mental illness in this study, sleep-disordered participants had higher rates of negative and depressive symptoms on the Positive and Negative Syndrome Scale, as well as significantly lower function via the global assessment of functioning.11,12
Additional data suggests simply being awake during the night (00:00-05:59) elevates risk of suicide. The mean incident rate of completed suicide in one study was a striking four times the rate noted during daytime hours (06:00-23:59 ) (P < .001).13
Although insomnia symptoms can resolve after relief from a particular life stressor, as many as half of patients with more severe symptoms develop a chronic course.14 This then leads to an extended use of many types of sedative-hypnotics designed and studied primarily for short-term use.15 In a survey reviewing national use of prescription drugs for insomnia, as many as 20% of individuals use a medication to target insomnia in a given month.16
Fortunately, despite the many challenges posed by COVID-19, particularly for those with psychiatric illness and limited access to care, telehealth has become more readily available. Additionally, digital versions of evidence-based treatments specifically for sleep problems, such as cognitive-behavioral therapy for insomnia (CBT-I), are regularly being developed.
The benefits of CBT-I have been demonstrated repeatedly and it is recommended as the first line treatment for insomnia by the Clinical Guidelines of the American Academy of Sleep Medicine, the Centers for Disease Control and Prevention, and the National Institutes of Health.17-21 Studies suggest benefits persist long-term, even after completing the therapy sessions, which differ in durability from medication choices.18
One group that may be particularly suited for treatment with CBT-I is women with insomnia during pregnancy or the postpartum period. In these women, options for treatment may be limited by risk of medication during breastfeeding, as well as difficulty traveling to a physician’s or therapist’s office to receive psychotherapy. However, two recent studies evaluated the use of digital CBT-I to treat insomnia during pregnancy and in the postpartum period, respectively.22-23
In both studies,the same group of women with insomnia diagnosed during pregnancy were given six weekly 20-minute sessions of digital CBT-I or standard treatment for insomnia, including medication and psychotherapy per their usual provider.
By study end, the pregnant women receiving the CBT-I intervention not only had significantly improved severity of insomnia, they also experienced improved depression and anxiety symptoms, and a decrease in the use of prescription or over-the-counter sleep aides, compared with the standard treatment group, lowering the fetal exposure to medication during pregnancy.22
In the more recent study, the same group was followed for 6 months post partum.23 Results were again notable, with the women who received CBT-I reporting significantly less insomnia, as well as significantly lower rates of probable major depression at 3 and 6 months (18% vs. 4%, 10% vs. 0%, respectively.) They also exhibited lower rates of moderate to severe anxiety (17% vs. 4%) at 3 months, compared with those receiving standard care. With as many as one in seven women suffering from postpartum depression, these findings represent a substantial public health benefit.
In summary, insomnia is a critical area of focus for any provider diagnosing and treating psychiatric illness. Attempts to optimize sleep, whether through CBT-I or other psychotherapy approaches, or evidence-based medications dosed for appropriate lengths and at safe doses, should be a part of most, if not all, clinical encounters.
Dr. Reid is a board-certified psychiatrist and award-winning medical educator with a private practice in Philadelphia, as well as a clinical faculty role at the University of Pennsylvania, also in Philadelphia. She attended medical school at Columbia University, New York, and completed her psychiatry residency at the University of California, Los Angeles. Dr. Reid is a regular contributor to Psychology Today with her blog, “Think Like a Shrink,” and writes and podcasts as The Reflective Doc.
References
1. Voitsidis P et al. Psychiatry Res. 2020 Jul;289:113076. doi: 10.1016/j.psychres.2020.113076.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, Va.: American Psychiatric Publishing, 2013.
3. Ford DE and Kamerow DB. JAMA. 1989;262(11):1479-84. doi: 10.1001/jama.1989.03430110069030.
4. Ohayon MM and Roth T. J Psychiatr Res. Jan-Feb 2003;37(1):9-15. doi: 10.1016/s0022-3956(02)00052-3.
5. Seow LSE et al. J Ment Health. 2016 Dec;25(6):492-9. doi: 10.3109/09638237.2015.1124390.
6. Thase ME. J Clin Psychiatry. 1999;60 Suppl 17:28-31; discussion 46-8.
7. Baglioni C et al. J Affect Disord. 2011 Dec;135(1-3):10-9. doi: 10.1016/j.jad.2011.01.011.
8. Hertenstein E et al. Sleep Med Rev. 2019 Feb;43:96-105. doi: 10.1016/j.smrv.2018.10.006.
9. Brower KJ et al. Medical Hypotheses. 2010;74(5):928-33. doi: 10.1016/j.mehy.2009.10.020.
10. Laskemoen JF et al. Compr Psychiatry. 2019 May;91:6-12. doi: 10.1016/j.comppsych.2019.02.006.
11. Kay SR et al. Schizophr Bull. 1987;13(2):261-76. doi: 10.1093/schbul/13.2.261.
12. Hall R. Psychosomatics. May-Jun 1995;36(3):267-75. doi: 10.1016/S0033-3182(95)71666-8.
13. Perlis ML et al. J Clin Psychiatry. 2016 Jun;77(6):e726-33. doi: 10.4088/JCP.15m10131.
14. Morin CM et al. Arch Intern Med. 2009 Mar 9. doi: 10.1001/archinternmed.2008.610.
15. Cheung J et al. Sleep Med Clin. 2019 Jun;14(2):253-65. doi: 10.1016/j.jsmc.2019.01.006.
16. Bertisch SM et al. Sleep. 2014 Feb 1. doi: 10.5665/sleep.3410.
17. Okajima I et al. Sleep Biol Rhythms. 2010 Nov 28. doi: 10.1111/j.1479-8425.2010.00481.x.
18. Trauer JM et al. Ann Intern Med. 2015 Aug 4. doi: 10.7326/M14-2841.
19. Edinger J et al. J Clin Sleep Med. 2021 Feb 1. doi: 10.5664/jcsm.8986.
20. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/for-clinicians.html.
21. National Institutes of Health. Sleep Health. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/sleep-health.
22. Felder JN et al. JAMA Psychiatry. 2020;77(5):484-92. doi:10.1001/jamapsychiatry.2019.4491.
23. Felder JN et al. Sleep. 2022 Feb 14. doi: 10.1093/sleep/zsab280.
Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
Mr. B, age 64, is being treated in the psychiatric clinic for generalized anxiety disorder. He also has a history of type 2 diabetes mellitus and osteoarthritis. His present medications include metformin 500 mg twice daily, escitalopram 20 mg/d, and a multivitamin.
Three months after a shingles outbreak on his left trunk, Mr. B develops a sharp, burning pain and hypersensitivity to light in the same area as the shingles flare-up. He is diagnosed with postherpetic neuralgia. Despite a 12-week trial of cognitive-behavioral therapy, Mr. B continues to report excessive worry, irritability, poor concentration, psychomotor restlessness, and poor sleep.
Contrasting with the serendipitous discovery of iproniazid and chlorpromazine leading to the development of the current spectrum of antidepressant and antipsychotic agents, discovery of the benefits various antiepileptic agents have in bipolar disorder has not led to a similar proliferation of medication development for bipolar mania or depression.1-3 Divalproex, one of the most commonly used antiepileptic drugs (AEDs) in psychiatry, was thought to be an inactive organic solvent until it was used in 1962 to test the anticonvulsant activity of other compounds. This led to the discovery and subsequent use of divalproex in patients with epilepsy, followed by FDA approval in bipolar disorder.4,5 Off-label use of many AEDs as mood-stabilizing agents in bipolar disorder led to the emergence of carbamazepine, divalproex, and lamotrigine, which joined lithium as classic mood-stabilizing agents.4,6-8 Amid varying definitions of “mood stabilizer,” many AEDs have failed to demonstrate mood-stabilizing effects in bipolar disorder and therefore should not all be considered mood stabilizers.9 Nonetheless, the dual use of a single AED for both psychiatric and nonpsychiatric indications can decrease polypharmacy and increase acceptability of medications in patients who have low insight into their illness.10,11
Because AEDs were originally purposed to treat neurologic disease, psychiatric indications must first be established before considering other indications. AEDs as a class have broad pharmacologic actions, but are generally CNS depressants, decreasing brain signaling through mechanisms such as ion channel antagonism (carbamazepine, gabapentin) or alterations to gamma-aminobutyric acid/glutamate signaling (divalproex, topiramate).4,6,12,13 Compared to antidepressants and antipsychotics, whose primary use for psychiatric conditions is firmly rooted in evidence, rational use of AEDs for psychiatric conditions and symptoms depends on the agent-specific efficacy. Patients with comorbid psychiatric and neurologic disorders are ideal candidates for dually indicated AEDs due to these agents’ class effects rooted in epilepsy. Due to the history of positive psychiatric benefits with AEDs, newer agents may be psychiatrically beneficial but will likely follow the discovery of these benefits in patients for whom epilepsy is the primary diagnosis.
Consider the limitations
Using AEDs to reduce polypharmacy should be done judiciously from a drug-drug interaction perspective, because certain AEDs (eg, carbamazepine, divalproex) can greatly influence the metabolism of other medications, which may defeat the best intentions of the original intervention.4,6
Several other limitations should be considered. This article does not include all AEDs, only those commonly used for psychiatric indications with known nonpsychiatric benefits. Some may worsen psychiatric conditions (such as rage and irritability in the case of levetiracetam), and all AEDs have an FDA warning regarding suicidal behaviors and ideation.14,15 Another important limitation is the potential for differential dosing across indications; tolerability concerns may limit adequate dosing across multiple uses. For example, topiramate’s migraine prophylaxis effect can be achieved at much lower doses than the patient-specific efficacy dosing seen in binge eating disorder, with higher doses increasing the propensity for adverse effects.13,16Dual-use AEDs should be considered wherever possible, but judicious review of evidence is necessary to appropriately adjudicate a specific patient’s risk vs benefit. The Table4,6-9,12,13,16-68 provides information on select AEDs with both psychiatric and nonpsychiatric indications, including both FDA-approved and common off-label uses. These indications are limited to adult use only.
CASE CONTINUED
After reviewing Mr. B’s medical history, the treating medical team decides to cross-taper escitalopram to duloxetine 30 mg twice daily. Though his pain lessens after several weeks, it persists enough to interfere with Mr. B’s daily life. In addition to duloxetine, he is started on pregabalin 50 mg 3 times a day. Mr. B’s pain decreases to a tolerable level, and he reports decreased worrying and restlessness, and improvements in concentration and sleep.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.
1. Meyer JM. A concise guide to monoamine oxidase inhibitors. Current Psychiatry. 2017;16(12):14-16,18-23,47,A.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. López-Mun
4. Depakote [package insert]. North Chicago, IL: AbbVie, Inc; 2021.
5. Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37 Suppl 2:5-16.
6. Tegretol and Tegretol-XR [package insert]. East Hanover, NJ: Pharmaceuticals Co.; 2020.
7. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
8. Lithobid [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc; 2009.
9. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
10. National Alliance on Mental Illness. Anosognosia. Common with mental illness. Accessed March 3, 2022. https://www.nami.org/About-Mental-Illness/Common-with-Mental-Illness/Anosognosia
11. Hales CM, Servais J, Martin CB, et al. Prescription drug use among adults aged 40-79 in the United States and Canada. NCHS Data Brief. 2019(347):1-8.
12. Neurontin [package insert]. New York, NY: Pfizer; 2017.
13. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
14. Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79-81.
15. U.S. Food & Drug Administration. FDA Statistical Review and Evaluation. Antiepileptic Drugs and Suicidality. 2008. Accessed March 3, 2022. https://www.fda.gov/files/drugs/published/Statistical-Review-and-Evaluation--Antiepileptic-Drugs-and-Suicidality.pdf
16. McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61(9):1039-1048.
17. Zhang ZJ, Kang WH, Tan QR, et al. Adjunctive herbal medicine with carbamazepine for bipolar disorders: a double-blind, randomized, placebo-controlled study. J Psychiatr Res. 2007;41(3-4):360-369.
18. Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42 Suppl 1:2-10.
19. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
20. Davis LL, Bartolucci A, Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord. 2005;85(3):259-266.
21. Gyulai L, Bowden CL, McElroy SL, et al. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology. 2003;28(7):1374-1382.
22. Limdi NA, Shimpi AV, Faught E, et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353-355.
23. Temkin NR, Dikmen SS, Anderson GD, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999; 91(4):593-600.
24. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90.
25. US Dept of Veterans Affairs, US Dept of Defense, The Management of Substance Use Disorders Work Group. VA/DoD clinical practice guideline for the management of substance use disorders. US Dept of Veterans Affairs/Dept of Defense; 2015. Accessed March 3, 2022. http://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
26. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
27. Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
28. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19(4):341-348.
29. Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583-1589.
30. Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27-44.
31. Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336-1344.
32. Alonso-Navarro H, Rubio L, Jiménez-Jiménez FJ. Refractory hiccup: successful treatment with gabapentin. Clin Neuropharmacol. 2007;30(3):186-187.
33. Jatzko A, Stegmeier-Petroianu A, Petroianu GA. Alpha-2-delta ligands for singultus (hiccup) treatment: three case reports. J Pain Symptom Manage. 2007;33(6):756-760.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
35. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938.
36. Yuan M, Zhou HY, Xiao ZL, et al. Efficacy and safety of gabapentin vs. carbamazepine in the treatment of trigeminal neuralgia: a meta-analysis. Pain Pract. 2016;16(8):1083-1091.
37. Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563-581.
38. Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-Foundation. Sleep Med. 2016;21:1-11.
39. Cobin RH, Goodman NF; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause—2017 update [published correction appears in Endocr Pract. 2017;23 (12):1488]. Endocr Pract. 2017;23(7):869-880.
40. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder: Lamictal 614 Study Group. J Clin Psychiatry. 2000;60(11):841-850.
41. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066-1077.
42. Stein G, Bernadt M. Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry. 1993;162:634-640.
43. Craft M, Ismail IA, Krishnamurti D, et al. Lithium in the treatment of aggression in mentally handicapped patients: a double-blind trial. Br J Psychiatry. 1987;150:685-689.
44. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
45. Dickstein G, Shechner C, Adawi F, et al. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454-458.
46. Bogazzi F, Bartalena L, Brogioni S, et al. Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84(2):499-503.
47. Lyrica [package insert]. New York, NY: Parke-Davis, Division of Pfizer Inc; 2020.
48. Lydiard RB, Rickels K, Herman B, et al. Comparative efficacy of pregabalin and benzodiazepines in treating the psychic and somatic symptoms of generalized anxiety disorder. Int J Neuropsychopharmacol. 2010;13(2):229-241.
49. Pande AC, Feltner DE, Jefferson JW, et al. Efficacy of the novel anxiolytic pregabalin in social anxiety disorder: a placebo-controlled, multicenter study. J Clin Psychopharmacol. 2004;24(2):141-149.
50. Vertigan AE, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: a randomized controlled trial. Chest. 2016;149(3):639-648.
51. Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619-625.e6.
52. Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
53. Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1 [published correction appears in J Clin Oncol. 2010;28(10):1808]. J Clin Oncol. 2010;28(4):641-647.
54. Dunlop BW, Papp L, Garlow SJ, et al. Tiagabine for social anxiety disorder. Hum Psychopharmacol. 2007;22(4):241-244.
55. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. J Psychopharmacol. 2010;24(9):1375-1380.
56. Gabitril [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc; 2015.
57. Johnson BA, Ait-Daoud N, Bowden C, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685.
58. Linde M, Mulleners WM, Chronicle EP, et al. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010610.
59. Pascual J, Láinez MJ, Dodick D, et al. Antiepileptic drugs for the treatment of chronic and episodic cluster headache: a review. Headache. 2007;47(1):81-89.
60. Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66(5):672-677.
61. Ko YH, Joe SH, Jung IK, et al. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol. 2005;28(4):169-175.
62. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410.
63. Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007; 30(6):1480-1486.
64. McElroy SL, Kotwal R, Guerdjikova AI, et al. Zonisamide in the treatment of binge eating disorder with obesity: a randomized controlled trial. J Clin Psychiatry. 2006;67(12):1897-1906.
65. Zonegran [package insert]. Teaneck, NJ: Eisai Inc; 2006.
66. Drake ME Jr, Greathouse NI, Renner JB, et al. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004;27(6):278-280.
67. Matsunaga S, Kishi T, Iwata N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: a meta-analysis. J Alzheimers Dis. 2017;56(4):1229-1239.
68. Gadde KM, Kopping MF, Wagner HR 2nd, et al. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557-1564.