User login
Does An Elevated Lp(a) Call for Low-dose Aspirin?
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
How Well Do Clinicians Support Patients’ Sexual Health?
From adolescence onward, the need for sexual health is particularly important. Yet, information and healthcare services are limited, which often leaves patients in distress and subject to misconceptions. What are the specific issues related to sexuality in adolescence, middle age, and beyond? This news organization interviewed Carol Burté, MD, a specialist in sexual medicine from Monaco.
Question: Regarding young individuals, what about sex education in schools?
Dr. Burté: The French law of 2018 specifies that at least three annual sessions must be devoted to sex education in elementary school, middle school, and high school.
In practice, this is not always the case, and interventions are very focused on prevention and rules. Sexuality is almost always absent from the program. Sexuality means: What does it mean to have desire? How does pleasure work? At what age do we have sex? etc. Young people receive prevention advice, but the link with sexuality is not made.
Sexuality remains taboo. You know, like in books: “They got married and had many children ...” End of the story, we don’t know more [laughs].
Question: And outside the school setting, do doctors sufficiently address sexual health issues with adolescents?
Dr. Burté: Rarely. I understand that a general practitioner has little time, but they can still ask the young person if they have any questions. They can refer them to someone or provide reading recommendations. Regarding sex education on the Internet, there are many well-made websites, such as the one by the national education system.
Also, it is important to give young people lifestyle advice to combat overweight, sedentary behavior, etc., by explaining to them that these factors can lead to sexual disorders later as well as infertility.
Another very important point: There is an inequality between boys and girls, but this time, to the disadvantage of boys. We have a sexual health consultation dedicated to young girls for the pill, but no one examines the boys. However, testicular cancer or undescended testicles can occur. I think we really need to change things and establish a clinical examination for boys in adolescence.
Question: More and more young people identify as asexual. What do you think of this?
Dr. Burté: People who identify as asexual represent about 1% of the population. These are individuals who are not attracted to having sexual relationships with someone. This does not prevent them from having a boyfriend, a girlfriend, masturbating, etc. It is sexual intercourse that does not interest them. These young people often say they have done it all. They have seen a lot of images, viewed sexuality as gymnastics with all the positions, tricks. They are jaded. Also, when you are faced with an image that provides a very strong and rapid stimulation, human relationships seem much more difficult because, obviously, you will never reproduce that sensation when you are with your partner with whom you must connect. The relationship is no longer emotional and shared. Yet, sexuality is emotional, relational, intellectual.
I think people go through phases. At a certain point, they feel asexual, but they can change their minds and think differently if they have real encounters, encounters that are increasingly difficult. Today, we are witnessing a loss of confidence. Young people, but also others, want to protect themselves from everything, especially from falling in love, not get back into a relationship because it is constraining.
Question: Data show that young people are exposed to pornography at an increasingly early age. Is this a problem for their future sexuality?
Dr. Burté: The exposure to pornography at an early age, around 11 years old, has only been a reality for the past decade. It is too early to say how it will impact their sexuality. When examining the literature on this subject, some publications indicate that the consequences can be dramatic for children. Others show that children can distinguish between reality and fantasy.
Whenever I see young people in consultation, I ask them whether they feel pornography has helped or hindered them, whether it is the cause of the issue they are facing. I would say that, other than those who have viewed pornography under duress, which is of the order of violence, pornography does not seem to pose a problem. It can even provide certain knowledge.
Question: What about sexual violence in children? What are the consequences?
Dr. Burté: In sexual medicine, this is one of the questions we ask systematically because it is very common. It is important to keep in mind that this not only affects girls; boys are also sexually abused. The consequences are dramatic in terms of psychosexual development. Each case is different.
Question: At the other end of life, is it “normal” to have sexual disorders at a certain age? Should we resign ourselves?
Dr. Burté: When it comes to sexuality, people have many misconceptions and beliefs that are conveyed through media and the Internet. One of them is to believe that because we are aging, we cannot have a proper sexuality. Sexuality slows down with age, as all sensitivities decrease, but desire is something present throughout life. Yet, seniors are rarely questioned about their sexual health by the media.
Note that older people in institutions face an additional obstacle: lack of privacy. Is this normal? Sexuality releases endorphins, oxytocin, it is well-being that costs nothing. It is something that should be prescribed!
Question: Chronic diseases, disabilities with incidence increases with age — are they not inevitable obstacles to a fulfilling sexuality?
Dr. Burté: It is possible to have a sexual life regardless of the disease one has, cancer, diabetes, rheumatic disease — regardless of the disability.
A collaboration with the National Cancer Institute on the preservation of sexual health after cancer in which I participated shows that people are extremely demanding of care and that this care is still very insufficient, unfortunately, even in the case of prostate cancer, for example, when it should be obvious.
Question: But aging itself brings challenges in terms of sexuality.
Dr. Burté: Yes, in men, the consequences of low testosterone levels are well known. Therefore, we must stop thinking that men do not have their “menopause.” Men often have a testosterone deficiency after a certain age. This is very annoying because they have many symptoms that are truly unpleasant and yet can be corrected by completely reliable treatments.
Men are very misinformed on this subject. We talk about gender inequality, but in this area, a young woman who has her first period knows very well that one day she will go through menopause, but a boy has no idea that one day he will have hormone problems.
Question: Therefore, is it important to question men past the age of 50 years?
Dr. Burté: Yes. Faced with sexual symptoms or simply fatigue, or among those who are a bit depressed, investigating a testosterone deficiency should be part of the reflexes.
Also, if you ask a man in general, “How is it going from a sexual point of view,” and he answers that everything is going well, this means he has good arteries, good veins, a good nervous system, sufficient hormones, and psychologically, everything is going rather well. Conversely, erectile dysfunction can be one of the first symptoms of cardiovascular pathologies.
After a certain age, there is no test that provides as much information about people’s health as this question about sexual health.
Question: On their side, are women better cared for at menopause?
Dr. Burté: Yes, but women still lack explanations. I work in sexual medicine, and in my consultation, I see women who come simply to get information about menopause.
Women must know that menopause is a turning point in life because they will spend 30%-40% of their lives without hormones.
It is important to explain that indeed, after menopause, without treatment, it is not the same. There are genital and urinary, psychological, sexual, and skin consequences. It is important to provide true data on the influence of hormonal treatments. Today, hormone fear is not over. I think we need to rehabilitate treatments, care for women.
Question: So we must not forget men or women.
Dr. Burté: Yes. It is also very important to adopt a perspective not only for the individual but also for the couple. If you treat a man with testosterone, after 3 months, he will be in great shape. However, if the couple has long been accustomed to having a limited sexual life, if the woman is not supported on her side, the couple will be unbalanced. The couple is concerned with managing the hormonal changes of both.
Question: Sexual medicine is essential, yet it seems inaccessible.
Dr. Burté: There are very few specialists in sexual medicine because there is no legal provision for it. These consultations are lengthy but not valued. Who wants to work for that?
If there was reimbursement for sexual medicine consultations at age 15 years, at menopause, and for men around the age of 50 years, it would change mentalities. Sexual medicine must be integrated into medicine. It should also be noted that not all sexologists are physicians.
Some people are very well trained through universities, and others are not. Ideally, someone with a sexual disorder should first have a sexual medicine consultation to understand the situation. Then, the physician can refer the patient to a competent sexologist because we work in a network.
Dr. Burté has no conflicts of interest related to the subject.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
From adolescence onward, the need for sexual health is particularly important. Yet, information and healthcare services are limited, which often leaves patients in distress and subject to misconceptions. What are the specific issues related to sexuality in adolescence, middle age, and beyond? This news organization interviewed Carol Burté, MD, a specialist in sexual medicine from Monaco.
Question: Regarding young individuals, what about sex education in schools?
Dr. Burté: The French law of 2018 specifies that at least three annual sessions must be devoted to sex education in elementary school, middle school, and high school.
In practice, this is not always the case, and interventions are very focused on prevention and rules. Sexuality is almost always absent from the program. Sexuality means: What does it mean to have desire? How does pleasure work? At what age do we have sex? etc. Young people receive prevention advice, but the link with sexuality is not made.
Sexuality remains taboo. You know, like in books: “They got married and had many children ...” End of the story, we don’t know more [laughs].
Question: And outside the school setting, do doctors sufficiently address sexual health issues with adolescents?
Dr. Burté: Rarely. I understand that a general practitioner has little time, but they can still ask the young person if they have any questions. They can refer them to someone or provide reading recommendations. Regarding sex education on the Internet, there are many well-made websites, such as the one by the national education system.
Also, it is important to give young people lifestyle advice to combat overweight, sedentary behavior, etc., by explaining to them that these factors can lead to sexual disorders later as well as infertility.
Another very important point: There is an inequality between boys and girls, but this time, to the disadvantage of boys. We have a sexual health consultation dedicated to young girls for the pill, but no one examines the boys. However, testicular cancer or undescended testicles can occur. I think we really need to change things and establish a clinical examination for boys in adolescence.
Question: More and more young people identify as asexual. What do you think of this?
Dr. Burté: People who identify as asexual represent about 1% of the population. These are individuals who are not attracted to having sexual relationships with someone. This does not prevent them from having a boyfriend, a girlfriend, masturbating, etc. It is sexual intercourse that does not interest them. These young people often say they have done it all. They have seen a lot of images, viewed sexuality as gymnastics with all the positions, tricks. They are jaded. Also, when you are faced with an image that provides a very strong and rapid stimulation, human relationships seem much more difficult because, obviously, you will never reproduce that sensation when you are with your partner with whom you must connect. The relationship is no longer emotional and shared. Yet, sexuality is emotional, relational, intellectual.
I think people go through phases. At a certain point, they feel asexual, but they can change their minds and think differently if they have real encounters, encounters that are increasingly difficult. Today, we are witnessing a loss of confidence. Young people, but also others, want to protect themselves from everything, especially from falling in love, not get back into a relationship because it is constraining.
Question: Data show that young people are exposed to pornography at an increasingly early age. Is this a problem for their future sexuality?
Dr. Burté: The exposure to pornography at an early age, around 11 years old, has only been a reality for the past decade. It is too early to say how it will impact their sexuality. When examining the literature on this subject, some publications indicate that the consequences can be dramatic for children. Others show that children can distinguish between reality and fantasy.
Whenever I see young people in consultation, I ask them whether they feel pornography has helped or hindered them, whether it is the cause of the issue they are facing. I would say that, other than those who have viewed pornography under duress, which is of the order of violence, pornography does not seem to pose a problem. It can even provide certain knowledge.
Question: What about sexual violence in children? What are the consequences?
Dr. Burté: In sexual medicine, this is one of the questions we ask systematically because it is very common. It is important to keep in mind that this not only affects girls; boys are also sexually abused. The consequences are dramatic in terms of psychosexual development. Each case is different.
Question: At the other end of life, is it “normal” to have sexual disorders at a certain age? Should we resign ourselves?
Dr. Burté: When it comes to sexuality, people have many misconceptions and beliefs that are conveyed through media and the Internet. One of them is to believe that because we are aging, we cannot have a proper sexuality. Sexuality slows down with age, as all sensitivities decrease, but desire is something present throughout life. Yet, seniors are rarely questioned about their sexual health by the media.
Note that older people in institutions face an additional obstacle: lack of privacy. Is this normal? Sexuality releases endorphins, oxytocin, it is well-being that costs nothing. It is something that should be prescribed!
Question: Chronic diseases, disabilities with incidence increases with age — are they not inevitable obstacles to a fulfilling sexuality?
Dr. Burté: It is possible to have a sexual life regardless of the disease one has, cancer, diabetes, rheumatic disease — regardless of the disability.
A collaboration with the National Cancer Institute on the preservation of sexual health after cancer in which I participated shows that people are extremely demanding of care and that this care is still very insufficient, unfortunately, even in the case of prostate cancer, for example, when it should be obvious.
Question: But aging itself brings challenges in terms of sexuality.
Dr. Burté: Yes, in men, the consequences of low testosterone levels are well known. Therefore, we must stop thinking that men do not have their “menopause.” Men often have a testosterone deficiency after a certain age. This is very annoying because they have many symptoms that are truly unpleasant and yet can be corrected by completely reliable treatments.
Men are very misinformed on this subject. We talk about gender inequality, but in this area, a young woman who has her first period knows very well that one day she will go through menopause, but a boy has no idea that one day he will have hormone problems.
Question: Therefore, is it important to question men past the age of 50 years?
Dr. Burté: Yes. Faced with sexual symptoms or simply fatigue, or among those who are a bit depressed, investigating a testosterone deficiency should be part of the reflexes.
Also, if you ask a man in general, “How is it going from a sexual point of view,” and he answers that everything is going well, this means he has good arteries, good veins, a good nervous system, sufficient hormones, and psychologically, everything is going rather well. Conversely, erectile dysfunction can be one of the first symptoms of cardiovascular pathologies.
After a certain age, there is no test that provides as much information about people’s health as this question about sexual health.
Question: On their side, are women better cared for at menopause?
Dr. Burté: Yes, but women still lack explanations. I work in sexual medicine, and in my consultation, I see women who come simply to get information about menopause.
Women must know that menopause is a turning point in life because they will spend 30%-40% of their lives without hormones.
It is important to explain that indeed, after menopause, without treatment, it is not the same. There are genital and urinary, psychological, sexual, and skin consequences. It is important to provide true data on the influence of hormonal treatments. Today, hormone fear is not over. I think we need to rehabilitate treatments, care for women.
Question: So we must not forget men or women.
Dr. Burté: Yes. It is also very important to adopt a perspective not only for the individual but also for the couple. If you treat a man with testosterone, after 3 months, he will be in great shape. However, if the couple has long been accustomed to having a limited sexual life, if the woman is not supported on her side, the couple will be unbalanced. The couple is concerned with managing the hormonal changes of both.
Question: Sexual medicine is essential, yet it seems inaccessible.
Dr. Burté: There are very few specialists in sexual medicine because there is no legal provision for it. These consultations are lengthy but not valued. Who wants to work for that?
If there was reimbursement for sexual medicine consultations at age 15 years, at menopause, and for men around the age of 50 years, it would change mentalities. Sexual medicine must be integrated into medicine. It should also be noted that not all sexologists are physicians.
Some people are very well trained through universities, and others are not. Ideally, someone with a sexual disorder should first have a sexual medicine consultation to understand the situation. Then, the physician can refer the patient to a competent sexologist because we work in a network.
Dr. Burté has no conflicts of interest related to the subject.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
From adolescence onward, the need for sexual health is particularly important. Yet, information and healthcare services are limited, which often leaves patients in distress and subject to misconceptions. What are the specific issues related to sexuality in adolescence, middle age, and beyond? This news organization interviewed Carol Burté, MD, a specialist in sexual medicine from Monaco.
Question: Regarding young individuals, what about sex education in schools?
Dr. Burté: The French law of 2018 specifies that at least three annual sessions must be devoted to sex education in elementary school, middle school, and high school.
In practice, this is not always the case, and interventions are very focused on prevention and rules. Sexuality is almost always absent from the program. Sexuality means: What does it mean to have desire? How does pleasure work? At what age do we have sex? etc. Young people receive prevention advice, but the link with sexuality is not made.
Sexuality remains taboo. You know, like in books: “They got married and had many children ...” End of the story, we don’t know more [laughs].
Question: And outside the school setting, do doctors sufficiently address sexual health issues with adolescents?
Dr. Burté: Rarely. I understand that a general practitioner has little time, but they can still ask the young person if they have any questions. They can refer them to someone or provide reading recommendations. Regarding sex education on the Internet, there are many well-made websites, such as the one by the national education system.
Also, it is important to give young people lifestyle advice to combat overweight, sedentary behavior, etc., by explaining to them that these factors can lead to sexual disorders later as well as infertility.
Another very important point: There is an inequality between boys and girls, but this time, to the disadvantage of boys. We have a sexual health consultation dedicated to young girls for the pill, but no one examines the boys. However, testicular cancer or undescended testicles can occur. I think we really need to change things and establish a clinical examination for boys in adolescence.
Question: More and more young people identify as asexual. What do you think of this?
Dr. Burté: People who identify as asexual represent about 1% of the population. These are individuals who are not attracted to having sexual relationships with someone. This does not prevent them from having a boyfriend, a girlfriend, masturbating, etc. It is sexual intercourse that does not interest them. These young people often say they have done it all. They have seen a lot of images, viewed sexuality as gymnastics with all the positions, tricks. They are jaded. Also, when you are faced with an image that provides a very strong and rapid stimulation, human relationships seem much more difficult because, obviously, you will never reproduce that sensation when you are with your partner with whom you must connect. The relationship is no longer emotional and shared. Yet, sexuality is emotional, relational, intellectual.
I think people go through phases. At a certain point, they feel asexual, but they can change their minds and think differently if they have real encounters, encounters that are increasingly difficult. Today, we are witnessing a loss of confidence. Young people, but also others, want to protect themselves from everything, especially from falling in love, not get back into a relationship because it is constraining.
Question: Data show that young people are exposed to pornography at an increasingly early age. Is this a problem for their future sexuality?
Dr. Burté: The exposure to pornography at an early age, around 11 years old, has only been a reality for the past decade. It is too early to say how it will impact their sexuality. When examining the literature on this subject, some publications indicate that the consequences can be dramatic for children. Others show that children can distinguish between reality and fantasy.
Whenever I see young people in consultation, I ask them whether they feel pornography has helped or hindered them, whether it is the cause of the issue they are facing. I would say that, other than those who have viewed pornography under duress, which is of the order of violence, pornography does not seem to pose a problem. It can even provide certain knowledge.
Question: What about sexual violence in children? What are the consequences?
Dr. Burté: In sexual medicine, this is one of the questions we ask systematically because it is very common. It is important to keep in mind that this not only affects girls; boys are also sexually abused. The consequences are dramatic in terms of psychosexual development. Each case is different.
Question: At the other end of life, is it “normal” to have sexual disorders at a certain age? Should we resign ourselves?
Dr. Burté: When it comes to sexuality, people have many misconceptions and beliefs that are conveyed through media and the Internet. One of them is to believe that because we are aging, we cannot have a proper sexuality. Sexuality slows down with age, as all sensitivities decrease, but desire is something present throughout life. Yet, seniors are rarely questioned about their sexual health by the media.
Note that older people in institutions face an additional obstacle: lack of privacy. Is this normal? Sexuality releases endorphins, oxytocin, it is well-being that costs nothing. It is something that should be prescribed!
Question: Chronic diseases, disabilities with incidence increases with age — are they not inevitable obstacles to a fulfilling sexuality?
Dr. Burté: It is possible to have a sexual life regardless of the disease one has, cancer, diabetes, rheumatic disease — regardless of the disability.
A collaboration with the National Cancer Institute on the preservation of sexual health after cancer in which I participated shows that people are extremely demanding of care and that this care is still very insufficient, unfortunately, even in the case of prostate cancer, for example, when it should be obvious.
Question: But aging itself brings challenges in terms of sexuality.
Dr. Burté: Yes, in men, the consequences of low testosterone levels are well known. Therefore, we must stop thinking that men do not have their “menopause.” Men often have a testosterone deficiency after a certain age. This is very annoying because they have many symptoms that are truly unpleasant and yet can be corrected by completely reliable treatments.
Men are very misinformed on this subject. We talk about gender inequality, but in this area, a young woman who has her first period knows very well that one day she will go through menopause, but a boy has no idea that one day he will have hormone problems.
Question: Therefore, is it important to question men past the age of 50 years?
Dr. Burté: Yes. Faced with sexual symptoms or simply fatigue, or among those who are a bit depressed, investigating a testosterone deficiency should be part of the reflexes.
Also, if you ask a man in general, “How is it going from a sexual point of view,” and he answers that everything is going well, this means he has good arteries, good veins, a good nervous system, sufficient hormones, and psychologically, everything is going rather well. Conversely, erectile dysfunction can be one of the first symptoms of cardiovascular pathologies.
After a certain age, there is no test that provides as much information about people’s health as this question about sexual health.
Question: On their side, are women better cared for at menopause?
Dr. Burté: Yes, but women still lack explanations. I work in sexual medicine, and in my consultation, I see women who come simply to get information about menopause.
Women must know that menopause is a turning point in life because they will spend 30%-40% of their lives without hormones.
It is important to explain that indeed, after menopause, without treatment, it is not the same. There are genital and urinary, psychological, sexual, and skin consequences. It is important to provide true data on the influence of hormonal treatments. Today, hormone fear is not over. I think we need to rehabilitate treatments, care for women.
Question: So we must not forget men or women.
Dr. Burté: Yes. It is also very important to adopt a perspective not only for the individual but also for the couple. If you treat a man with testosterone, after 3 months, he will be in great shape. However, if the couple has long been accustomed to having a limited sexual life, if the woman is not supported on her side, the couple will be unbalanced. The couple is concerned with managing the hormonal changes of both.
Question: Sexual medicine is essential, yet it seems inaccessible.
Dr. Burté: There are very few specialists in sexual medicine because there is no legal provision for it. These consultations are lengthy but not valued. Who wants to work for that?
If there was reimbursement for sexual medicine consultations at age 15 years, at menopause, and for men around the age of 50 years, it would change mentalities. Sexual medicine must be integrated into medicine. It should also be noted that not all sexologists are physicians.
Some people are very well trained through universities, and others are not. Ideally, someone with a sexual disorder should first have a sexual medicine consultation to understand the situation. Then, the physician can refer the patient to a competent sexologist because we work in a network.
Dr. Burté has no conflicts of interest related to the subject.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Primary Care: Try These Steps to Boost Lung Cancer Screens
A few years ago, Kim Lori Sandler, MD, realized many patients newly diagnosed with lung cancer had never been screened for the disease — they received CT scans only because they were symptomatic.
But Dr. Sandler, a radiologist at Vanderbilt University Medical Center in Nashville, Tennessee, could see in medical charts that most of these patients had been eligible for a screening before becoming symptomatic. And for women, most had received decades worth of mammograms. She saw an opportunity and launched a study to find out if an intervention would work.
Low-dose CT and mammography services often are available in the same imaging facility, so women who qualified for a lung cancer screening were offered the scan during their mammography visit. Over a 3-year period, monthly rates of lung scans in women rose by 50% at one facility and 36% at the other.
“What we found is that women are really receptive, if you talk to them about it,” Dr. Sandler said. “I don’t think that lung cancer is thought of as a disease in women.”
Although lung cancer is the leading cause of cancer deaths in the United States, a recent study in JAMA Internal Medicine found only 18% of eligible patients were screened in 2022, a far cry from the rates of 72% for colon cancer — which itself falls short of goals from US medical groups like the American Cancer Society (ACS). Among those eligible, rates of lung screenings were lowest among younger people without comorbid conditions, who did not have health insurance or a usual source of care, and those living in southern states and states that did not expand Medicaid as part of the Affordable Care Act.
Getting patients screened is lifesaving: 27% of people with lung cancer survive 5 years after diagnosis. But the survival rate rises to 63% when cases are diagnosed at an early stage.
Increasing Uptake
The formal recommendation to use low-dose chest CT to screen for lung cancer is only a decade old. The approach was first endorsed by the United States Preventive Services Task Force (USPSTF) on the basis of an influential trial that found such testing was linked to a 20% reduction in mortality from the disease. Updated 2021 USPSTF guidelines call for annual screening of people aged 50-80 years who have a 20 pack-year history of smoking and currently smoke or have quit within the past 15 years.
But implementing the recommendation is not always simple. Unlike a colorectal or breast cancer screening, which is recommended primarily on patient age, eligibility for a lung cancer screening requires calculating pack-years of smoking, and, for past smokers, knowledge of when they quit.
The structured fields in most electronic medical records (EMRs) inquire about current or past use of cigarettes and the number of daily packs smoked. But few EMRs can calculate when a patient starts smoking two cigarettes a day but then increases to a pack a day and cuts down again. EMRs also do not track when a patient has stopped smoking permanently. Individual clinicians or health systems must identify patients who are eligible for screening, but the lack of automated calculations makes that job more difficult.
Dr. Sandler and colleagues turned to the informatics team at Vanderbilt to develop a natural language processing approach that extracts smoking data directly from clinician notes instead of using standard variables in their EMR.
The number of patients identified as needing a screening using the algorithm nearly doubled from baseline, from 5887 to 10,231 over a 3-year period, according to results from another study that Dr. Sandler published.
Although the algorithm may occasionally flag someone who does not need screening as eligible, “you can always have a conversation with the patient to determine if they actually meet eligibility criteria,” Dr. Sandler said.
Patient Navigators to the Rescue?
About a decade ago, Travis Baggett, MD, MPH, an associate professor of internal medicine at Harvard Medical School, Boston, Massachusetts, received pilot funding from the ACS to study cancer epidemiology among patients at Boston Health Care for the Homeless Program (BHCHP), which serves nearly 10,000 patients at a variety of Boston-area clinics each year.
“We found that both the incidence and mortality rates for lung cancer were more than twofold higher than in the general population,” Dr. Baggett, who is also the director of research at BHCHP, said.
He also discovered that BHCHP patients were diagnosed at significantly later stages than people in the general population for malignancies like breast and colorectal cancer.
Screening for lung cancer was a new recommendation at the time. With additional funding from the ACS, he launched a clinical trial in 2020 that randomized patients who were eligible for lung cancer screening to either work with a patient navigator or receive usual care.
The navigators eased the burden on primary care clinicians: They facilitated shared decision-making visits, helped participants make and attend appointments for low-dose CT, assisted with transportation, and arranged follow-up as needed.
The 3-year study found 43% of patients who received navigation services underwent screening for lung cancer, compared with 9% in the usual-care arm. Participants said the navigators played a critical role in educating them about the importance of screening, coordinating care, and providing emotional support.
“At the root of it all, it was quite clear that one thing that made the navigator successful was their interpersonal qualities and having someone that the patient could trust to help guide them through the process,” Dr. Baggett said.
The navigator program, however, stopped when the funding for the study ended.
But another health system has implemented navigators in a sustainable way through a quality improvement project. Michael Gieske, MD, director of lung cancer screening at St. Elizabeth Healthcare in Edgewood, Kentucky, starts his Friday morning meeting with a multidisciplinary group, including a thoracic surgeon, radiologist, pulmonologist, and several screening nurse navigators. They review the week’s chest CTs, with approximately one-third from patients who underwent lung cancer screening.
Nurse navigators at St. Elizabeth Healthcare follow up with any patient whose scan is suspicious for lung cancer and guide them through the process of seeing specialists and obtaining additional testing.
“They essentially hold the patient’s hand through this scary time in their life and make sure that everything flows smoothly and efficiently,” said Dr. Gieske, a family medicine physician.
St. Elizabeth’s program also draws on several evidence-based strategies used for other cancer screening programs, such as patient and provider education and quarterly feedback to their 194 primary care clinicians on rates of lung cancer screening among their eligible patients.
Several requirements for reimbursement for a lung cancer screening from the US Centers for Medicare & Medicaid Services can also serve as barriers to getting patients screened: Clinicians must identify who is eligible, provide tobacco cessation counseling, and document the shared decision-making process.
To streamline the steps, St. Elizabeth’s clinicians use an EMR smart set that reminds clinicians to verify smoking history and helps them document the required counseling.
Last year, 47% of eligible patients received their recommended screening, and Dr. Gieske said he expects even more improvement.
“We’re on track this year to complete 60% uptake if things continue,” he said, adding that 76% of the new cases of lung cancer are now diagnosed in stage I, with only 5% diagnosed in stage IV.
Dr. Gieske has shared his experience with many clinics in Appalachia, home to some of the highest rates of mortality from lung cancer in the country. A major part of his role with the Appalachian Community Cancer Alliance is helping educate primary care clinicians in the region about the importance of early detection of lung cancer.
“I think one of the most important things is just to convey a message of hope,” he said. “We’re trying to get the good word out there that if you screen individuals, you’re going to catch it early, when you have an extremely high chance of curing the lung cancer.”
Dr. Baggett reported support from grants from the ACS and the Massachusetts General Hospital Research Scholars Program. Dr. Sandler and Dr. Gieske reported no financial conflicts.
A version of this article first appeared on Medscape.com.
A few years ago, Kim Lori Sandler, MD, realized many patients newly diagnosed with lung cancer had never been screened for the disease — they received CT scans only because they were symptomatic.
But Dr. Sandler, a radiologist at Vanderbilt University Medical Center in Nashville, Tennessee, could see in medical charts that most of these patients had been eligible for a screening before becoming symptomatic. And for women, most had received decades worth of mammograms. She saw an opportunity and launched a study to find out if an intervention would work.
Low-dose CT and mammography services often are available in the same imaging facility, so women who qualified for a lung cancer screening were offered the scan during their mammography visit. Over a 3-year period, monthly rates of lung scans in women rose by 50% at one facility and 36% at the other.
“What we found is that women are really receptive, if you talk to them about it,” Dr. Sandler said. “I don’t think that lung cancer is thought of as a disease in women.”
Although lung cancer is the leading cause of cancer deaths in the United States, a recent study in JAMA Internal Medicine found only 18% of eligible patients were screened in 2022, a far cry from the rates of 72% for colon cancer — which itself falls short of goals from US medical groups like the American Cancer Society (ACS). Among those eligible, rates of lung screenings were lowest among younger people without comorbid conditions, who did not have health insurance or a usual source of care, and those living in southern states and states that did not expand Medicaid as part of the Affordable Care Act.
Getting patients screened is lifesaving: 27% of people with lung cancer survive 5 years after diagnosis. But the survival rate rises to 63% when cases are diagnosed at an early stage.
Increasing Uptake
The formal recommendation to use low-dose chest CT to screen for lung cancer is only a decade old. The approach was first endorsed by the United States Preventive Services Task Force (USPSTF) on the basis of an influential trial that found such testing was linked to a 20% reduction in mortality from the disease. Updated 2021 USPSTF guidelines call for annual screening of people aged 50-80 years who have a 20 pack-year history of smoking and currently smoke or have quit within the past 15 years.
But implementing the recommendation is not always simple. Unlike a colorectal or breast cancer screening, which is recommended primarily on patient age, eligibility for a lung cancer screening requires calculating pack-years of smoking, and, for past smokers, knowledge of when they quit.
The structured fields in most electronic medical records (EMRs) inquire about current or past use of cigarettes and the number of daily packs smoked. But few EMRs can calculate when a patient starts smoking two cigarettes a day but then increases to a pack a day and cuts down again. EMRs also do not track when a patient has stopped smoking permanently. Individual clinicians or health systems must identify patients who are eligible for screening, but the lack of automated calculations makes that job more difficult.
Dr. Sandler and colleagues turned to the informatics team at Vanderbilt to develop a natural language processing approach that extracts smoking data directly from clinician notes instead of using standard variables in their EMR.
The number of patients identified as needing a screening using the algorithm nearly doubled from baseline, from 5887 to 10,231 over a 3-year period, according to results from another study that Dr. Sandler published.
Although the algorithm may occasionally flag someone who does not need screening as eligible, “you can always have a conversation with the patient to determine if they actually meet eligibility criteria,” Dr. Sandler said.
Patient Navigators to the Rescue?
About a decade ago, Travis Baggett, MD, MPH, an associate professor of internal medicine at Harvard Medical School, Boston, Massachusetts, received pilot funding from the ACS to study cancer epidemiology among patients at Boston Health Care for the Homeless Program (BHCHP), which serves nearly 10,000 patients at a variety of Boston-area clinics each year.
“We found that both the incidence and mortality rates for lung cancer were more than twofold higher than in the general population,” Dr. Baggett, who is also the director of research at BHCHP, said.
He also discovered that BHCHP patients were diagnosed at significantly later stages than people in the general population for malignancies like breast and colorectal cancer.
Screening for lung cancer was a new recommendation at the time. With additional funding from the ACS, he launched a clinical trial in 2020 that randomized patients who were eligible for lung cancer screening to either work with a patient navigator or receive usual care.
The navigators eased the burden on primary care clinicians: They facilitated shared decision-making visits, helped participants make and attend appointments for low-dose CT, assisted with transportation, and arranged follow-up as needed.
The 3-year study found 43% of patients who received navigation services underwent screening for lung cancer, compared with 9% in the usual-care arm. Participants said the navigators played a critical role in educating them about the importance of screening, coordinating care, and providing emotional support.
“At the root of it all, it was quite clear that one thing that made the navigator successful was their interpersonal qualities and having someone that the patient could trust to help guide them through the process,” Dr. Baggett said.
The navigator program, however, stopped when the funding for the study ended.
But another health system has implemented navigators in a sustainable way through a quality improvement project. Michael Gieske, MD, director of lung cancer screening at St. Elizabeth Healthcare in Edgewood, Kentucky, starts his Friday morning meeting with a multidisciplinary group, including a thoracic surgeon, radiologist, pulmonologist, and several screening nurse navigators. They review the week’s chest CTs, with approximately one-third from patients who underwent lung cancer screening.
Nurse navigators at St. Elizabeth Healthcare follow up with any patient whose scan is suspicious for lung cancer and guide them through the process of seeing specialists and obtaining additional testing.
“They essentially hold the patient’s hand through this scary time in their life and make sure that everything flows smoothly and efficiently,” said Dr. Gieske, a family medicine physician.
St. Elizabeth’s program also draws on several evidence-based strategies used for other cancer screening programs, such as patient and provider education and quarterly feedback to their 194 primary care clinicians on rates of lung cancer screening among their eligible patients.
Several requirements for reimbursement for a lung cancer screening from the US Centers for Medicare & Medicaid Services can also serve as barriers to getting patients screened: Clinicians must identify who is eligible, provide tobacco cessation counseling, and document the shared decision-making process.
To streamline the steps, St. Elizabeth’s clinicians use an EMR smart set that reminds clinicians to verify smoking history and helps them document the required counseling.
Last year, 47% of eligible patients received their recommended screening, and Dr. Gieske said he expects even more improvement.
“We’re on track this year to complete 60% uptake if things continue,” he said, adding that 76% of the new cases of lung cancer are now diagnosed in stage I, with only 5% diagnosed in stage IV.
Dr. Gieske has shared his experience with many clinics in Appalachia, home to some of the highest rates of mortality from lung cancer in the country. A major part of his role with the Appalachian Community Cancer Alliance is helping educate primary care clinicians in the region about the importance of early detection of lung cancer.
“I think one of the most important things is just to convey a message of hope,” he said. “We’re trying to get the good word out there that if you screen individuals, you’re going to catch it early, when you have an extremely high chance of curing the lung cancer.”
Dr. Baggett reported support from grants from the ACS and the Massachusetts General Hospital Research Scholars Program. Dr. Sandler and Dr. Gieske reported no financial conflicts.
A version of this article first appeared on Medscape.com.
A few years ago, Kim Lori Sandler, MD, realized many patients newly diagnosed with lung cancer had never been screened for the disease — they received CT scans only because they were symptomatic.
But Dr. Sandler, a radiologist at Vanderbilt University Medical Center in Nashville, Tennessee, could see in medical charts that most of these patients had been eligible for a screening before becoming symptomatic. And for women, most had received decades worth of mammograms. She saw an opportunity and launched a study to find out if an intervention would work.
Low-dose CT and mammography services often are available in the same imaging facility, so women who qualified for a lung cancer screening were offered the scan during their mammography visit. Over a 3-year period, monthly rates of lung scans in women rose by 50% at one facility and 36% at the other.
“What we found is that women are really receptive, if you talk to them about it,” Dr. Sandler said. “I don’t think that lung cancer is thought of as a disease in women.”
Although lung cancer is the leading cause of cancer deaths in the United States, a recent study in JAMA Internal Medicine found only 18% of eligible patients were screened in 2022, a far cry from the rates of 72% for colon cancer — which itself falls short of goals from US medical groups like the American Cancer Society (ACS). Among those eligible, rates of lung screenings were lowest among younger people without comorbid conditions, who did not have health insurance or a usual source of care, and those living in southern states and states that did not expand Medicaid as part of the Affordable Care Act.
Getting patients screened is lifesaving: 27% of people with lung cancer survive 5 years after diagnosis. But the survival rate rises to 63% when cases are diagnosed at an early stage.
Increasing Uptake
The formal recommendation to use low-dose chest CT to screen for lung cancer is only a decade old. The approach was first endorsed by the United States Preventive Services Task Force (USPSTF) on the basis of an influential trial that found such testing was linked to a 20% reduction in mortality from the disease. Updated 2021 USPSTF guidelines call for annual screening of people aged 50-80 years who have a 20 pack-year history of smoking and currently smoke or have quit within the past 15 years.
But implementing the recommendation is not always simple. Unlike a colorectal or breast cancer screening, which is recommended primarily on patient age, eligibility for a lung cancer screening requires calculating pack-years of smoking, and, for past smokers, knowledge of when they quit.
The structured fields in most electronic medical records (EMRs) inquire about current or past use of cigarettes and the number of daily packs smoked. But few EMRs can calculate when a patient starts smoking two cigarettes a day but then increases to a pack a day and cuts down again. EMRs also do not track when a patient has stopped smoking permanently. Individual clinicians or health systems must identify patients who are eligible for screening, but the lack of automated calculations makes that job more difficult.
Dr. Sandler and colleagues turned to the informatics team at Vanderbilt to develop a natural language processing approach that extracts smoking data directly from clinician notes instead of using standard variables in their EMR.
The number of patients identified as needing a screening using the algorithm nearly doubled from baseline, from 5887 to 10,231 over a 3-year period, according to results from another study that Dr. Sandler published.
Although the algorithm may occasionally flag someone who does not need screening as eligible, “you can always have a conversation with the patient to determine if they actually meet eligibility criteria,” Dr. Sandler said.
Patient Navigators to the Rescue?
About a decade ago, Travis Baggett, MD, MPH, an associate professor of internal medicine at Harvard Medical School, Boston, Massachusetts, received pilot funding from the ACS to study cancer epidemiology among patients at Boston Health Care for the Homeless Program (BHCHP), which serves nearly 10,000 patients at a variety of Boston-area clinics each year.
“We found that both the incidence and mortality rates for lung cancer were more than twofold higher than in the general population,” Dr. Baggett, who is also the director of research at BHCHP, said.
He also discovered that BHCHP patients were diagnosed at significantly later stages than people in the general population for malignancies like breast and colorectal cancer.
Screening for lung cancer was a new recommendation at the time. With additional funding from the ACS, he launched a clinical trial in 2020 that randomized patients who were eligible for lung cancer screening to either work with a patient navigator or receive usual care.
The navigators eased the burden on primary care clinicians: They facilitated shared decision-making visits, helped participants make and attend appointments for low-dose CT, assisted with transportation, and arranged follow-up as needed.
The 3-year study found 43% of patients who received navigation services underwent screening for lung cancer, compared with 9% in the usual-care arm. Participants said the navigators played a critical role in educating them about the importance of screening, coordinating care, and providing emotional support.
“At the root of it all, it was quite clear that one thing that made the navigator successful was their interpersonal qualities and having someone that the patient could trust to help guide them through the process,” Dr. Baggett said.
The navigator program, however, stopped when the funding for the study ended.
But another health system has implemented navigators in a sustainable way through a quality improvement project. Michael Gieske, MD, director of lung cancer screening at St. Elizabeth Healthcare in Edgewood, Kentucky, starts his Friday morning meeting with a multidisciplinary group, including a thoracic surgeon, radiologist, pulmonologist, and several screening nurse navigators. They review the week’s chest CTs, with approximately one-third from patients who underwent lung cancer screening.
Nurse navigators at St. Elizabeth Healthcare follow up with any patient whose scan is suspicious for lung cancer and guide them through the process of seeing specialists and obtaining additional testing.
“They essentially hold the patient’s hand through this scary time in their life and make sure that everything flows smoothly and efficiently,” said Dr. Gieske, a family medicine physician.
St. Elizabeth’s program also draws on several evidence-based strategies used for other cancer screening programs, such as patient and provider education and quarterly feedback to their 194 primary care clinicians on rates of lung cancer screening among their eligible patients.
Several requirements for reimbursement for a lung cancer screening from the US Centers for Medicare & Medicaid Services can also serve as barriers to getting patients screened: Clinicians must identify who is eligible, provide tobacco cessation counseling, and document the shared decision-making process.
To streamline the steps, St. Elizabeth’s clinicians use an EMR smart set that reminds clinicians to verify smoking history and helps them document the required counseling.
Last year, 47% of eligible patients received their recommended screening, and Dr. Gieske said he expects even more improvement.
“We’re on track this year to complete 60% uptake if things continue,” he said, adding that 76% of the new cases of lung cancer are now diagnosed in stage I, with only 5% diagnosed in stage IV.
Dr. Gieske has shared his experience with many clinics in Appalachia, home to some of the highest rates of mortality from lung cancer in the country. A major part of his role with the Appalachian Community Cancer Alliance is helping educate primary care clinicians in the region about the importance of early detection of lung cancer.
“I think one of the most important things is just to convey a message of hope,” he said. “We’re trying to get the good word out there that if you screen individuals, you’re going to catch it early, when you have an extremely high chance of curing the lung cancer.”
Dr. Baggett reported support from grants from the ACS and the Massachusetts General Hospital Research Scholars Program. Dr. Sandler and Dr. Gieske reported no financial conflicts.
A version of this article first appeared on Medscape.com.
Dengue Surge in US Cases This Year
Federal health officials with the US Centers for Disease Control and Prevention (CDC) have issued an alert, warning health professionals and the public about an increased risk for dengue virus infections in the United States.
The global incidence of dengue in 2024 is the highest on record, reported the agency.
In the United States, Puerto Rico has declared a public health emergency, with 1498 dengue cases reported so far and a “higher-than-expected” number of dengue cases having been identified among US travelers in the first half of this year at 745 cases, according to the alert.
The CDC reports 197 dengue cases in Florida, 134 in New York, 50 in Massachusetts, 40 in California, 14 in Colorado, nine in Arizona, and eight in the District of Columbia, among others.
Transmitted by infected Aedes genus mosquitoes, dengue is the most common arboviral disease globally and is a nationally notifiable disease in the United States.
The six US territories and freely associated states with frequent or continuous dengue transmission are Puerto Rico, American Samoa, the US Virgin Islands, the Federated States of Micronesia, the Republic of the Marshall Islands, and the Republic of Palau.
Monitoring for Dengue
With rising global and domestic cases of dengue, the CDC urges healthcare providers to monitor for dengue:
- Maintain a high index of suspicion in patients with fever who have been in areas with frequent or continuous dengue transmission within 14 days before illness onset.
- Order diagnostic tests for acute dengue infection such as reverse transcription polymerase chain reaction and immunoglobulin M (IgM) antibody tests or nonstructural protein 1 antigen tests and IgM antibody tests.
- Ensure timely reporting of dengue cases to public health authorities.
- Promote mosquito bite prevention measures among people living in or visiting areas with frequent or continuous dengue transmission.
Roughly one in four dengue virus infections are symptomatic and can be mild or severe. Symptoms begin after an incubation period of about 5-7 days.
Symptoms include fever accompanied by nonspecific signs and symptoms such as nausea, vomiting, rash, muscle aches, joint pain, bone pain, pain behind the eyes, headache, or low white blood cell counts.
Disease Progression
Warning signs that may predict progression to severe disease include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and progressive increase in hematocrit or liver enlargement.
One in 20 people with symptomatic dengue will develop severe disease, with bleeding, shock, or respiratory distress caused by plasma leakage or end-organ impairment.
Infants aged a year or younger, pregnant people, adults aged 65 years or older, people with certain medical conditions, and those with previous dengue infections are at increased risk for severe dengue.
“Healthcare providers should be prepared to recognize, diagnose, manage, and report dengue cases to health authorities; public health partners should investigate cases and disseminate clear prevention messages to the public,” the alert stated.
The CDC is actively implementing several strategies to address the increase in cases of dengue in the United States. In early April, the agency launched a program-led emergency response and is providing monthly situational updates on dengue to partners, stakeholders, and jurisdictions.
The CDC is also expanding laboratory capacity to improve laboratory testing approaches; collaborating with state, tribal, local, and territorial health departments to strengthen dengue surveillance and recommend prevention strategies; and working to educate the public on dengue prevention.
A version of this article first appeared on Medscape.com.
Federal health officials with the US Centers for Disease Control and Prevention (CDC) have issued an alert, warning health professionals and the public about an increased risk for dengue virus infections in the United States.
The global incidence of dengue in 2024 is the highest on record, reported the agency.
In the United States, Puerto Rico has declared a public health emergency, with 1498 dengue cases reported so far and a “higher-than-expected” number of dengue cases having been identified among US travelers in the first half of this year at 745 cases, according to the alert.
The CDC reports 197 dengue cases in Florida, 134 in New York, 50 in Massachusetts, 40 in California, 14 in Colorado, nine in Arizona, and eight in the District of Columbia, among others.
Transmitted by infected Aedes genus mosquitoes, dengue is the most common arboviral disease globally and is a nationally notifiable disease in the United States.
The six US territories and freely associated states with frequent or continuous dengue transmission are Puerto Rico, American Samoa, the US Virgin Islands, the Federated States of Micronesia, the Republic of the Marshall Islands, and the Republic of Palau.
Monitoring for Dengue
With rising global and domestic cases of dengue, the CDC urges healthcare providers to monitor for dengue:
- Maintain a high index of suspicion in patients with fever who have been in areas with frequent or continuous dengue transmission within 14 days before illness onset.
- Order diagnostic tests for acute dengue infection such as reverse transcription polymerase chain reaction and immunoglobulin M (IgM) antibody tests or nonstructural protein 1 antigen tests and IgM antibody tests.
- Ensure timely reporting of dengue cases to public health authorities.
- Promote mosquito bite prevention measures among people living in or visiting areas with frequent or continuous dengue transmission.
Roughly one in four dengue virus infections are symptomatic and can be mild or severe. Symptoms begin after an incubation period of about 5-7 days.
Symptoms include fever accompanied by nonspecific signs and symptoms such as nausea, vomiting, rash, muscle aches, joint pain, bone pain, pain behind the eyes, headache, or low white blood cell counts.
Disease Progression
Warning signs that may predict progression to severe disease include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and progressive increase in hematocrit or liver enlargement.
One in 20 people with symptomatic dengue will develop severe disease, with bleeding, shock, or respiratory distress caused by plasma leakage or end-organ impairment.
Infants aged a year or younger, pregnant people, adults aged 65 years or older, people with certain medical conditions, and those with previous dengue infections are at increased risk for severe dengue.
“Healthcare providers should be prepared to recognize, diagnose, manage, and report dengue cases to health authorities; public health partners should investigate cases and disseminate clear prevention messages to the public,” the alert stated.
The CDC is actively implementing several strategies to address the increase in cases of dengue in the United States. In early April, the agency launched a program-led emergency response and is providing monthly situational updates on dengue to partners, stakeholders, and jurisdictions.
The CDC is also expanding laboratory capacity to improve laboratory testing approaches; collaborating with state, tribal, local, and territorial health departments to strengthen dengue surveillance and recommend prevention strategies; and working to educate the public on dengue prevention.
A version of this article first appeared on Medscape.com.
Federal health officials with the US Centers for Disease Control and Prevention (CDC) have issued an alert, warning health professionals and the public about an increased risk for dengue virus infections in the United States.
The global incidence of dengue in 2024 is the highest on record, reported the agency.
In the United States, Puerto Rico has declared a public health emergency, with 1498 dengue cases reported so far and a “higher-than-expected” number of dengue cases having been identified among US travelers in the first half of this year at 745 cases, according to the alert.
The CDC reports 197 dengue cases in Florida, 134 in New York, 50 in Massachusetts, 40 in California, 14 in Colorado, nine in Arizona, and eight in the District of Columbia, among others.
Transmitted by infected Aedes genus mosquitoes, dengue is the most common arboviral disease globally and is a nationally notifiable disease in the United States.
The six US territories and freely associated states with frequent or continuous dengue transmission are Puerto Rico, American Samoa, the US Virgin Islands, the Federated States of Micronesia, the Republic of the Marshall Islands, and the Republic of Palau.
Monitoring for Dengue
With rising global and domestic cases of dengue, the CDC urges healthcare providers to monitor for dengue:
- Maintain a high index of suspicion in patients with fever who have been in areas with frequent or continuous dengue transmission within 14 days before illness onset.
- Order diagnostic tests for acute dengue infection such as reverse transcription polymerase chain reaction and immunoglobulin M (IgM) antibody tests or nonstructural protein 1 antigen tests and IgM antibody tests.
- Ensure timely reporting of dengue cases to public health authorities.
- Promote mosquito bite prevention measures among people living in or visiting areas with frequent or continuous dengue transmission.
Roughly one in four dengue virus infections are symptomatic and can be mild or severe. Symptoms begin after an incubation period of about 5-7 days.
Symptoms include fever accompanied by nonspecific signs and symptoms such as nausea, vomiting, rash, muscle aches, joint pain, bone pain, pain behind the eyes, headache, or low white blood cell counts.
Disease Progression
Warning signs that may predict progression to severe disease include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and progressive increase in hematocrit or liver enlargement.
One in 20 people with symptomatic dengue will develop severe disease, with bleeding, shock, or respiratory distress caused by plasma leakage or end-organ impairment.
Infants aged a year or younger, pregnant people, adults aged 65 years or older, people with certain medical conditions, and those with previous dengue infections are at increased risk for severe dengue.
“Healthcare providers should be prepared to recognize, diagnose, manage, and report dengue cases to health authorities; public health partners should investigate cases and disseminate clear prevention messages to the public,” the alert stated.
The CDC is actively implementing several strategies to address the increase in cases of dengue in the United States. In early April, the agency launched a program-led emergency response and is providing monthly situational updates on dengue to partners, stakeholders, and jurisdictions.
The CDC is also expanding laboratory capacity to improve laboratory testing approaches; collaborating with state, tribal, local, and territorial health departments to strengthen dengue surveillance and recommend prevention strategies; and working to educate the public on dengue prevention.
A version of this article first appeared on Medscape.com.
More Evidence PTSD Tied to Obstructive Sleep Apnea Risk
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder (PTSD) may enhance the risk for obstructive sleep apnea (OSA) in older male veterans, the results of a cross-sectional twin study suggested. However, additional high-quality research is needed and may yield important mechanistic insights into both conditions and improve treatment, experts said.
“The strength of the association was a bit surprising,” said study investigator Amit J. Shah, MD, MSCR, Emory University, Atlanta, Georgia. “Many physicians and scientists may otherwise assume that the relationship between PTSD and sleep apnea would be primarily mediated by obesity, but we did not find that obesity explained our findings.”
The study was published online in JAMA Network Open.
A More Rigorous Evaluation
“Prior studies have shown an association between PTSD and sleep apnea, but the size of the association was not as strong,” Dr. Shah said, possibly because many were based on symptomatic patients referred for clinical evaluation of OSA and some relied on self-report of a sleep apnea diagnosis.
The current study involved 181 male twins, aged 61-71 years, including 66 pairs discordant for PTSD symptoms and 15 pairs discordant for PTSD diagnosis, who were recruited from the Vietnam Era Twin Registry and underwent a formal psychiatric and polysomnography evaluation as follow-up of the Emory Twin Study.
PTSD symptom severity was assessed using the self-administered Posttraumatic Stress Disorder Checklist (PCL). OSA was mild in 74% of participants, moderate to severe in 40%, and severe in 18%.
The mean apnea-hypopnea index (AHI) was 17.7 events per hour, and the mean proportion of the night with SaO2 less than 90% was 8.9%.
In fully adjusted models, each 15-point within-pair difference in PCL score was associated with a 4.6 events-per-hour higher AHI, a 6.4 events-per-hour higher oxygen desaturation index, and a 4.8% greater sleep duration with SaO2 less than 90%.
A current PTSD diagnosis is associated with an approximate 10-unit higher adjusted AHI in separate models involving potential cardiovascular mediators (10.5-unit; 95% CI, 5.7-15.3) and sociodemographic and psychiatric confounders (10.7-unit; 95% CI, 4.0-17.4).
The investigators called for more research into the underlying mechanisms but speculated that pharyngeal collapsibility and exaggerated loop gain, among others, may play a role.
“Our findings broaden the concept of OSA as one that may involve stress pathways in addition to the traditional mechanisms involving airway collapse and obesity,” Dr. Shah said. “We should be more suspicious of OSA as an important comorbidity in PTSD, given the high OSA prevalence that we found in PTSD veterans.”
Questions Remain
In an accompanying editorial, Steven H. Woodward, PhD, and Ruth M. Benca, MD, PhD, VA Palo Alto Health Care Systems, Palo Alto, California, noted the study affirmatively answers the decades-old question of whether rates of OSA are elevated in PTSD and “eliminates many potential confounders that might cast doubt on the PTSD-OSA association.”
However, they noted, it’s difficult to ascertain the directionality of this association and point out that, in terms of potential mechanisms, the oft-cited 1994 study linking sleep fragmentation with upper airway collapsibility has never been replicated and that a recent study found no difference in airway collapsibility or evidence of differential loop gain in combat veterans with and without PTSD.
Dr. Woodward and Dr. Benca also highlighted the large body of evidence that psychiatric disorders such as bipolar disorder, schizophrenia, and, in particular, major depressive disorder, are strongly associated with higher rates of OSA.
“In sum, we do not believe that a fair reading of the current literature supports a conclusion that PTSD bears an association with OSA that does not overlap with those manifested by other psychiatric disorders,” they wrote.
“This commentary is not intended to discourage any specific line of inquiry. Rather, we seek to keep the door open as wide as possible to hypotheses and research designs aimed at elucidating the relationships between OSA and psychiatric disorders,” Dr. Woodward and Dr. Benca concluded.
In response, Dr. Shah said the editorialists’ “point about psychiatric conditions other than PTSD also being important in OSA is well taken. In our own cohort, we did not see such an association, but that does not mean that this does not exist.
“Autonomic physiology, which we plan to study next, may underlie not only the PTSD-OSA relationship but also the relationship between other psychiatric factors and OSA,” he added.
The study was funded by grants from the National Institutes of Health (NIH). One study author reported receiving personal fees from Idorsia, and another reported receiving personal fees from Clinilabs, Eisai, Ferring Pharmaceuticals, Huxley, Idorsia, and Merck Sharp & Dohme. Dr. Benca reported receiving grants from the NIH and Eisai and personal fees from Eisai, Idorsia, Haleon, and Sage Therapeutics. Dr. Woodward reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
New Insight Into CVD, Stroke Risk in Migraine
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Researchers are unraveling the complex relationship between cardiovascular (CV)- and stroke-related outcomes in migraine with, and without, aura.
“We confirmed that aura increases the risk for these cerebrovascular and cardiovascular outcomes in people with migraine and that there’s an increased risk of these MACE events in men with migraine,” said study investigator Gina Dumkrieger, PhD, principal data science analyst and assistant professor of neurology, Mayo Clinic, Phoenix, Arizona.
The findings were presented at the annual meeting of the American Headache Society.
Few Data on Migraine and Stroke Risk
The extent to which migraine increases the risk for stroke CV outcomes has not been extensively studied.
“We’re trying to find out whether migraine-related factors make it more likely that you’re going to have one of these events,” said Dr. Dumkrieger. “Knowing a particular factor increases the risk is something patients and medical providers would want to know.”
Using Mayo Clinic electronic health records, which cover all three sites (Florida, Minnesota, and Arizona), researchers identified individuals with migraine using diagnostic codes. They also looked at data on sex, race, and the presence of aura.
They investigated whether a history of MACE risk factors — including atrial fibrillation, diabetes, hyperlipidemia, hypertension, and tobacco use — affected risk and the potential interaction of aura with these risk factors.
MACE events included cerebral infarction, intracerebral hemorrhage, and acute myocardial infarction.
The analysis included 130,126 participants (80% women, 95% White individuals). Of these, 6% experienced a MACE event, and 94% did not.
“We confirmed that aura does increase the risk for a MACE event, and all of the known risk factors that we included were also significant,” said Dr. Dumkrieger.
Odds ratios (ORs) were 3.82 for atrial fibrillation, 3.11 for hypertension, and 3.06 for hyperlipidemia.
It was surprising, said Dr. Dumkrieger, that male sex was tied to an increased risk for a MACE event (OR, 1.40). “This is not something that was known before,” she said.
The link between migraine and ischemic stroke, particularly with aura, was stronger in women — particularly young women.
Investigators also found an interaction between male sex and aura, when it comes to MACE outcomes, said Dr. Dumkrieger. “Males in general are at higher risk, and people with aura are at higher risk. Males with aura are also at higher risk, but maybe not as much as you would think they would be. It’s not a purely additive thing. This is something we need to look into more,” she said.
The study also revealed an interaction between aura and hypertension as well as aura and tobacco use, but here too, it was not an additive risk, said Dr. Dumkrieger. However, she added, the presence of aura does not moderate the risk for hyperlipidemia, diabetes, or atrial fibrillation.
The research also showed a significant interaction between male sex and Black race which was additive. “There’s apparently increased risk if you are male and Black or African American that’s greater than what you would expect. We should be especially concerned about these individuals,” she said.
Unanswered Questions
The current analysis is part of a larger study that will more closely examine these relationships. “We want to learn, for example, why aura moderates some of the risk factors but not others,” said Dr. Dumkrieger.
The researchers also plan to investigate other migraine features, including headache frequency, and headache sensations such as pulsating or throbbing.
Dr. Dumkrieger was an investigator of another study, also presented at the AHS meeting, that’s investigating the role of migraine-specific features and imaging results in the complex interrelationship between migraine and MACE risk.
That study, which also used the Mayo Clinic electronic health record data, included 60,454 migraine patients diagnosed with migraine after 2010.
Researchers divided participants into those with a MACE outcome (1107) and those without such an outcome (59,347) after at least 2 years of follow-up. They created a propensity cohort of individuals matched for age and risk factors for MACE outcome.
The final cohort consisted of 575 patients with and 652 patients without MACE outcome.
One of the most interesting early results from this study was that those with a MACE outcome had significantly more white matter hyperintensities than those with no MACE outcome, at 64% versus 51%, respectively.
This and other findings need to be validated in a different cohort with an electronic health records database from another institution. In future, the team plans to focus on identifying specific migraine features and medications and their relative contributions to MACE risk in migraine patients.
Yet another study featured at the AHS meeting confirmed the increased risk for stroke among migraine patients using a large database with over 410,000 subjects.
Results showed stroke was more than three times more common in those with a migraine diagnosis than in those without (risk ratio, [RR] 3.23; P < .001). The RR for hemorrhagic stroke (3.15) was comparable with that of ischemic stroke (3.20).
The overall stroke RR for chronic migraine versus controls without migraine was 3.68 (P < .001). The RR for migraine with aura versus migraine without aura was 1.37 (P < .001).
Useful Data
Commenting on the research, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, Arizona, described this new information as “very useful.”
The fact that there are more white matter lesions on MRI scans in migraine patients with MACE needs further exploration, said Dr. VanderPluym.
“Understanding how much of that relates to migraine, how much relates to other comorbid conditions, and what this all means together, is very important, particularly because MACE can be life-threatening and life-altering,” she added.
Learning how migraine medications may impact MACE risk is also something that needs to be examined in greater depth, she said. “I would think that migraines that are controlled might have a different risk for MACE than uncontrolled migraine,” she said.
The investigators reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM AHS 2024
Olive Oil Shows Promise for Wound Healing of Ulcers
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Practice Points
- Interventions that effectively reduce excessive and prolonged inflammation can help promote timely wound healing. Consider integrating anti-inflammatory treatments into wound care protocols to enhance healing outcomes.
- Utilization of olive oil in wound therapy, particularly for conditions such as diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers, has shown promise for promoting healing.
- Regularly review and incorporate findings from recent studies on the use of olive oil and other novel interventions in wound therapy to ensure the application of the most current and effective treatment strategies.
Plantar Hyperpigmentation
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Use of Hypoglossal Nerve Stimulation for Treating OSA in Military Patient Populations
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
Obstructive sleep apnea (OSA), the repetitive collapse of posterior oropharynx during sleep resulting in hypoxia and/or arousals from sleep, is the most common form of sleep disordered breathing and a common chronic respiratory disorders among middle-aged adults. OSA can lead to significant health problems, such as worsened cardiometabolic disease and cognitive impairment, which can increase morbidity and mortality.1
The gold standard for OSA diagnosis is polysomnography (PSG), although home sleep studies can be performed for select patients. OSA diagnoses are based on the number of times per hour of sleep a patient’s airway narrows or collapses, reducing or stopping airflow, scored as hypopnea or apnea events, respectively. An Apnea-Hypopnea Index (AHI) score of 5 to 14 events/hour is considered mild OSA, 15 to 30 events/hour moderate OSA, and ≥ 30 events/hour severe OSA.2
Treatment commonly includes positive airway pressure (PAP) but more than one-half of patients are not adherent to continuous PAP (CPAP) treatment after about 90 days.3 Efficacy of treatments vary as a function of disease severity and etiology, which—in addition to the classic presentation of obesity with large neck/narrowupper airway—includes craniofacial abnormalities, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck.
Background
The American Academy of Sleep Medicine (AASM) estimates that 10% to 17% of adults in the United States have OSA.4 Compared with civilians, the military population generally is younger and healthier. Service members have access to regular health care with yearly physical examinations, exercise scheduled into the workday, and mandatory height/weight and fitness standards. Because obesity is a major risk factor for OSA, and the incidence of obesity is relatively low in the military population (estimated at 18.8% in 2021 vs 39.8% among all US adults aged 20 to 39 years), it might be expected that incidence of OSA would be correspondingly low.5,6 However, there is evidence of a rapidly increasing incidence of OSA in military populations. A 2021 study revealed that OSA incidence rates increased from 11 to 333 per 10,000 between 2005 and 2019 across all military branches and demographics, with the highest rate among Army personnel.7 An earlier study revealed a 600% increase in OSA incidence among Army personnel between 2003 and 2011.8
Several factors likely contributed to this increase, including expanding obesity and greater physician awareness and availability of sleep study centers. Rogers and colleagues found that 40% to 50% of incident OSA diagnoses among military personnel occur within 12 months of separation, suggesting that the secondary gains associated with military disability benefits might motivate OSA evaluation.9 It is possible that secondary gain is a factor because an OSA diagnosis can range from a 0% to 100% disability rating, depending on the severity.10 This disability claim is based on evidence that untreated OSA can negatively affect long-term health and mission readiness.8 For example, untreated OSA can lead to hypertension, which contributes to a long list of adverse health and wellness consequences. Most importantly for the military, OSA has been shown to increase daytime sleepiness and reduce cognitive performance.10
The current first-line treatment for OSA is CPAP, which improves symptoms of daytime sleepiness, hypertension management, and daytime alertness.11 Despite its efficacy, nonadherence rates range from 29% to 83%.12-15 Nonadherence factors include lifestyle changes, adverse effects (eg, nasal congestion), and lack of education on proper use.11 Lifestyle changes needed to increase the likelihood of successful therapy, such as regular sleep schedules and proper CPAP cleaning and maintenance, are difficult for military personnel because of the nature of continuous or sustained operations that might require shift work and/or around-the-clock (ie, 24-hour, 7 days a week) task performance. Traveling with CPAP is an added burden for service members deployed to combat operations (ie, added luggage, weight, maintenance). Although alternate treatments such as oral appliances (ie, custom dental devices) are available, they generally are less effective than CPAP.2 Oral appliances could be a reasonable alternative treatment for some patients who cannot manage their OSA with behavioral modifications and are intolerant or unable to effectively use CPAP. This could include patients in the military who are deployed to austere environments.
Surgically implanted hypoglossal nerve stimulator (HGNS) treatment may provide long-term health benefits to service members. After the device is implanted near the hypoglossal nerve, electrical stimulation causes the tongue to move forward, which opens the airway in the anteroposterior dimension. The most important consideration is the mechanism of airway collapse. HGNS is not effective for patients whose OSA events are caused by circumferential collapse of other airway muscles. The cause of airway collapse is ascertained before surgery with drug-induced sleep endoscopy, a procedure that allows visualization of conformational changes in the upper airway during OSA events.
The US Food and Drug Administration (FDA) approved HGNS in 2014. However, it is not considered a first-line treatment for OSA by the AASM. Original candidate criteria for HGNS included an AHI score of 15 to 65 events/hour, age ≥ 18 years, failed CPAP use, body mass index (BMI) < 32, absence of palatal complete concentric collapse, and central apneas comprising < 25% of total events.16 In June 2023, the FDA expanded approval to increase the upper limit of AHI to 100 events/hour and the BMI to < 40.17
HGNS has been reported to be effective in appropriately selected patients with OSA at tertiary care centers with established multidisciplinary sleep surgical programs. These benefits have not been confirmed in larger, community-based settings, where most of these surgeries occur. In community practice, there is significant confusion among patients and clinicians about the optimal pathway for patient selection and clinical follow-up. Many patients view HGNS as a viable alternative to CPAP, but initially do not understand that it requires surgery. Surgical treatments for OSA, such as HGNS, are appealing because they suggest a 1-time intervention that permanently treats the condition, without need for follow-up or equipment resupply. HGNS might be an appealing treatment option because it is less obtrusive than CPAP and requires fewer resources for set-up and maintenance. Also, it does not cause skin irritation (a possible adverse effect of nightly use of a CPAP mask), allows the individual to sleep in a variety of positions, has less impact on social and sex life, and does not require an electric outlet. In the long term, HGNS might be more cost effective because there is no yearly physician follow-up or equipment resupply and/or maintenance.
The military population has specific demands that impact delivery and effectiveness of health care. Among service members with OSA, CPAP treatment can be challenging because of low adherence, required annual follow-up despite frequent moving cycles that pose a challenge for care continuity, and duty limitations for affected service members (ie, the requirement for a waiver to deploy and potential medical separation if symptoms are not adequately controlled). As the incidence of OSA continues to increase among service members, so does the need for OSA treatment options that are efficacious as CPAP but better tolerated and more suitable for use during military operations. The aim of this review is to assess the effectiveness of HGNS and its potential use by the military OSA patient population.
METHODS
To identify eligible studies, we employed PICOS: Population (patients aged ≥ 18 years with a history of OSA), Intervention (HGNS), Comparator (standard of care PAP therapy), Outcome (AHI or Epworth Sleepiness Scale [ESS], and Study (randomized control trial [RCT] or clinical trial). Studies were excluded if they were not written in English or included pediatric populations. The ESS is a subjective rating scale used to determine and quantify a patient’s level of daytime sleepiness, using a 4-point scale for the likelihood of falling asleep totaled across 8 different situations.18 Daytime sleepiness is considered lower normal(0-5 points), higher normal (6-10 points), mild or moderate excessive (11-15 points), and severe excessive (16-24 points).
Literature Search
We conducted a review of PubMed and Scopus for RCTs and controlled trials published from 2013 to 2023 that included the keywords and phrases: obstructive sleep apnea and either hypoglossal nerve stimulation or upper airway stimulation. The final literature search was performed December 8, 2023.
Two authors independently assessed the titles and abstracts of studies identified in the literature search based on the predefined inclusion criteria. If it was not clear whether an article met inclusion criteria based on its title and/or abstract, the 2 review authors assessed the full text of study and resolved any disagreement through consensus. If consensus was not obtained, a third author was consulted. No duplicates were identified. The PRISMA study selection process is presented in the Figure.
Data extraction was performed by 1 independent reviewer. A second author reviewed the extracted data. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third author was consulted. Study data included methods (study design and study objective), participants mean age, inclusion criteria, exclusion criteria, interventions and comparators, and primary study outcomes.
The quality of evidence was assessed using a rating of 1 to 5 based on a modified version of the Oxford Centre for Evidence-based Medicine Levels of Evidence and Grades of Recommendation.19 A rating of 1 indicated a properly powered and conducted RCT, 2 demonstrated a well-designed controlled trial without randomization or prospective comparative cohort trial, 3 designated a case-control study or retrospective cohort study, 4 signified a case series with or without intervention or a cross-sectional study, and 5 denoted an opinion of respected authorities or case reports. Two reviewers independently evaluated the quality of evidence. Any identified discrepancies were resolved through discussion and consensus. If consensus was not obtained, a third review author was consulted.
RESULTS
We identified 30 studies; 19 articles did not meet inclusion criteria. The remaining 11 articles were divided into 4 cohorts. Five articles were based on data from the STAR trial, a multicenter study that included adults with moderate-to-severe OSA and inadequate adherence to CPAP.20-24 Four articles used the same patient selection criteria as the STAR trial for a long-term German postmarket study of upper airway stimulation efficacy with OSA.25-28 The third and fourth cohorts each consist of 31 patients with moderate-to-severe OSA with CPAP nonadherence or failure.29,30 The STAR trial included follow-up at 5 years, and the German-postmarket had a follow-up at3 years. The remaining 2 cohorts have 1-year follow-ups.
The Scopus review identified 304 studies; 299 did not meet inclusion criteria and 1 was part of the STAR trial.31 The remaining 4 articles were classified as distinct cohorts. Huntley and colleagues included patients from Thomas Jefferson University (TJU) and University of Pittsburgh (UP) academic medical centers.32 The Pordzik and colleagues cohort received implantation at a tertiary medical center, an RCCT, and a 1:1 comparator trial (Table 1).33-35
STAR Trial
This multicenter, prospective, single-group cohort study was conducted in the US, Germany, Belgium, Netherlands, and France. The STAR trial included 126 patients who were not CPAP therapy adherent. Patients were excluded if they had AHI < 20 or > 50, central sleep apnea > 25% of total AHI, anatomical abnormalities that prevent effective assessment of upper-airway stimulation, complete concentric collapse of the retropalatal airway during drug-induced sleep, neuromuscular disease, hypoglossal-nerve palsy, severe restrictive or obstructive pulmonary disease, moderate-to-severe pulmonary arterial hypertension, severe valvular heart disease, New York Heart Association class III or IV heart failure, recent myocardial infarction or severe cardiac arrhythmias (within the past 6 months), persistent uncontrolled hypertension despite medication use, active psychiatric illness, or coexisting nonrespiratory sleep disorders that would confound functional sleep assessment. Primary outcome measures included the AHI and oxygen desaturation index (ODI) with secondary outcomes using the ESS, the Functional Outcomes of Sleep Questionnaire (FOSQ), and the percentage of sleep time with oxygen saturation < 90%. Of 126 patients who received implantation, 71 underwent an overnight PSG evaluation at 5-year follow-up. Mean (SD) AHI at baseline was reduced with HGNS treatment to from 32.0 (11.8) to 12.4 (16.3). Mean (SD) ESS for 92 participants with 2 measurements declined from 11.6 (5.0) at baseline to 6.9 (4.7) at 5-year follow-up.
The STAR trial included a randomized controlled withdrawal study for 46 patients who had a positive response to therapy to evaluate efficacy and durability of upper airway stimulation. Patients were randomly assigned to therapy maintenance or therapy withdrawal groups for ≥ 1 week. The short-term withdrawal effect was assessed using the original trial outcome measures and indicated that both the withdrawal and maintenance groups showed improvements at 12 months compared with the baseline. However, after the randomized withdrawal, the withdrawal group’s outcome measures deteriorated to baseline levels while the maintenance group showed no change. At 18 months of therapy, outcome measures for both groups were similar to those observed with therapy at 12 months.24 The STAR trial included self-reported outcomes at baseline, 12 months, and 24 months that used ESS to measure daytime sleepiness. These results included subsequent STAR trial reports.20-24,31
The German Postmarket Cohort
This multicenter, prospective, single-arm study used selection criteria that were based on those used in the STAR trial and included patients with moderate-to-severe OSA and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, AHI < 15 or > 65; central apnea index > 25% of total AHI; or complete concentric collapse at the velopharynx during drug-induced sleep. Measured outcomes included AHI, ODI, FOSQ, and ESS. Among the 60 participants, 38 received implantation and a 3-year follow-up. Mean (SD) AHI decreased from 31.2 (13.2) at baseline to 13.1 (14.1) at follow-up, while mean (SD) ESS decreased from 12.8 (5.3) at baseline to 6.0 (3.2) at follow-up.25-28
Munich Cohort
This single-center, prospective clinical trial included patients with AHI > 15 and < 65, central apnea index < 25% of total AHI, and nonadherence to CPAP. Patients were excluded if they had a BMI > 35, anatomical abnormalities that would prevent effective assessment of upper-airway stimulation; all other exclusion criteria matched those used in the STAR trial. Among 31 patients who received implants and completed a 1-year follow-up, mean (SD) AHI decreased from 32.9 (11.2) at baseline to 7.1 (5.9) at follow-up and mean (SD) ESS decreased from 12.6 (5.6) at baseline to 5.9 (5.2) at follow-up.29
Kezirian and Colleagues Cohort
This prospective, single-arm, open-label study was conducted at 4 Australian and 4 US sites. Selection criteria included moderate-to-severe OSA with failure of CPAP, AHI of 20 to 100 with ≥ 15 events/hour occurring in sleep that was non-REM (rapid eye movement) sleep, BMI ≤ 40 (Australia) or ≤ 37 (US), and a predominance of hypopneas (≥ 80% of disordered breathing events during sleep). Patients were excluded if they had earlier upper airway surgery, markedly enlarged tonsils, uncontrolled nasal obstruction, severe retrognathia, > 5% central or mixed apneic events, incompletely treated sleep disorders other than OSA, or a major disorder of the pulmonary, cardiac, renal, or nervous systems. Data were reported for 31 patients whose mean (SD) AHI declined from 45.4 (17.5) at baseline to 25.3 (20.6) at 1-year follow-up and mean (SD) ESS score declined from 12.1 (4.6) at baseline to 7.9 (3.8) 1 year later.30
TJU and UP Cohorts
The TJU and UP cohorts are composed of patients who underwent implantation between May 2014 and August 2016 at 2 academic centers.31,32 Selection criteria was consistent with that used in the STAR trial, and patients completed postoperative titration PSG and outpatient follow-up (48 patients at TJU and 49 at UP). Primary outcomes included AHI, ESS, and O2 nadir. Secondary outcomes consisted of surgical success and percentage of patients tolerating optimal titration setting at follow-up. Postoperative outcomes were assessed during the titration PSG. Time from initial ESS to postoperative PSG at TJU was 1.7 years and at UP was 1.9 years. Time from initial AHI to postoperative PSG at TJU was 90.4 days and 85.2 days at UP. At TJU, mean (SD) AHI and ESS dropped from 35.9 (20.8) and 11.1 (3.8), respectively at baseline to 6.3 (11.5) and 5.8 (3.4), respectively at follow-up. At UP, mean (SD) AHI and ESS fell from 35.3 (15.3) and 10.9 (4.9), respectively at baseline to 6.3 (6.1) and 6.6 (4.5), respectively at follow-up. There were no site-related differences in rates of AHI, ESS, or surgical success.31
Pordzik and Colleagues Cohort
This cohort of 29 patients underwent implantation between February 2020 and June 2022 at a tertiary university medical center with both pre- and postoperative PSG. Selection criteria was consistent with that of the German postmarket cohort. Postoperative PSG was completed a mean (SD) 96.3 (27.0) days after device activation. Mean (SD) AHI dropped from 38.6 (12.7) preoperatively to 24.4 (13.3) postoperatively. Notably, this cohort showed a much lower decrease of postoperative AHI than reported by the STAR trial and UP/TJU cohort.33
Stimulation vs Sham Trial
This multicenter, double-blinded, randomized, crossover trial assessed the effect of HGNS (stim) vs sham stimulation (sham) in 86 patients that completed both phases of the trial. Primary outcomes included AHI and ESS. Secondary outcomes included FOSQ. No carryover effect was found during the crossover phase. The difference between the phases was−15.5 (95% CI, −18.3 to −12.8) for AHI and −3.3 (95% CI, −4.4 to −2.2) for ESS.34
Comparator
The comparator study used propensity score matching to compare outcomes of HGNS and PAP therapy. Primary outcomes included sleepiness, AHI, and effectiveness with outcome measures of AHI and ESS collected at baseline and 12 months postimplantation. The article reported that 126 of 227 patients were matched 1:1. Both groups showed improvement in AHI and ESS. Mean (SD) AHI for the HGNS group at baseline started at 33.9 (15.1) and decreased to 8.1 (6.3). Mean (SD) ESS for the HGNS group at baseline was 15.4 (3.5) and decreased to 7.5 (4.7). In the PAP comparator group, mean (SD) baseline AHI was 36.8 (21.6) and at follow-up was 6.6 (8.0) and mean (SD) ESS was 14.6 (3.9) at baseline and 10.8 (5.6) at follow-up.35
DISCUSSION
The current clinical data on HGNS suggest that this treatment is effective in adults with moderate-to-severe OSA and effects are sustained at long-term follow-up, as measured by AHI reduction and improvements in sleep related symptoms and quality of life (Table 2). These results have been consistent across several sites.
The STAR trial included a randomized control withdrawal group, for whom HGNS treatment was withdrawn after the 12-month follow-up, and then restored at 18 months.21 This revealed that withdrawal of HGNS treatment resulted in deterioration of both objective and subjective measures of OSA and sleepiness. The beneficial effects of HGNS were restored when treatment was resumed.24 Additionally, the RCCT revealed that therapeutic stimulation via HGNS significantly reduced subjective and objective measures of OSA.34 These studies provide definitive evidence of HGNS efficacy.
Currently, a diagnosis of OSA on PAP is classified as a 50% military disability rating. This rating is based primarily on epidemiologic evidence that untreated OSA is a costly disease that leads to other chronic illnesses that increases health care utilization.9 HGNS requires an initially invasive procedure and higher upfront costs, but it could result in reduced health care use and long-term costs because of improved adherence to treatment—compared with CPAP—that results in better outcomes.
Limitations to OSA Studies
The reviewed studies have several limitations that warrant caution when determining the possible benefits of HGNS treatment. The primary limitation is the lack of active control groups, therefore precluding a direct comparison of the short- and long-term effectiveness of HGNS vs other treatments (eg, CPAP). This is especially problematic because in the reviewed studies HGNS treatment efficacy is reported as a function of the mean—and SD—percent reduction in the AHI, whereas the efficacy of CPAP treatment usually is defined in terms of “adequacy of titration” as suggested by the AASM.36 It has been reported that with CPAP treatment, 50% to 60% of OSA patients achieve AASM-defined optimal improvement of respiratory disturbance index of < 5/hour during a polysomnographic sleep recording of ≥ 15 minutes duration that includes REM sleep in the supine position.37 In most of the reviewed studies, treatment success was more liberally defined as a decrease of AHI by ≥ 50%, regardless of the resulting AHI. It is notable that among the reviewed HGNS studies, the TJU and UP cohorts achieved the best outcome in short-term follow-up of 2 months with a mean (SD) AHI of 6.3 (11.5) and 6.4 (6.1), respectively. Among those cohortsassessed at a 12-month follow-up, the Munich cohort achieved the best outcome with a mean (SD) AHI of 7.1 (5.9).
Although the metrics reported in the reviewed studies are not directly comparable, the reported findings strongly suggest that HGNS generally is less effective than CPAP. How important are these differences? With findings that HGNS “reliably produces clinically meaningful (positive) effects on daytime sleepiness, daytime functioning, and sleep quality,” does it really matter if the outcome metrics for HGNS are a little less positive than those produced by CPAP?38 For individual military OSA patients the answer is yes. This is because in military operational environments—especially during deployment—sleep restriction is nearly ubiquitous, therefore any mild residual deficits in sleep quality and daytime alertness resulting from nominally adequate, but suboptimal OSA treatment, could be exacerbated by sleep restriction, therefore placing the service member and the mission at increased risk.39
Another limitation is the narrow inclusion criteria these studies employed, which limits the generalizability of the findings. Participants in the reviewed clinical trials were selected from a patient population that was mostly middle-aged, White, and obese or overweight. In a Medical Surveillance Monthly Report study, OSA was found to be highest among service members aged > 40 years, male, obese, and Black/non-Hispanic (although it should be noted that more than one-half of enlisted service members aged ≤ 25 years).40,41 Obesity has been noted as a growing concern for the military as the military population is beginning to mirror the civilian population in terms of being overweight or obese despite height and weight standards. HGNS might not be as successful in military populations with different demographics. Moreover, HGNS has been shown to have greater AHI reduction among those with higher BMI.30 Although obese service members have a 6-fold higher 12-year incidence rate of OSA than service members without obesity, this nevertheless suggests that general level of HGNS efficacy might be lower among the military patient population, because obesity is less prevalent in the military than the general population.9
Ethnicity has been found to be a relevant factor, with the highest incidence rate of OSA among non-Hispanic Black males, a demographic that was underrepresented in cohorts included in this review. Further studies will be needed to determine the extent to which findings from HGNS treatment studies are generalizable to the broader OSA patient population.
HGNS Implementation Challenges
Current impediments to widespread use of HGNS as an OSA treatment include no standardized guidance for titration and follow-on care, which varies based on the resources available. Titrating a new device for HGNS requires experienced sleep technicians who have close relationships with device representatives and can troubleshoot problems. Technical expertise, which currently is rare, is required if there are complications after placement or if adjustments to voltage settings are needed over time. In addition, patients may require multiple specialists making it easy to get lost to follow-up after implantation. This is particularly challenging in a transient community, such as the military, because there is no guarantee that a service member will have access to the same specialty care at the next duty station.
Although some evidence suggests that HGNS is a viable alternative treatment for some patients with OSA, the generalizability of these findings to the military patient population is unclear. Specialized facilities and expertise are needed for the surgical procedure and follow-up requirements, which currently constitute significant logistical constraints. As with any implantable device, there is a risk of complications including infection that could result in medical evacuation from a theater of operations. If the device malfunctions or loses effectiveness in a deployed environment, the service member might not have immediate access to medical support, potentially leading to undertreatment of OSA. In future battlefield scenarios in multidomain operations, prolonged, far-forward field care will become the new normal because the military is not expected to have air superiority or the ability to quickly evacuate service members to a higher level of medical care.42
In deployed environments, the potential limitations of HGNS become increasingly risky for the service member and the overall mission. Considering these factors, it will be important to evaluate the practicality of HGNS as a treatment option in military populations. Military-specific challenges associated with HGNS that require further study, include guidance for patient selection outside academic centers, guidance on long-term postsurgical care and device maintenance, duty limitation and military retention considerations, and limitations in training and combat environments. The military medical community needs to conduct its own studies in appropriately selected service members to guide clinical practice.
CONCLUSIONS
HGNS treatment results in improvement of both AHI and ESS scores and could be a deployable treatment option for military patients with OSA. However, HGNS has not been found to be as effective as CPAP, although the current literature is limited by small sample sizes, homogeneous populations that do not reflect the demographics of the military, and mostly short follow-up periods. Future studies should be focused on collecting data on HGNS from demographic groups that are more representative of the military OSA patient population and identifying the subpopulation of patients who derive the greatest benefit from HGNS, so that this treatment can be better individually targeted. Until data on existing military patients is published, it is not possible to fully weigh risks and benefits in this population and generalize civilian guidance to the military.
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
1. Cumpston E, Chen P. Sleep Apnea Syndrome. PubMed. Updated September 4, 2023. Published January 2024. https://www.ncbi.nlm.nih.gov/books/NBK564431/
2. American Academy of Sleep Medicine. Obstructive sleep apnea. Accessed November 27, 2023. https://aasm.org/resources/factsheets/sleepapnea.pdf
3. Cowen J, Harrison S, Thom L, et al. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with Osa. Sleep Breath. 2023;27(5):1899-1908. doi:10.1007/s11325-023-02806-3
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
5. Stiegmann RA, Payne CB, Kiel MA, Stahlman SL. Increased Prevalence of Overweight and Obesity and Incidence of Prediabetes and Type 2 Diabetes During the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR. 2023;30(1):11-18. Published 2023 Jan 20.
6. Adult obesity facts. Centers for Disease Control and Prevention. Updated May 17, 2022. Accessed November 27, 2023. https://www.cdc.gov/obesity/data/adult.html
7. Moore BA, Tison LM, Palacios JG, Peterson AL, Mysliwiec V. Incidence of insomnia and obstructive sleep apnea in active duty United States military service members. Sleep. 2021;44(7):zsab024. doi:10.1093/sleep/zsab024
8. Caldwell JA, Knapik JJ, Shing TL, Kardouni JR, Lieberman HR. The association of insomnia and sleep apnea with deployment and combat exposure in the entire population of US army soldiers from 1997 to 2011: a retrospective cohort investigation. Sleep. 2019;42(8):zsz112. doi:10.1093/sleep/zsz112
9. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
10. Veterans Affairs 38 C.F.R. § 4.97-13, Code 6847.
11. Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323-335. doi:10.1007/s11325-010-0391-y
12. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173-178. doi:10.1513/pats.200708-119mg
13. Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430-435. doi:10.1378/chest.121.2.430
14. Nowak C, Bourgin P, Portier F, Genty E, Escourrou P, Bobin S. Obstruction nasale et compliance à la ventilation nasale à pression positive [Nasal obstruction and compliance to nasal positive airway pressure]. Ann Otolaryngol Chir Cervicofac. 2003;120(3):161-166.
15. Brin YS, Reuveni H, Greenberg S, Tal A, Tarasiuk A. Determinants affecting initiation of continuous positive airway pressure treatment. Isr Med Assoc J. 2005;7(1):13-18.
16. Suurna MV, Jacobowitz O, Chang J, et al. Improving outcomes of hypoglossal nerve stimulation therapy: current practice, future directions, and research gaps. Proceedings of the 2019 International Sleep Surgery Society Research Forum. J Clin Sleep Med. 2021;17(12):2477-2487. doi:10.5664/jcsm.9542
17. Inspire Medical Systems, Inc. Announces FDA approval for apnea hypopnea index indication expansion and increased body mass index labeling. Inspire Medical Systems, Inc. Accessed July 14, 2023. https://investors.inspiresleep.com/investors/press-releases/press-release-details/2023/Inspire-Medical-Systems-Inc.-Announces-FDA-Approval-for-Apnea-Hypopnea-Index-Indication-Expansion-and-Increased-Body-Mass-Index-Labeling/default.aspx
18. Lapin BR, Bena JF, Walia HK, Moul DE. The Epworth Sleepiness Scale: Validation of one-dimensional factor structure in a large clinical sample. J Clin Sleep Med. 2018;14(08):1293-1301. Published 2018 Aug 15. doi:10.5664/jcsm.7258
19. The Centre for Evidence-Based Medicine. November 25, 2020. http://www.cebm.net/index.aspx?o=5653
20. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
21. Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598. Published 2015 Oct 1. doi:10.5665/sleep.5054
22. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188. doi:10.1177/0194599815616618
23. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202. doi:10.1177/0194599818762383
24. Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151(5):880-887. doi:10.1177/0194599814544445
25. Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-384. doi:10.1177/0194599816683378
26. Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128(2):509-515. doi:10.1002/lary.26688
27. Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2020;24(3):979-984. doi:10.1007/s11325-019-01933-028.
28. Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol. 2018;275(7):1913-1919. doi:10.1007/s00405-018-5017-129.
29. Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274(3):1727-1734. doi:10.1007/s00405-016-4297-6
30. Kezirian EJ, Goding GS Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77-83. doi:10.1111/jsr.12079
31. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48. doi:10.5664/jcsm.5390
32. Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med. 2017;13(9):1075-1079. Published 2017 Sep 15. doi:10.5664/jcsm.6726

33. Pordzik J, Seifen C, Ludwig K, et al. Short-term outcome of unilateral inspiration-coupled hypoglossal nerve stimulation in patients with obstructive sleep apnea. Int J Environ Res Public Health. 2022;19(24):16443. Published 2022 Dec 8. doi:10.3390/ijerph192416443
34. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. Published 2021 Jun 29. doi:10.3390/jcm1013288035.
35. Heiser C, Steffen A, Strollo PJ Jr, Giaie-Miniet C, Vanderveken OM, Hofauer B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023;27(2):693-701. doi:10.1007/s11325-022-02663-6
36. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171.
37. Freedman N, Johnson K. Positive airway pressure treatment for obstructive sleep apnea. In: Kryger MH, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. Elsevier; 2022:1260-1283.
38. Braun M, Stoerzel M, Wollny M, Schoebel C, Ulrich Sommer J, Heiser C. Patient-reported outcomes with hypoglossal nerve stimulation for treatment of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280(10):4627-4639. doi:10.1007/s00405-023-08062-1
39. Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189-1195. doi:10.5665/SLEEP.1236
40. Rogers AE, Stahlman S, Hunt DJ, Oh GT, Clark LL. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004-May 2016. MSMR. 2016;23(10):2-11.
41. Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy. 2017 Demographics: Profile of the Military Community. US Dept of Defense;2017. Accessed April 4, 2024. http://download.militaryonesource.mil/12038/MOS/Reports/2017-demographics-report.pdf
42. Remondelli MH, Remick KN, Shackelford SA, et al. Casualty care implications of large-scale combat operations. J Trauma Acute Care Surg. 2023;95(2S Suppl 1): S180-S184. doi:10.1097/TA.0000000000004063
Recalcitrant Folliculitis Decalvans Treatment Outcomes With Biologics and Small Molecule Inhibitors
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).
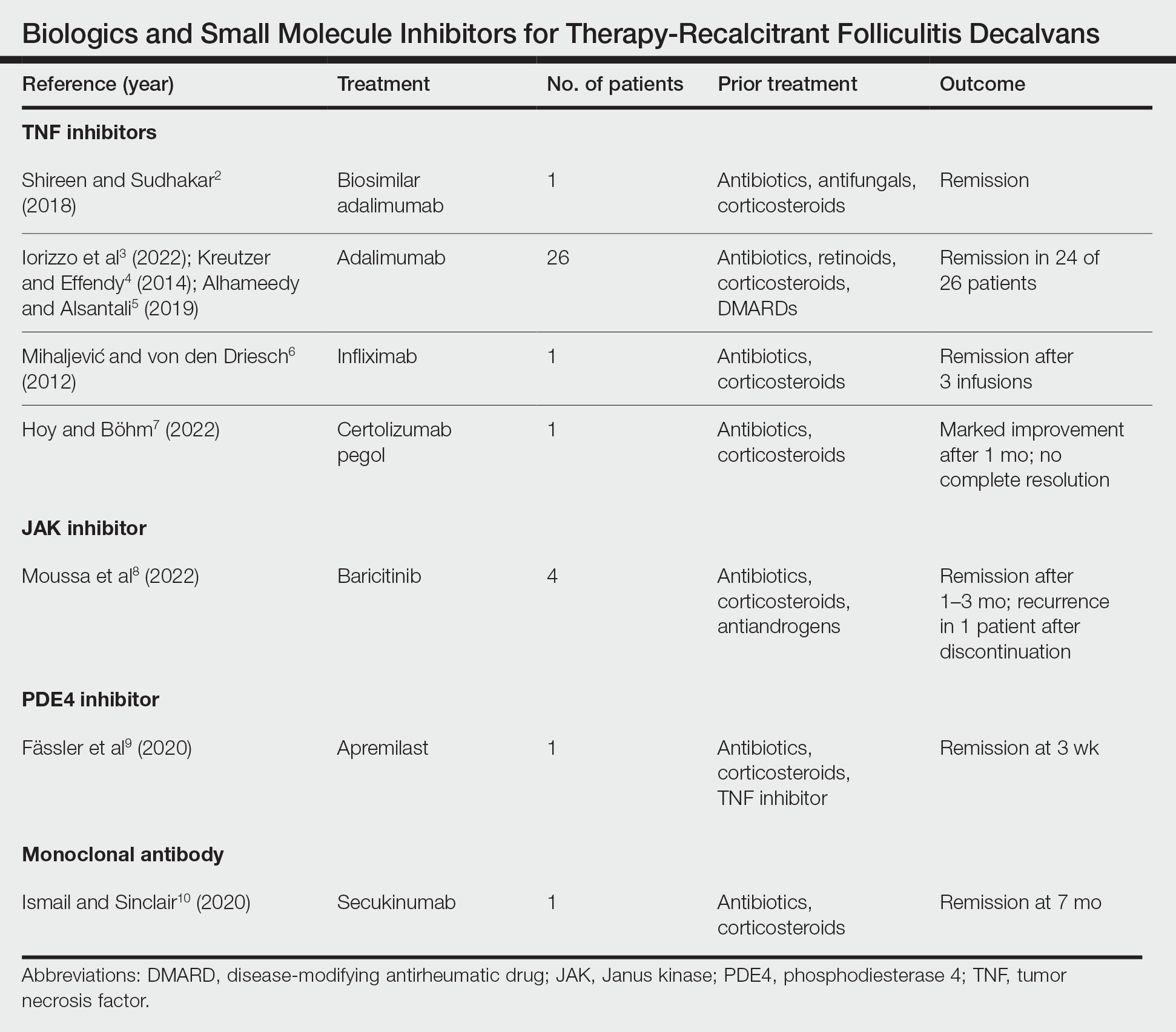
The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Practice Points
- Tumor necrosis factor inhibitors, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and monoclonal antibodies have shown success in the treatment of folliculitis decalvans resistant to traditional therapies.
- The true etiology of folliculitis decalvans is still unknown, but possible factors include Staphylococcus aureus infection and an impaired host immune system, which may benefit from treatment with biologics and small molecule inhibitors.