User login
‘Impressive’ outcomes sans chemo in poor-prognosis ALL
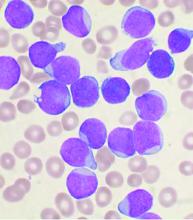
The days of using chemotherapy to treat Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) may be numbered.
In a phase 2 trial, upfront chemotherapy-free induction/consolidation with the tyrosine kinase inhibitor dasatinib (Sprycel) and the bispecific T-cell engager antibody blinatumomab (Blincyto) yielded high rates of molecular response, “impressive” survival at 18 months, and few toxic effects of grade 3 or higher, say researchers.
With this approach, “60% of adult Ph+ ALL patients, of all ages, can obtain a molecular response, and this percent can increase further with more cycles of blinatumomab,” lead researcher Robin Foà, MD, from Sapienza University of Rome, said in an interview.
“The rates of disease-free survival and overall survival at 18 months are highly favorable, and the protocol is associated with limited toxicity,” Dr. Foà added.
“I see this chemo-free approach becoming a realistic approach for a substantial proportion of adult Ph+ ALL patients, particularly for the older patients, keeping in mind that the incidence of Ph+ ALL increases with age,” Dr. Foà said.
The results of the study were published Oct. 22 in the New England Journal of Medicine.
‘Innovative’ and ‘highly successful’
This “innovative” chemotherapy-free approach proved “highly successful” with “surprisingly” few toxic effects, Dieter Hoelzer, MD, PhD, University of Frankfurt (Germany), wrote in a linked editorial.
The Italian GIMEMA LAL2116 D-ALBA trial enrolled 63 adults (median age, 54 years; range, 24-82 years) with newly diagnosed Ph+ ALL. All patients received a glucocorticoid for 31 days beginning 7 days before starting treatment with dasatinib.
Dasatinib (140 mg once daily) induction therapy lasted 85 days. All patients who completed the induction phase received blinatumomab (28 mcg/d) consolidation therapy. Dexamethasone (20 mg) was administered before each blinatumomab cycle. To prevent central nervous system adverse events, levetiracetam (500 mg twice daily) was administered.
All but two patients completed dasatinib induction. One was a 73-year-old woman who withdrew from the trial because of toxic effects after 10 days of dasatinib treatment. She later died of pneumonia. The other was an 82-year-old woman who had a complete hematologic response but left the trial because of pneumonia and pneumonitis.
At the end of the induction phase, 98% of the patients (62 of 63) had a complete hematologic response, including the patient with a complete hematologic response who withdrew; 29% (17 of 59 patients) had a molecular response.
Of the 61 patients who completed the induction phase, 58 received one cycle of blinatumomab, 56 received two cycles, 45 received three cycles, 37 received four cycles, and 29 received five cycles. At the end of the second blinatumomab cycle, 60% of the patients (33 of 55 patients) had a molecular response.
The percentage of patients with a molecular response increased further after receiving additional cycles of blinatumomab – to 70% (28 of 40 patients) after the third cycle, 81% (29 of 36 patients) after the fourth cycle, and 72% (21 of 29 patients) after the fifth cycle.
At a median follow-up of 18 months, overall survival was 95%, and disease-free survival was 88%.
There were no significant differences in DFS between patients with p190-kd fusion protein (85%) and those with p210-kd fusion protein (95%). However, DFS was lower in patients with IKZF1 deletion plus additional genetic aberrations (CDKN2A or CDKN2B, PAX5, or both).
ABL1 mutations were present in six patients who had increased minimal residual disease during induction therapy. All these mutations were cleared by blinatumomab.
There were six relapses, of which three were hematologic. One occurred in a patient with a major protocol violation (a delay of more than 2 months in receiving blinatumomab), one occurred after 12 months in the patient who discontinued the trial after receiving dasatinib for 12 days, and one occurred in a patient after the second cycle of blinatumomab.
A total of 21 adverse events of grade 3 or higher were noted. They included cytomegalovirus reactivation or infection in six patients; neutropenia in four patients; persistent fever in two patients; and pleural effusion, pulmonary hypertension, and a neurologic disorder in one patient each.
Of the 24 patients who received a stem-cell allograft, two died, but only one death was related to transplant (4%).
The very low nonrelapse mortality among patients who underwent transplant during remission is “remarkable,” Dr. Hoelzer wrote. It suggests that toxicity from induction chemotherapy puts the patient at risk for toxic effects and death from subsequent stem-cell transplant – “a consequence that is avoided with targeted therapy.”
Unanswered questions
“Will the excellent outcomes be preserved with longer follow-up? The answer is probably yes, given that the majority of relapses in ALL occur within the first 1.5 to 2.0 years after the initiation of treatment,” Dr. Hoelzer wrote.
He said other outstanding questions include whether long-term outcomes will differ between patients who undergo transplant and those who do not; whether ABL1 mutations emerge; whether minimal residual disease recurs with longer follow-up; and whether this treatment approach can be used for patients with other subtypes of ALL, such as Ph-negative, B-lineage ALL, or even T-cell ALL.
“If these promising trial results hold, chemotherapy-free induction without the immediate and long-term toxic effects of intensive chemotherapy regimens could also be used in adolescents and, finally, in children. These questions will need to be addressed with longer follow-up and large, prospective trials,” Dr. Hoelzer concluded.
The study was supported by grants from the Italian Association for Cancer Research and Sapienza University of Rome.
A version of this article originally appeared on Medscape.com
The days of using chemotherapy to treat Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) may be numbered.
In a phase 2 trial, upfront chemotherapy-free induction/consolidation with the tyrosine kinase inhibitor dasatinib (Sprycel) and the bispecific T-cell engager antibody blinatumomab (Blincyto) yielded high rates of molecular response, “impressive” survival at 18 months, and few toxic effects of grade 3 or higher, say researchers.
With this approach, “60% of adult Ph+ ALL patients, of all ages, can obtain a molecular response, and this percent can increase further with more cycles of blinatumomab,” lead researcher Robin Foà, MD, from Sapienza University of Rome, said in an interview.
“The rates of disease-free survival and overall survival at 18 months are highly favorable, and the protocol is associated with limited toxicity,” Dr. Foà added.
“I see this chemo-free approach becoming a realistic approach for a substantial proportion of adult Ph+ ALL patients, particularly for the older patients, keeping in mind that the incidence of Ph+ ALL increases with age,” Dr. Foà said.
The results of the study were published Oct. 22 in the New England Journal of Medicine.
‘Innovative’ and ‘highly successful’
This “innovative” chemotherapy-free approach proved “highly successful” with “surprisingly” few toxic effects, Dieter Hoelzer, MD, PhD, University of Frankfurt (Germany), wrote in a linked editorial.
The Italian GIMEMA LAL2116 D-ALBA trial enrolled 63 adults (median age, 54 years; range, 24-82 years) with newly diagnosed Ph+ ALL. All patients received a glucocorticoid for 31 days beginning 7 days before starting treatment with dasatinib.
Dasatinib (140 mg once daily) induction therapy lasted 85 days. All patients who completed the induction phase received blinatumomab (28 mcg/d) consolidation therapy. Dexamethasone (20 mg) was administered before each blinatumomab cycle. To prevent central nervous system adverse events, levetiracetam (500 mg twice daily) was administered.
All but two patients completed dasatinib induction. One was a 73-year-old woman who withdrew from the trial because of toxic effects after 10 days of dasatinib treatment. She later died of pneumonia. The other was an 82-year-old woman who had a complete hematologic response but left the trial because of pneumonia and pneumonitis.
At the end of the induction phase, 98% of the patients (62 of 63) had a complete hematologic response, including the patient with a complete hematologic response who withdrew; 29% (17 of 59 patients) had a molecular response.
Of the 61 patients who completed the induction phase, 58 received one cycle of blinatumomab, 56 received two cycles, 45 received three cycles, 37 received four cycles, and 29 received five cycles. At the end of the second blinatumomab cycle, 60% of the patients (33 of 55 patients) had a molecular response.
The percentage of patients with a molecular response increased further after receiving additional cycles of blinatumomab – to 70% (28 of 40 patients) after the third cycle, 81% (29 of 36 patients) after the fourth cycle, and 72% (21 of 29 patients) after the fifth cycle.
At a median follow-up of 18 months, overall survival was 95%, and disease-free survival was 88%.
There were no significant differences in DFS between patients with p190-kd fusion protein (85%) and those with p210-kd fusion protein (95%). However, DFS was lower in patients with IKZF1 deletion plus additional genetic aberrations (CDKN2A or CDKN2B, PAX5, or both).
ABL1 mutations were present in six patients who had increased minimal residual disease during induction therapy. All these mutations were cleared by blinatumomab.
There were six relapses, of which three were hematologic. One occurred in a patient with a major protocol violation (a delay of more than 2 months in receiving blinatumomab), one occurred after 12 months in the patient who discontinued the trial after receiving dasatinib for 12 days, and one occurred in a patient after the second cycle of blinatumomab.
A total of 21 adverse events of grade 3 or higher were noted. They included cytomegalovirus reactivation or infection in six patients; neutropenia in four patients; persistent fever in two patients; and pleural effusion, pulmonary hypertension, and a neurologic disorder in one patient each.
Of the 24 patients who received a stem-cell allograft, two died, but only one death was related to transplant (4%).
The very low nonrelapse mortality among patients who underwent transplant during remission is “remarkable,” Dr. Hoelzer wrote. It suggests that toxicity from induction chemotherapy puts the patient at risk for toxic effects and death from subsequent stem-cell transplant – “a consequence that is avoided with targeted therapy.”
Unanswered questions
“Will the excellent outcomes be preserved with longer follow-up? The answer is probably yes, given that the majority of relapses in ALL occur within the first 1.5 to 2.0 years after the initiation of treatment,” Dr. Hoelzer wrote.
He said other outstanding questions include whether long-term outcomes will differ between patients who undergo transplant and those who do not; whether ABL1 mutations emerge; whether minimal residual disease recurs with longer follow-up; and whether this treatment approach can be used for patients with other subtypes of ALL, such as Ph-negative, B-lineage ALL, or even T-cell ALL.
“If these promising trial results hold, chemotherapy-free induction without the immediate and long-term toxic effects of intensive chemotherapy regimens could also be used in adolescents and, finally, in children. These questions will need to be addressed with longer follow-up and large, prospective trials,” Dr. Hoelzer concluded.
The study was supported by grants from the Italian Association for Cancer Research and Sapienza University of Rome.
A version of this article originally appeared on Medscape.com
The days of using chemotherapy to treat Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) may be numbered.
In a phase 2 trial, upfront chemotherapy-free induction/consolidation with the tyrosine kinase inhibitor dasatinib (Sprycel) and the bispecific T-cell engager antibody blinatumomab (Blincyto) yielded high rates of molecular response, “impressive” survival at 18 months, and few toxic effects of grade 3 or higher, say researchers.
With this approach, “60% of adult Ph+ ALL patients, of all ages, can obtain a molecular response, and this percent can increase further with more cycles of blinatumomab,” lead researcher Robin Foà, MD, from Sapienza University of Rome, said in an interview.
“The rates of disease-free survival and overall survival at 18 months are highly favorable, and the protocol is associated with limited toxicity,” Dr. Foà added.
“I see this chemo-free approach becoming a realistic approach for a substantial proportion of adult Ph+ ALL patients, particularly for the older patients, keeping in mind that the incidence of Ph+ ALL increases with age,” Dr. Foà said.
The results of the study were published Oct. 22 in the New England Journal of Medicine.
‘Innovative’ and ‘highly successful’
This “innovative” chemotherapy-free approach proved “highly successful” with “surprisingly” few toxic effects, Dieter Hoelzer, MD, PhD, University of Frankfurt (Germany), wrote in a linked editorial.
The Italian GIMEMA LAL2116 D-ALBA trial enrolled 63 adults (median age, 54 years; range, 24-82 years) with newly diagnosed Ph+ ALL. All patients received a glucocorticoid for 31 days beginning 7 days before starting treatment with dasatinib.
Dasatinib (140 mg once daily) induction therapy lasted 85 days. All patients who completed the induction phase received blinatumomab (28 mcg/d) consolidation therapy. Dexamethasone (20 mg) was administered before each blinatumomab cycle. To prevent central nervous system adverse events, levetiracetam (500 mg twice daily) was administered.
All but two patients completed dasatinib induction. One was a 73-year-old woman who withdrew from the trial because of toxic effects after 10 days of dasatinib treatment. She later died of pneumonia. The other was an 82-year-old woman who had a complete hematologic response but left the trial because of pneumonia and pneumonitis.
At the end of the induction phase, 98% of the patients (62 of 63) had a complete hematologic response, including the patient with a complete hematologic response who withdrew; 29% (17 of 59 patients) had a molecular response.
Of the 61 patients who completed the induction phase, 58 received one cycle of blinatumomab, 56 received two cycles, 45 received three cycles, 37 received four cycles, and 29 received five cycles. At the end of the second blinatumomab cycle, 60% of the patients (33 of 55 patients) had a molecular response.
The percentage of patients with a molecular response increased further after receiving additional cycles of blinatumomab – to 70% (28 of 40 patients) after the third cycle, 81% (29 of 36 patients) after the fourth cycle, and 72% (21 of 29 patients) after the fifth cycle.
At a median follow-up of 18 months, overall survival was 95%, and disease-free survival was 88%.
There were no significant differences in DFS between patients with p190-kd fusion protein (85%) and those with p210-kd fusion protein (95%). However, DFS was lower in patients with IKZF1 deletion plus additional genetic aberrations (CDKN2A or CDKN2B, PAX5, or both).
ABL1 mutations were present in six patients who had increased minimal residual disease during induction therapy. All these mutations were cleared by blinatumomab.
There were six relapses, of which three were hematologic. One occurred in a patient with a major protocol violation (a delay of more than 2 months in receiving blinatumomab), one occurred after 12 months in the patient who discontinued the trial after receiving dasatinib for 12 days, and one occurred in a patient after the second cycle of blinatumomab.
A total of 21 adverse events of grade 3 or higher were noted. They included cytomegalovirus reactivation or infection in six patients; neutropenia in four patients; persistent fever in two patients; and pleural effusion, pulmonary hypertension, and a neurologic disorder in one patient each.
Of the 24 patients who received a stem-cell allograft, two died, but only one death was related to transplant (4%).
The very low nonrelapse mortality among patients who underwent transplant during remission is “remarkable,” Dr. Hoelzer wrote. It suggests that toxicity from induction chemotherapy puts the patient at risk for toxic effects and death from subsequent stem-cell transplant – “a consequence that is avoided with targeted therapy.”
Unanswered questions
“Will the excellent outcomes be preserved with longer follow-up? The answer is probably yes, given that the majority of relapses in ALL occur within the first 1.5 to 2.0 years after the initiation of treatment,” Dr. Hoelzer wrote.
He said other outstanding questions include whether long-term outcomes will differ between patients who undergo transplant and those who do not; whether ABL1 mutations emerge; whether minimal residual disease recurs with longer follow-up; and whether this treatment approach can be used for patients with other subtypes of ALL, such as Ph-negative, B-lineage ALL, or even T-cell ALL.
“If these promising trial results hold, chemotherapy-free induction without the immediate and long-term toxic effects of intensive chemotherapy regimens could also be used in adolescents and, finally, in children. These questions will need to be addressed with longer follow-up and large, prospective trials,” Dr. Hoelzer concluded.
The study was supported by grants from the Italian Association for Cancer Research and Sapienza University of Rome.
A version of this article originally appeared on Medscape.com
Immunotherapy for ALL: Roles emerge in R/R disease, MRD+ disease
“Most of the emerging therapies in ALL are immunotherapies that have really made an impact in the relapsed and refractory setting,” he said during a presentation at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress. “Another very exciting development is that these immunotherapies are now demonstrating efficacy and increased tolerability over chemotherapy in the minimal residual disease (MRD)-positive setting up front.”
Dr. Brown, director of the pediatric leukemia program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, focused on blinatumomab, inotuzumab, and chimeric antigen receptor (CAR) T-cell therapy for ALL, and explained the rationale for their use.
Why immunotherapy?
“It turns out that in normal B cell development there are a number of proteins that are expressed on the surface of B cells and these same proteins are expressed on the surface of many B-cell malignancies,” he said, noting that ALL is “probably the least differentiated of the B-lineage malignancies,” but the vast majority of ALL cases will express CD19 and CD22, and – in adults more often than pediatric patients – CD20.
These antigens make good targets for ALL therapy because they aren’t expressed on bone marrow stem cells or other tissues in the body.
“They really are specific for B cells,” he said, explaining that inotuzumab, a CD22 antibody drug conjugate (ADC), and blinatumomab, a bi-specific T cell-engaging antibody (BiTE) that targets CD19, are antibody-based immunotherapies, whereas CAR T-cell therapies are a separate category that can be single- or multi-antigen targeted.
Inotuzumab, blinatumomab, and CAR T cells
Inotuzumab targets the CD22 immunotoxin antigen via a T-cell independent process and is delivered as a once-weekly 1-hour infusion. It is approved for adult relapsed/refractory B-ALL. Blinatumomab binds CD19 on the surface of the tumor cells and CD3 on the surface of any T cell in the vicinity of the tumor cell.
“The recognition that blinatumomab allows between the tumor cell and the T cell is independent of the specificity of the T-cell receptor. It also does not require [major histocompatibility complex] class 1 or peptide antigens on the surface of the T cell,” he said, adding that it does, however, rely on a functional endogenous cytotoxic T-cell response, unlike inotuzumab. “It’s also very difficult technically to give because it’s given as a 28-day continuous IV infusion with bag changes required every 4-7 days.”
Blinatumomab is approved for adult and pediatric Philadelphia chromosome-negative relapsed/refractory B-cell precursor ALL and MRD-positive B-cell precursor ALL.
CAR T-cell therapy, an autologous immunotherapy, is “really kind of the pinnacle of technological advances in immunotherapy in that it combine three different modalities into one: cellular therapy, gene therapy, and immunotherapy,” he said, noting that the process of genetically engineering T cells to express a CAR is complex and costly and access is limited, but expanding with about 90 centers in the U.S. now providing CAR T-cell therapy.
Response rates with each of these therapies represent a paradigm shift in the relapsed/refractory ALL setting, Dr. Brown said.
Studies have shown complete remission (CR) and minimum residual disease (MRD)-negative CR rates of 81% and 78%, respectively, with inotuzumab, and 43% and 33%, respectively, with blinatumomab.
“This depth of remission was really not seen with prior salvage therapies,” he noted, but added that neither has shown significant durable improvement in overall survival (OS) rates.
CAR T-cell therapy, however, has the highest response rates, with tisagenlecleucel – which targets CD19 and was the first CAR T-cell therapy approved for refractory or second or greater relapse in patients up to age 26 years – showing 81% CR and MRD-negative CR rates and providing a durable survival advantage without subsequent therapy in 40-50% of patients.
“So CAR T cells can represent definitive therapy in a subset of patients,” Dr. Brown said. “One thing we’re struggling with is to be able to predict which patients those are, and there are some emerging biomarkers that may help us with that, but as of now it’s very difficult to predict which patients, when you’re treating them, are going to be in [that group].”
Toxicities and limitations
Cytokine release syndrome and neurotoxicity are the primary toxicities associated with both blinatumomab and tisagenlecleucel. Hepatotoxicity is a major concern with inotuzumab.
“This is particularly important because that hepatotoxicity appears to be primarily a problem in patients who receive inotuzumab either after or prior to hematopoietic stem cell transplant, and since this therapy does not represent definitive therapy and often is really a bridge to transplant, this ... can be a significant limitation to this product,” Dr. Brown said.
A limitation of CAR T cells is failure to manufacture the product, which occurs most often in very young and heavily pretreated patients in whom it can be difficult to obtain enough functional T cells to create the product. Failure to engraft or lack of persistence of the CAR T cells can also occur.
Endogenous or CAR T-cell exhaustion is another potential limitation with blinatumomab and CAR T-cell therapy, and antigen escape can occur with both therapies, as well.
Strategies are being investigated to overcome treatment challenges, Dr. Brown noted.
Examples include efforts to develop universal “off-the-shelf” allogeneic CAR T-cell products to address failure to manufacture, working on more co-stimulatory domains that may be more effective to promote engraftment and persistence, adding immune checkpoint inhibitors to therapy to combat endogenous or CAR T-cell exhaustion, and developing multi-antigen targeted approaches to overcome antigen escape, he said.
NCCN Treatment Guidelines
Based on the currently available data, the NCCN has included these immunotherapies in guidelines for both adolescent and young adult (AYA)/adult ALL and for pediatric ALL.
Each of the treatments is listed as an option to consider in both Philadelphia chromosome-positive and -negative AYA and adult patients under age 65 years. Additionally, blinatumomab is listed as an option for up-front treatment of MRD-positive Philadelphia chromosome-negative AYA patients and older patients.
Pediatric guidelines include blinatumomab and tisagenlecleucel as options for patients with MRD-positive disease after induction and for first relapse, and they include all three therapies as options in patients with multiple relapses or refractory disease, said Dr. Brown who chairs the NCCN Clinical Practice Guidelines panel for adult and pediatric ALL.
Treatment decision making
Asked by session moderator Ranjana H. Advani, MD, how to decide between the available immunotherapies, Dr. Brown said there is no one-size-fits-all answer.
“Is it availability, insurance coverage, the patient fits better with one therapy,” asked Dr. Advani, the Saul Rosenberg Professor of Lymphoma and the Physician Leader of the Lymphoma Clinical Care Program of Stanford Cancer Institute, Palo Alto, Calif.
“All of the above,” Dr. Brown said. “In 2020 with all these options available, we are a little bit spoiled for choice ... but every patient is an individual case and the risk -benefit ratios of all these therapies differ.”
An exception is that CAR T-cell therapy is a clear stand-out for the patient who isn’t transplant eligible, he noted, adding that CAR T cells “probably give that patient the best chance of survival.”
In a patient who could potentially go to transplant, selection is a bit more challenging, but given the risks associated with inotuzumab, blinatumomab is generally the preferred non-CAR T option, he said.
“It’s a complicated question, and the answer ... is [that it is] an individualized patient-by-patient decision,” he added.
Dr. Brown reported consulting, advisory board, or expert witness activity for Novartis Pharmaceuticals Corporation and Takeda Pharmaceuticals North America Inc.
“Most of the emerging therapies in ALL are immunotherapies that have really made an impact in the relapsed and refractory setting,” he said during a presentation at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress. “Another very exciting development is that these immunotherapies are now demonstrating efficacy and increased tolerability over chemotherapy in the minimal residual disease (MRD)-positive setting up front.”
Dr. Brown, director of the pediatric leukemia program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, focused on blinatumomab, inotuzumab, and chimeric antigen receptor (CAR) T-cell therapy for ALL, and explained the rationale for their use.
Why immunotherapy?
“It turns out that in normal B cell development there are a number of proteins that are expressed on the surface of B cells and these same proteins are expressed on the surface of many B-cell malignancies,” he said, noting that ALL is “probably the least differentiated of the B-lineage malignancies,” but the vast majority of ALL cases will express CD19 and CD22, and – in adults more often than pediatric patients – CD20.
These antigens make good targets for ALL therapy because they aren’t expressed on bone marrow stem cells or other tissues in the body.
“They really are specific for B cells,” he said, explaining that inotuzumab, a CD22 antibody drug conjugate (ADC), and blinatumomab, a bi-specific T cell-engaging antibody (BiTE) that targets CD19, are antibody-based immunotherapies, whereas CAR T-cell therapies are a separate category that can be single- or multi-antigen targeted.
Inotuzumab, blinatumomab, and CAR T cells
Inotuzumab targets the CD22 immunotoxin antigen via a T-cell independent process and is delivered as a once-weekly 1-hour infusion. It is approved for adult relapsed/refractory B-ALL. Blinatumomab binds CD19 on the surface of the tumor cells and CD3 on the surface of any T cell in the vicinity of the tumor cell.
“The recognition that blinatumomab allows between the tumor cell and the T cell is independent of the specificity of the T-cell receptor. It also does not require [major histocompatibility complex] class 1 or peptide antigens on the surface of the T cell,” he said, adding that it does, however, rely on a functional endogenous cytotoxic T-cell response, unlike inotuzumab. “It’s also very difficult technically to give because it’s given as a 28-day continuous IV infusion with bag changes required every 4-7 days.”
Blinatumomab is approved for adult and pediatric Philadelphia chromosome-negative relapsed/refractory B-cell precursor ALL and MRD-positive B-cell precursor ALL.
CAR T-cell therapy, an autologous immunotherapy, is “really kind of the pinnacle of technological advances in immunotherapy in that it combine three different modalities into one: cellular therapy, gene therapy, and immunotherapy,” he said, noting that the process of genetically engineering T cells to express a CAR is complex and costly and access is limited, but expanding with about 90 centers in the U.S. now providing CAR T-cell therapy.
Response rates with each of these therapies represent a paradigm shift in the relapsed/refractory ALL setting, Dr. Brown said.
Studies have shown complete remission (CR) and minimum residual disease (MRD)-negative CR rates of 81% and 78%, respectively, with inotuzumab, and 43% and 33%, respectively, with blinatumomab.
“This depth of remission was really not seen with prior salvage therapies,” he noted, but added that neither has shown significant durable improvement in overall survival (OS) rates.
CAR T-cell therapy, however, has the highest response rates, with tisagenlecleucel – which targets CD19 and was the first CAR T-cell therapy approved for refractory or second or greater relapse in patients up to age 26 years – showing 81% CR and MRD-negative CR rates and providing a durable survival advantage without subsequent therapy in 40-50% of patients.
“So CAR T cells can represent definitive therapy in a subset of patients,” Dr. Brown said. “One thing we’re struggling with is to be able to predict which patients those are, and there are some emerging biomarkers that may help us with that, but as of now it’s very difficult to predict which patients, when you’re treating them, are going to be in [that group].”
Toxicities and limitations
Cytokine release syndrome and neurotoxicity are the primary toxicities associated with both blinatumomab and tisagenlecleucel. Hepatotoxicity is a major concern with inotuzumab.
“This is particularly important because that hepatotoxicity appears to be primarily a problem in patients who receive inotuzumab either after or prior to hematopoietic stem cell transplant, and since this therapy does not represent definitive therapy and often is really a bridge to transplant, this ... can be a significant limitation to this product,” Dr. Brown said.
A limitation of CAR T cells is failure to manufacture the product, which occurs most often in very young and heavily pretreated patients in whom it can be difficult to obtain enough functional T cells to create the product. Failure to engraft or lack of persistence of the CAR T cells can also occur.
Endogenous or CAR T-cell exhaustion is another potential limitation with blinatumomab and CAR T-cell therapy, and antigen escape can occur with both therapies, as well.
Strategies are being investigated to overcome treatment challenges, Dr. Brown noted.
Examples include efforts to develop universal “off-the-shelf” allogeneic CAR T-cell products to address failure to manufacture, working on more co-stimulatory domains that may be more effective to promote engraftment and persistence, adding immune checkpoint inhibitors to therapy to combat endogenous or CAR T-cell exhaustion, and developing multi-antigen targeted approaches to overcome antigen escape, he said.
NCCN Treatment Guidelines
Based on the currently available data, the NCCN has included these immunotherapies in guidelines for both adolescent and young adult (AYA)/adult ALL and for pediatric ALL.
Each of the treatments is listed as an option to consider in both Philadelphia chromosome-positive and -negative AYA and adult patients under age 65 years. Additionally, blinatumomab is listed as an option for up-front treatment of MRD-positive Philadelphia chromosome-negative AYA patients and older patients.
Pediatric guidelines include blinatumomab and tisagenlecleucel as options for patients with MRD-positive disease after induction and for first relapse, and they include all three therapies as options in patients with multiple relapses or refractory disease, said Dr. Brown who chairs the NCCN Clinical Practice Guidelines panel for adult and pediatric ALL.
Treatment decision making
Asked by session moderator Ranjana H. Advani, MD, how to decide between the available immunotherapies, Dr. Brown said there is no one-size-fits-all answer.
“Is it availability, insurance coverage, the patient fits better with one therapy,” asked Dr. Advani, the Saul Rosenberg Professor of Lymphoma and the Physician Leader of the Lymphoma Clinical Care Program of Stanford Cancer Institute, Palo Alto, Calif.
“All of the above,” Dr. Brown said. “In 2020 with all these options available, we are a little bit spoiled for choice ... but every patient is an individual case and the risk -benefit ratios of all these therapies differ.”
An exception is that CAR T-cell therapy is a clear stand-out for the patient who isn’t transplant eligible, he noted, adding that CAR T cells “probably give that patient the best chance of survival.”
In a patient who could potentially go to transplant, selection is a bit more challenging, but given the risks associated with inotuzumab, blinatumomab is generally the preferred non-CAR T option, he said.
“It’s a complicated question, and the answer ... is [that it is] an individualized patient-by-patient decision,” he added.
Dr. Brown reported consulting, advisory board, or expert witness activity for Novartis Pharmaceuticals Corporation and Takeda Pharmaceuticals North America Inc.
“Most of the emerging therapies in ALL are immunotherapies that have really made an impact in the relapsed and refractory setting,” he said during a presentation at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress. “Another very exciting development is that these immunotherapies are now demonstrating efficacy and increased tolerability over chemotherapy in the minimal residual disease (MRD)-positive setting up front.”
Dr. Brown, director of the pediatric leukemia program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, focused on blinatumomab, inotuzumab, and chimeric antigen receptor (CAR) T-cell therapy for ALL, and explained the rationale for their use.
Why immunotherapy?
“It turns out that in normal B cell development there are a number of proteins that are expressed on the surface of B cells and these same proteins are expressed on the surface of many B-cell malignancies,” he said, noting that ALL is “probably the least differentiated of the B-lineage malignancies,” but the vast majority of ALL cases will express CD19 and CD22, and – in adults more often than pediatric patients – CD20.
These antigens make good targets for ALL therapy because they aren’t expressed on bone marrow stem cells or other tissues in the body.
“They really are specific for B cells,” he said, explaining that inotuzumab, a CD22 antibody drug conjugate (ADC), and blinatumomab, a bi-specific T cell-engaging antibody (BiTE) that targets CD19, are antibody-based immunotherapies, whereas CAR T-cell therapies are a separate category that can be single- or multi-antigen targeted.
Inotuzumab, blinatumomab, and CAR T cells
Inotuzumab targets the CD22 immunotoxin antigen via a T-cell independent process and is delivered as a once-weekly 1-hour infusion. It is approved for adult relapsed/refractory B-ALL. Blinatumomab binds CD19 on the surface of the tumor cells and CD3 on the surface of any T cell in the vicinity of the tumor cell.
“The recognition that blinatumomab allows between the tumor cell and the T cell is independent of the specificity of the T-cell receptor. It also does not require [major histocompatibility complex] class 1 or peptide antigens on the surface of the T cell,” he said, adding that it does, however, rely on a functional endogenous cytotoxic T-cell response, unlike inotuzumab. “It’s also very difficult technically to give because it’s given as a 28-day continuous IV infusion with bag changes required every 4-7 days.”
Blinatumomab is approved for adult and pediatric Philadelphia chromosome-negative relapsed/refractory B-cell precursor ALL and MRD-positive B-cell precursor ALL.
CAR T-cell therapy, an autologous immunotherapy, is “really kind of the pinnacle of technological advances in immunotherapy in that it combine three different modalities into one: cellular therapy, gene therapy, and immunotherapy,” he said, noting that the process of genetically engineering T cells to express a CAR is complex and costly and access is limited, but expanding with about 90 centers in the U.S. now providing CAR T-cell therapy.
Response rates with each of these therapies represent a paradigm shift in the relapsed/refractory ALL setting, Dr. Brown said.
Studies have shown complete remission (CR) and minimum residual disease (MRD)-negative CR rates of 81% and 78%, respectively, with inotuzumab, and 43% and 33%, respectively, with blinatumomab.
“This depth of remission was really not seen with prior salvage therapies,” he noted, but added that neither has shown significant durable improvement in overall survival (OS) rates.
CAR T-cell therapy, however, has the highest response rates, with tisagenlecleucel – which targets CD19 and was the first CAR T-cell therapy approved for refractory or second or greater relapse in patients up to age 26 years – showing 81% CR and MRD-negative CR rates and providing a durable survival advantage without subsequent therapy in 40-50% of patients.
“So CAR T cells can represent definitive therapy in a subset of patients,” Dr. Brown said. “One thing we’re struggling with is to be able to predict which patients those are, and there are some emerging biomarkers that may help us with that, but as of now it’s very difficult to predict which patients, when you’re treating them, are going to be in [that group].”
Toxicities and limitations
Cytokine release syndrome and neurotoxicity are the primary toxicities associated with both blinatumomab and tisagenlecleucel. Hepatotoxicity is a major concern with inotuzumab.
“This is particularly important because that hepatotoxicity appears to be primarily a problem in patients who receive inotuzumab either after or prior to hematopoietic stem cell transplant, and since this therapy does not represent definitive therapy and often is really a bridge to transplant, this ... can be a significant limitation to this product,” Dr. Brown said.
A limitation of CAR T cells is failure to manufacture the product, which occurs most often in very young and heavily pretreated patients in whom it can be difficult to obtain enough functional T cells to create the product. Failure to engraft or lack of persistence of the CAR T cells can also occur.
Endogenous or CAR T-cell exhaustion is another potential limitation with blinatumomab and CAR T-cell therapy, and antigen escape can occur with both therapies, as well.
Strategies are being investigated to overcome treatment challenges, Dr. Brown noted.
Examples include efforts to develop universal “off-the-shelf” allogeneic CAR T-cell products to address failure to manufacture, working on more co-stimulatory domains that may be more effective to promote engraftment and persistence, adding immune checkpoint inhibitors to therapy to combat endogenous or CAR T-cell exhaustion, and developing multi-antigen targeted approaches to overcome antigen escape, he said.
NCCN Treatment Guidelines
Based on the currently available data, the NCCN has included these immunotherapies in guidelines for both adolescent and young adult (AYA)/adult ALL and for pediatric ALL.
Each of the treatments is listed as an option to consider in both Philadelphia chromosome-positive and -negative AYA and adult patients under age 65 years. Additionally, blinatumomab is listed as an option for up-front treatment of MRD-positive Philadelphia chromosome-negative AYA patients and older patients.
Pediatric guidelines include blinatumomab and tisagenlecleucel as options for patients with MRD-positive disease after induction and for first relapse, and they include all three therapies as options in patients with multiple relapses or refractory disease, said Dr. Brown who chairs the NCCN Clinical Practice Guidelines panel for adult and pediatric ALL.
Treatment decision making
Asked by session moderator Ranjana H. Advani, MD, how to decide between the available immunotherapies, Dr. Brown said there is no one-size-fits-all answer.
“Is it availability, insurance coverage, the patient fits better with one therapy,” asked Dr. Advani, the Saul Rosenberg Professor of Lymphoma and the Physician Leader of the Lymphoma Clinical Care Program of Stanford Cancer Institute, Palo Alto, Calif.
“All of the above,” Dr. Brown said. “In 2020 with all these options available, we are a little bit spoiled for choice ... but every patient is an individual case and the risk -benefit ratios of all these therapies differ.”
An exception is that CAR T-cell therapy is a clear stand-out for the patient who isn’t transplant eligible, he noted, adding that CAR T cells “probably give that patient the best chance of survival.”
In a patient who could potentially go to transplant, selection is a bit more challenging, but given the risks associated with inotuzumab, blinatumomab is generally the preferred non-CAR T option, he said.
“It’s a complicated question, and the answer ... is [that it is] an individualized patient-by-patient decision,” he added.
Dr. Brown reported consulting, advisory board, or expert witness activity for Novartis Pharmaceuticals Corporation and Takeda Pharmaceuticals North America Inc.
FROM NCCN HEMATOLOGIC MALIGNANCIES
Are HMAS appropriate for posttransplant maintenance in acute leukemias?
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
FROM ALF 2020
Study: Complications from childhood ALL and its treatment are common, but can be managed
Despite survival after treatment of acute lymphoblastic leukemia (ALL), a high percentage of children suffered acute complications, even without relapse, according to a report published online in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study of 110 children with acute lymphoblastic leukemia (ALL), Ayse Pınar Öztürk, MD, and colleagues at Istanbul University, Cerrahpasa Faculty of Medicine, evaluated the acute complications that occurred during the treatment of childhood ALL and documented their survival rates. The 110 patients, comprising 65 boys and 45 girls, were all treated with the Children’s Oncology Group protocol from 1999 to 2014.
The mean age at admission was 8.3 years and 97 patients (88.2%) were diagnosed with pre–B-cell ALL, 11 (10%) with T-cell ALL, 1 (0.9%) with mixed phenotype acute leukemia, and 1 (0.9%) with mature B-cell acute leukemia. A total of 36.3% were evaluated to be in the standard-risk group and the rest were in the high-risk group. Regular follow-up and evaluation for acute complications was available for 105 of the patients.
Survival and complications
Of the 110 patients, 98 were assessed in the survival analyses. The 5- and 10-year overall survival rates were both 85.9%, while the relapse-free survival rates at 1, 3, and 5 years were 97.9%, 91.3%, and 86.3%, respectively. These results are favorable and in line with good results reported in the literature, according to the researchers.
In terms of acute complications, infection was the most common (88.5%), followed by gastrointestinal (27.6%), neurologic (26.6%), metabolic/endocrine (15.2%), drug-related hypersensitivity (15.2%), avascular necrosis (12.3%), thrombotic (10.4%), severe psychiatric (1.9%), and various other complications (11.4%).
In the present study, 13 of the 98 patients (13.3%) died. All 13 patients had been in the high-risk group and 9 had had relapsed ALL. Of the 13 deaths, 8 (8.2%) had resulted from treatment resistance and toxicity and 5 (5.1%) from severe infection (sepsis).
During ALL treatment, various complications can occur related to the disease itself or the treatment, according to the authors. However, they added that in regularly and closely monitored patients, complications can be effectively prevented, treated, and eliminated by aggressive observation and prompt intervention.
“In our study, the short hospitalization period, prompt implementation of protocol updates, rapid analysis of laboratory tests, continuous supportive care, efficient education given to the parents of children, and consistently undertaking patient care and treatment management by the same expert team increased the success of the therapy and ensured low complication rates,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Öztürk AP et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 17. doi: 10.1016/j.clml.2020.08.025.
Despite survival after treatment of acute lymphoblastic leukemia (ALL), a high percentage of children suffered acute complications, even without relapse, according to a report published online in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study of 110 children with acute lymphoblastic leukemia (ALL), Ayse Pınar Öztürk, MD, and colleagues at Istanbul University, Cerrahpasa Faculty of Medicine, evaluated the acute complications that occurred during the treatment of childhood ALL and documented their survival rates. The 110 patients, comprising 65 boys and 45 girls, were all treated with the Children’s Oncology Group protocol from 1999 to 2014.
The mean age at admission was 8.3 years and 97 patients (88.2%) were diagnosed with pre–B-cell ALL, 11 (10%) with T-cell ALL, 1 (0.9%) with mixed phenotype acute leukemia, and 1 (0.9%) with mature B-cell acute leukemia. A total of 36.3% were evaluated to be in the standard-risk group and the rest were in the high-risk group. Regular follow-up and evaluation for acute complications was available for 105 of the patients.
Survival and complications
Of the 110 patients, 98 were assessed in the survival analyses. The 5- and 10-year overall survival rates were both 85.9%, while the relapse-free survival rates at 1, 3, and 5 years were 97.9%, 91.3%, and 86.3%, respectively. These results are favorable and in line with good results reported in the literature, according to the researchers.
In terms of acute complications, infection was the most common (88.5%), followed by gastrointestinal (27.6%), neurologic (26.6%), metabolic/endocrine (15.2%), drug-related hypersensitivity (15.2%), avascular necrosis (12.3%), thrombotic (10.4%), severe psychiatric (1.9%), and various other complications (11.4%).
In the present study, 13 of the 98 patients (13.3%) died. All 13 patients had been in the high-risk group and 9 had had relapsed ALL. Of the 13 deaths, 8 (8.2%) had resulted from treatment resistance and toxicity and 5 (5.1%) from severe infection (sepsis).
During ALL treatment, various complications can occur related to the disease itself or the treatment, according to the authors. However, they added that in regularly and closely monitored patients, complications can be effectively prevented, treated, and eliminated by aggressive observation and prompt intervention.
“In our study, the short hospitalization period, prompt implementation of protocol updates, rapid analysis of laboratory tests, continuous supportive care, efficient education given to the parents of children, and consistently undertaking patient care and treatment management by the same expert team increased the success of the therapy and ensured low complication rates,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Öztürk AP et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 17. doi: 10.1016/j.clml.2020.08.025.
Despite survival after treatment of acute lymphoblastic leukemia (ALL), a high percentage of children suffered acute complications, even without relapse, according to a report published online in Clinical Lymphoma, Myeloma & Leukemia.
In a retrospective study of 110 children with acute lymphoblastic leukemia (ALL), Ayse Pınar Öztürk, MD, and colleagues at Istanbul University, Cerrahpasa Faculty of Medicine, evaluated the acute complications that occurred during the treatment of childhood ALL and documented their survival rates. The 110 patients, comprising 65 boys and 45 girls, were all treated with the Children’s Oncology Group protocol from 1999 to 2014.
The mean age at admission was 8.3 years and 97 patients (88.2%) were diagnosed with pre–B-cell ALL, 11 (10%) with T-cell ALL, 1 (0.9%) with mixed phenotype acute leukemia, and 1 (0.9%) with mature B-cell acute leukemia. A total of 36.3% were evaluated to be in the standard-risk group and the rest were in the high-risk group. Regular follow-up and evaluation for acute complications was available for 105 of the patients.
Survival and complications
Of the 110 patients, 98 were assessed in the survival analyses. The 5- and 10-year overall survival rates were both 85.9%, while the relapse-free survival rates at 1, 3, and 5 years were 97.9%, 91.3%, and 86.3%, respectively. These results are favorable and in line with good results reported in the literature, according to the researchers.
In terms of acute complications, infection was the most common (88.5%), followed by gastrointestinal (27.6%), neurologic (26.6%), metabolic/endocrine (15.2%), drug-related hypersensitivity (15.2%), avascular necrosis (12.3%), thrombotic (10.4%), severe psychiatric (1.9%), and various other complications (11.4%).
In the present study, 13 of the 98 patients (13.3%) died. All 13 patients had been in the high-risk group and 9 had had relapsed ALL. Of the 13 deaths, 8 (8.2%) had resulted from treatment resistance and toxicity and 5 (5.1%) from severe infection (sepsis).
During ALL treatment, various complications can occur related to the disease itself or the treatment, according to the authors. However, they added that in regularly and closely monitored patients, complications can be effectively prevented, treated, and eliminated by aggressive observation and prompt intervention.
“In our study, the short hospitalization period, prompt implementation of protocol updates, rapid analysis of laboratory tests, continuous supportive care, efficient education given to the parents of children, and consistently undertaking patient care and treatment management by the same expert team increased the success of the therapy and ensured low complication rates,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Öztürk AP et al. Clin Lymphoma Myeloma Leuk. 2020 Sep 17. doi: 10.1016/j.clml.2020.08.025.
FROM Clinical Lymphoma, Myeloma & Leukemia
Orthopedic problems in children can be the first indication of acute lymphoblastic leukemia
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
The diagnosis of acute lymphoblastic leukemia (ALL) can be delayed because of vague presentation and normal hematological results. Orthopedic manifestations may be the primary presentation of ALL to physicians, and such symptoms in children should be cause for suspicion, even in the absence of hematological abnormalities, according to a report published in the Journal of Orthopaedics.
The study retrospectively assessed 250 consecutive ALL patients at a single institution to identify the frequency of ALL cases presented to the orthopedic department and to determine the number of these patients presenting with normal hematological results, according to Amrath Raj BK, MD, and colleagues at the Manipal (India) Academy of Higher Education.
Suspicion warranted
Twenty-two of the 250 patients (8.8%) presented primarily to the orthopedic department (4 with vertebral compression fractures, 12 with joint pain, and 6 with bone pain), but were subsequently diagnosed with ALL. These results were comparable to previous studies. The mean patient age at the first visit was 5.6 years; 13 patients were boys, and 9 were girls. Six of these 22 patients (27.3%) had a normal peripheral blood smear, according to the researchers.
“Acute leukemia should be considered strongly as a differential diagnosis in children with severe osteoporosis and vertebral fractures. Initial orthopedic manifestations are not uncommon, and the primary physician should maintain a high index of suspicion as a peripheral smear is not diagnostic in all patients,” the researchers concluded.
The authors reported that there was no outside funding source and that they had no conflicts.
SOURCE: Raj BK A et al. Journal of Orthopaedics. 2020;22:326-330.
FROM THE JOURNAL OF ORTHOPAEDICS
Survey quantifies COVID-19’s impact on oncology
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
FROM ESMO 2020
TKI choice key for fit/unfit patients with Ph+ALL
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
FROM ASH HEMATOLOGIC MALIGNANCIES 2020
CSF metabolomic profile linked to cancer-related fatigue in children with ALL
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
FROM THE JOURNAL OF PAIN AND SYMPTOM MANAGEMENT
Polygenic risk score may predict VTE in adolescents, but not adults, with ALL
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
Although patients with acute lymphoblastic leukemia (ALL) are at known risk of venous thromboembolism (VTE), there was no overall genetic correlation found to be associated with that susceptibility in the overall population. However, a significant genetic predisposition to VTE was found in adolescent ALL patients, according to a report published in Thrombosis Research.
The researchers assessed the prospectively registered VTE events and collected germline DNA in patients aged 1-45.9 years in the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 study, which took place from 2008 to 2016. The researchers performed polygenic risk score (PRS) analysis on VTE development in the NOPHO cohort, according to Kirsten Brunsvig Jarvis, MD, of Oslo University Hospital, and colleagues.
The researchers used summary statistics from two large genomewide association studies on VTE in adults (the International Network of Venous Thromboembolism Clinical Research Networks [INVENT] consortium and the UK Biobank).
Of 1,252 patients with ALL in the genetic cohort, 89 developed VTE (2.5-year cumulative incidence, 7.2%; 95% confidence interval,5.7-8.6) at a median 12.7 weeks from diagnosis.
Overall, an analysis of single-nucleotide polymorphisms (SNPs) from INVENT and UK Biobank studies did not reveal evidence of polygenic correlation with VTE in patients with ALL, the researchers reported. However, when separating adolescents aged 10.0-17.9 years (n = 231) from adults aged 18 years or older (n = 127), they saw polygenic overlap between the INVENT study and thromboembolism development in the adolescent population.
The best-fit polygenic risk score, including 16,144 SNPs, was associated with VTE in adolescents with ALL at a hazard ratio of 1.76 (95% CI, 1.23-2.52; P = .02).
Adolescent vs. adult risk
The researchers expressed surprise that they did not find evidence of genetic overlap in adults. But they stated that, in general, VTE occurs more frequently in adults as part of natural aging, while children and adolescents are physiologically protected. This might explain why genetics might play a stronger role in the high-risk situation of cancer and chemotherapy in adolescents who do not have as many additional exogenic risk factors as adults.
“The usefulness of genetic studies on [V]TE in the general adult population is limited when it comes to understanding the etiology of [V]TE in patients with ALL. However, we found evidence of polygenic overlap in subgroup analysis of adolescents aged 10.0-17.9 years with ALL, and we believe the genetics of [V]TE in this group should be further explored in future risk prediction models for identification of those who might benefit from thromboprophylaxis,” the researchers concluded.
The study was supported by research grant from the South-Eastern Norway Regional Health Authority. The authors reported that they had no conflicts of interest.
SOURCE: Jarvis KB et al. Thromb Res. 2020 Aug 11.doi: 10.1016/j.thromres.2020.08.015.
FROM THROMBOSIS RESEARCH
Posaconazole prophylaxis was effective in children with ALL undergoing chemotherapy
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
FROM THE JOURNAL OF MICROBIOLOGY, IMMUNOLOGY AND INFECTION