User login
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
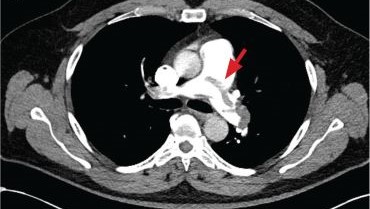
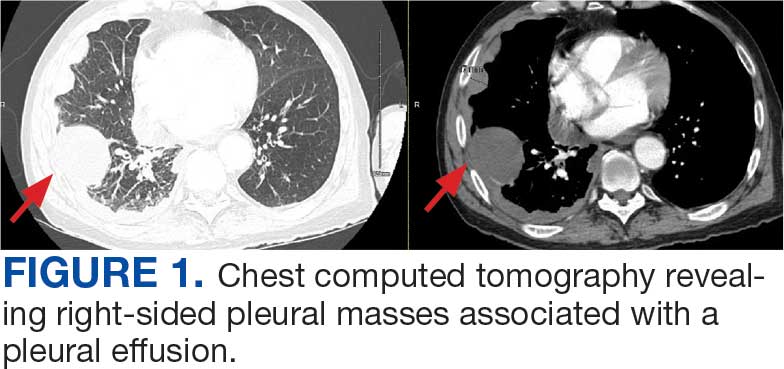
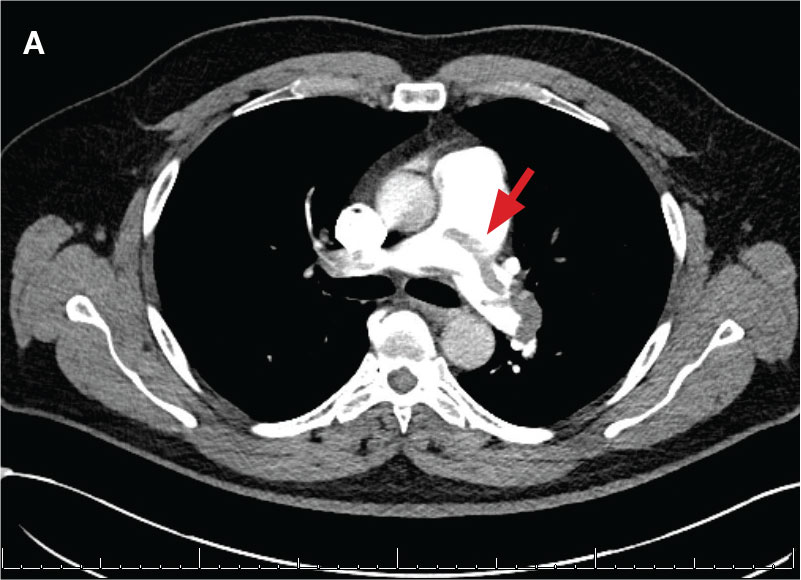
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.
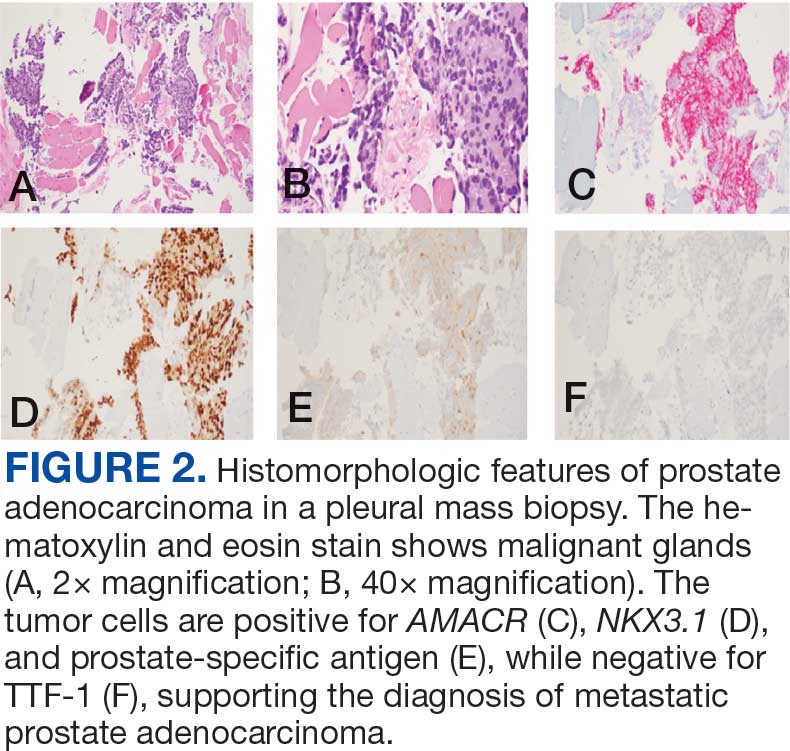
Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).
Case 2
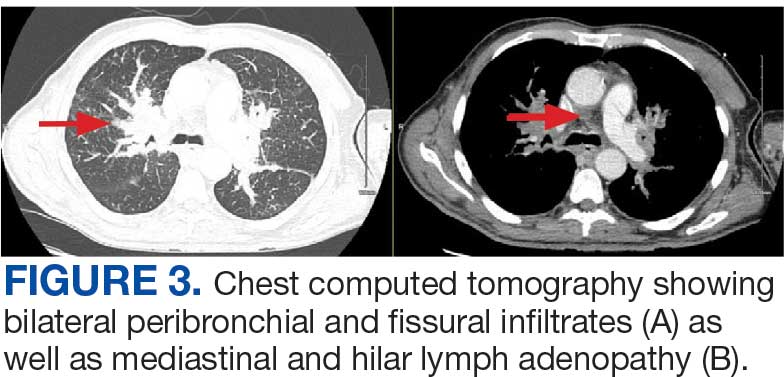
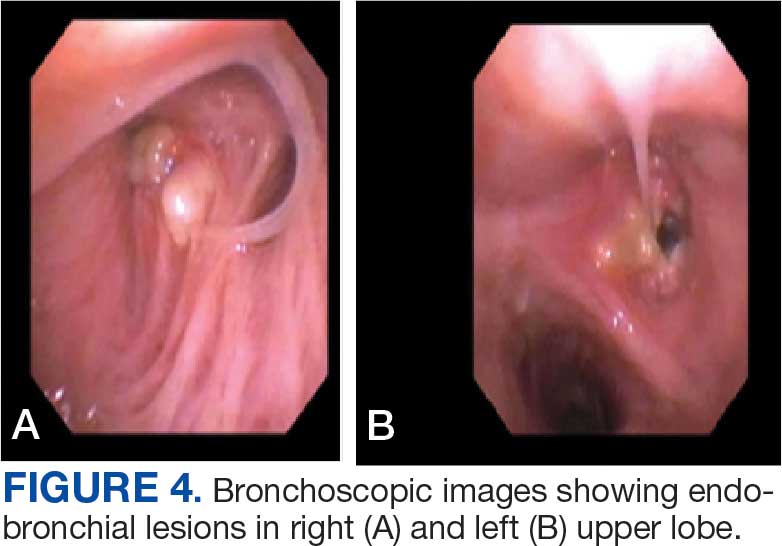
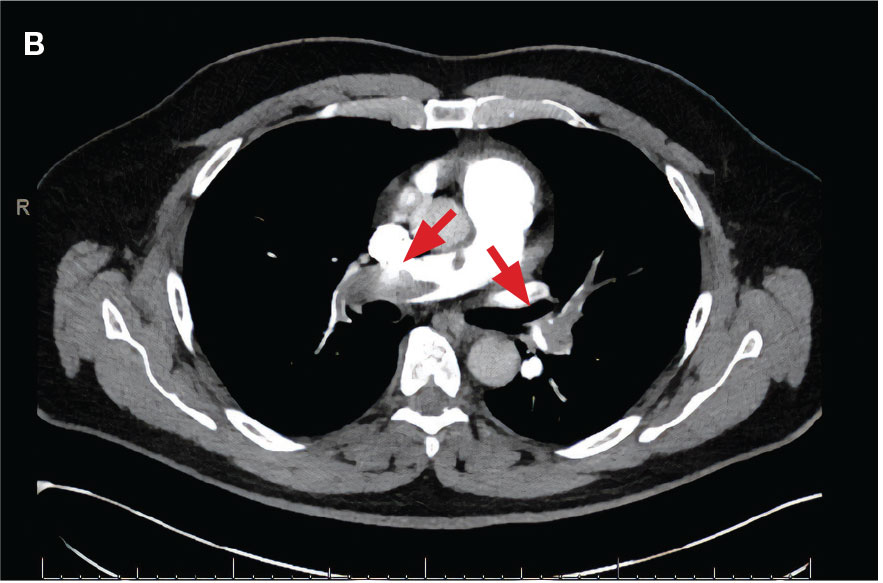
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).
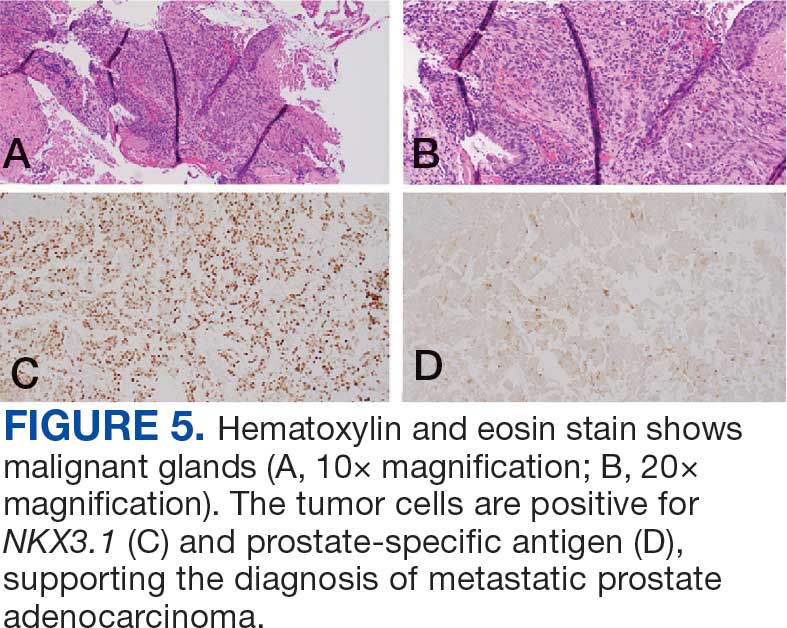
The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.
Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
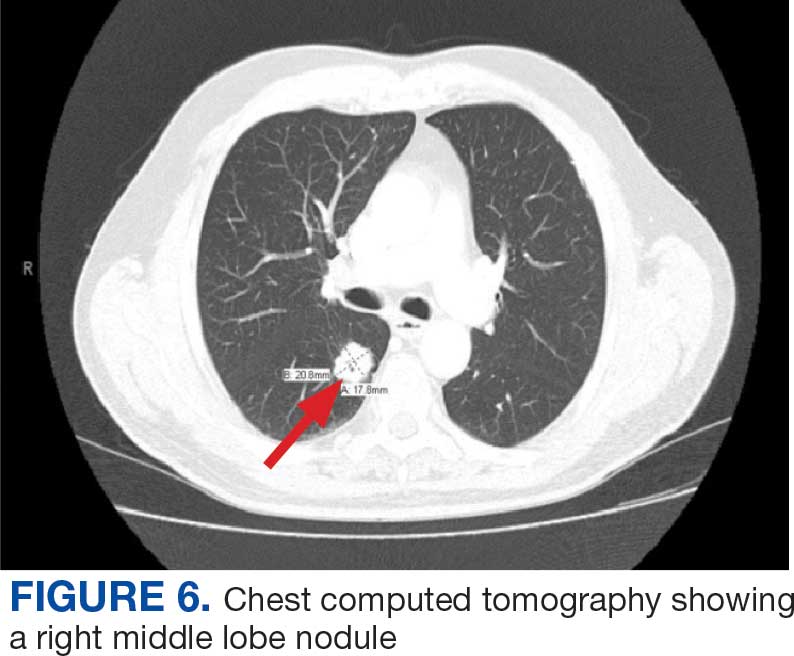
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.
The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
Prostate cancer is the most common noncutaneous cancer in men, accounting for 29% of all incident cancer cases.1 Typically, prostate cancer metastasizes to bone and regional lymph nodes.2 However, intrathoracic manifestation may occur. This report presents 3 cases of rare intrathoracic manifestations of metastatic prostate cancer with a review of the current literature.
CASE PRESENTATIONS
Case 1
A 71-year-old male who was an active smoker and a long-standing employment as a plumber was diagnosed with rectal cancer in 2022. He completed neoadjuvant capecitabine and radiation therapy followed by a rectosigmoidectomy. Several weeks after surgery, the patient presented to the emergency department (ED) with a dry cough and worsening shortness of breath. Point-of-care ultrasound of the lungs revealed a moderate right pleural effusion with several nodular pleural masses. A chest computed tomography (CT) confirmed these findings (Figure 1). A CT of the abdomen and pelvis revealed prostatomegaly with the medial lobe of the prostate protruding into the bladder; however, no enlarged retroperitoneal, mesenteric or pelvic lymph nodes were noted. The patient underwent a right pleural fluid drainage and pleural mass biopsy. Pleural mass histomorphology as well as immunohistochemical (IHC) stains were consistent with metastatic prostate adenocarcinoma. The pleural fluid cytology also was consistent with metastatic prostate adenocarcinoma.

Immunohistochemistry showed weak positive staining for prostate-specific NK3 homeobox 1 gene (NKX3.1), alpha-methylacyl-CoA racemase gene (AMACR), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), and prosaposin, and negative transcription termination factor (TTF-1), keratin-7 (CK7), keratin-20, and caudal type homeobox 2 gene (CDX2) (Figure 2) 2). The patient's prostate-specific antigen (PSA) was found to be elevated at 33.9 ng/mL (reference range, < 4 ng/mL).

Case 2
A 71-year-old male with a history of alcohol use disorder and a 30-year smoking history presented to the ED with worsening dyspnea on exertion. The patient’s baseline exercise tolerance decreased to walking for only 1 block. He reported unintentional weight loss of about 30 pounds over the prior year, no recent respiratory infections, no prior breathing problems, and no personal or family history of cancer. Chest CT revealed findings of bilateral peribronchial opacities as well as mediastinal and hilar lymphadenopathy (Figure 3). The patient developed hypoxic respiratory failure necessitating intubation, mechanical ventilation, and management in the medical intensive care unit, where he was treated for postobstructive pneumonia. Fiberoptic bronchoscopy revealed endobronchial lesions in the right and left upper lobe that were partially obstructing the airway (Figure 4).


The endobronchial masses were debulked using forceps, and samples were sent for surgical pathology evaluation. Staging was completed using linear endobronchial ultrasound, which revealed an enlarged subcarinal lymph node (S7). The surgical pathology of the endobronchial mass and the subcarinal lymph node cytology were consistent with metastatic adenocarcinoma of the prostate. The tumor cells were positive for AE1/AE3, PSA, and NKX3.1, but were negative for CK7 and TTF-1 (Figure 5). Further imaging revealed an enlarged heterogeneous prostate gland, prominent pelvic nodes, and left retroperitoneal lymphadenopathy, as well as sclerotic foci within the T10 vertebral body and right inferior pubic ramus. PSA was also found to be significantly elevated at 700 ng/mL.

Case 3
An 80-year-old male veteran with a history of prostate cancer and recently diagnosed T2N1M0 head and neck squamous cell carcinoma was referred to the Pulmonary service for evaluation of a pulmonary nodule. His medical history was notable for prostate cancer diagnosed 12 years earlier, with an unknown Gleason score. Initial treatment included prostatectomy followed by whole pelvic radiation therapy a year after, due to elevated PSA in surveillance monitoring. This treatment led to remission. After establishing remission for > 10 years, the patient was started on low-dose testosterone replacement therapy to address complications of radiation therapy, namely hypogonadism.
On evaluation, a chest CT was significant for a large 2-cm right middle lobe nodule (Figure 6). At that time, PSA was noted to be borderline elevated at 4.2 ng/mL, and whole-body imaging did not reveal any lesions elsewhere, specifically no bone metastasis. Biopsies of the right middle lobe lung nodule revealed adenocarcinoma consistent with metastatic prostate cancer. Testosterone therapy was promptly discontinued.

The patient initially refused androgen deprivation therapy owing to the antiandrogenic adverse effects. However, subsequent chest CTs revealed growing lung nodules, which convinced him to proceed with androgen deprivation therapy followed by palliative radiation, and chemotherapy and management of malignant pleural effusion with indwelling small bore pleural catheter for about 10 years. He died from COVID-19 during the pandemic.
DISCUSSION
These cases highlight the importance of including prostate cancer in the differential diagnoses of male patients with intrathoracic abnormalities, even in the absence of metastasis to the more common sites. In a large cohort study of 74,826 patients with metastatic prostate cancer, Gandaglia et al found that the most frequent sites of metastasis were bone (84.0%) and distant lymph nodes (10.6%).2 However, thoracic involvement was observed in 9.1% of cases, with isolated thoracic metastasis being rare. The cases described in this report exemplify exceptionally uncommon occurrences within that 9.1%.
Pleural metastases, as observed in Case 1, are a particularly rare manifestation. In a 10-year retrospective assessment, Vinjamoori et al discovered pleural nodules or masses in only 6 of 82 patients (7.3%) with atypical metastases.3 Adrenal and liver metastases accounted for 15% and 37% of cases with atypical distribution. As such, isolated pleural disease is rare even in atypical presentations.3
As seen in Case 2, endobronchial metastases producing airway obstruction are also rare, with the most common primary cancers associated with endobronchial metastasis being breast, colon, and renal cancer.4 The available literature on this presentation is confined to case reports. Hameed et al reported a case of synchronous biopsy-proven endobronchial metastasis from prostate cancer.5 These cases highlight the importance of maintaining a high level of clinical awareness when encountering endobronchial lesions in patients with prostate cancer.
Case 3 presents a unique situation of lung metastases without any involvement of the bones. It is well known—and was confirmed by Heidenreich et al—that lung metastases in prostate adenocarcinoma usually coincide with extensive osseous disease.6 This instance highlights the importance of watchful monitoring for unusual patterns of cancer recurrence.
Immunohistochemistry stains that are specific to prostate cancer include antibodies against PSA. Prostate-specific membrane antigen is another marker that is far more present in malignant than in benign prostate tissue.
The NKX3.1 gene encodes a homeobox protein, which is a transcription factor and tumor suppressor. In prostate cancer, there is loss of heterozygosity of the gene and stains for the IHC antibody to NKX3.1.7
On the other hand, lung cells stain positive for TTF-1, which is produced by surfactant-producing type 2 pneumocytes and club cells in the lung. Antibodies to TTF-1, a common IHC stain, are used to identify adenocarcinoma of lung origin and may carry a prognostic value.7
The immunohistochemistry profiles, specifically the presence of prostate-specific markers such as PSA and NKX3.1, played a vital role in making the diagnosis.
In Case 1, weak TTF-1 positivity was noted, an unusual finding in metastatic prostate adenocarcinoma. Marak et al documented a rare case of TTF-1–positive metastatic prostate cancer, illustrating the potential for diagnostic confusion with primary lung malignancies.8
The 3 cases described in this report demonstrate the importance of clinical consideration, serial follow-up of PSA levels, using more prostate-specific positron emission tomography tracers (eg, Pylarify) alongside traditional imaging, and tissue biopsy to detect unusual metastases.
CONCLUSIONS
Although thoracic metastases from prostate cancer are rare, these presentations highlight the importance of clinical awareness regarding atypical cases. Pleural disease, endobronchial lesions, and isolated pulmonary nodules might be the first clinical manifestation of metastatic prostate cancer. A high index of suspicion, appropriate imaging, and judicious use of immunohistochemistry are important to ensure accurate diagnosis and optimal patient management.
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-216. doi:10.1002/pros.22742
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199(2):367-372. doi:10.2214/AJR.11.7533
- Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996;62(4):249-252. doi:10.1002/(SICI)1096- 9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6
- Hameed M, Haq IU, Yousaf M, Hussein M, Rashid U, Al-Bozom I. Endobronchial metastases secondary to prostate cancer: a case report and literature review. Respir Med Case Rep. 2020;32:101326. doi:10.1016/j.rmcr.2020.101326
- Heidenreich A, Bastian PJ, Bellmunt J, et al; for the European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration- resistant prostate cancer. Eur Urol. 2014;65(2):467- 479. doi:10.1016/j.eururo.2013.11.002
- Schallenberg S, Dernbach G, Dragomir MP, et al. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. doi:10.1016/j.ejca.2023.113474
- Marak C, Guddati AK, Ashraf A, Smith J, Kaushik P. Prostate adenocarcinoma with atypical immunohistochemistry presenting with a Cheerio sign. AIM Clinical Cases. 2023;1:e220508. doi:10.7326/aimcc.2022.0508
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
Atypical Intrathoracic Manifestations of Metastatic Prostate Cancer: A Case Series
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.
indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
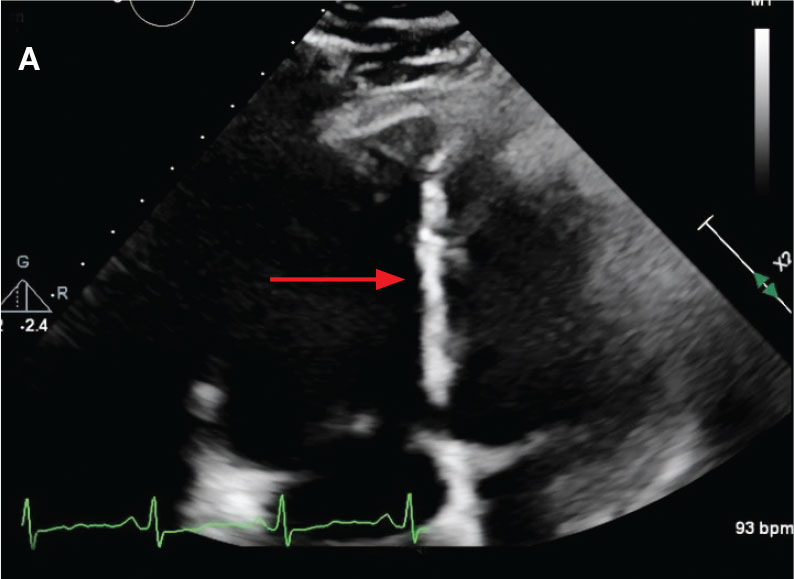
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.
flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
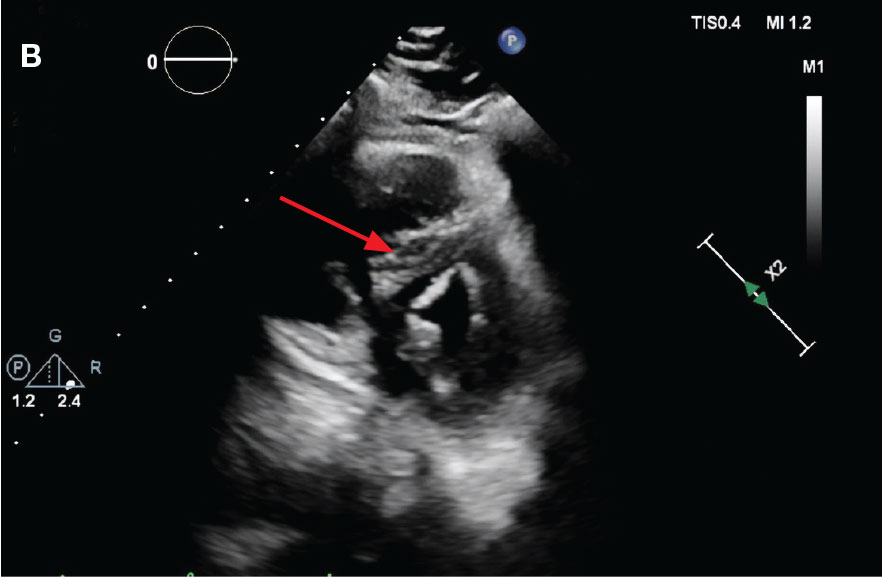
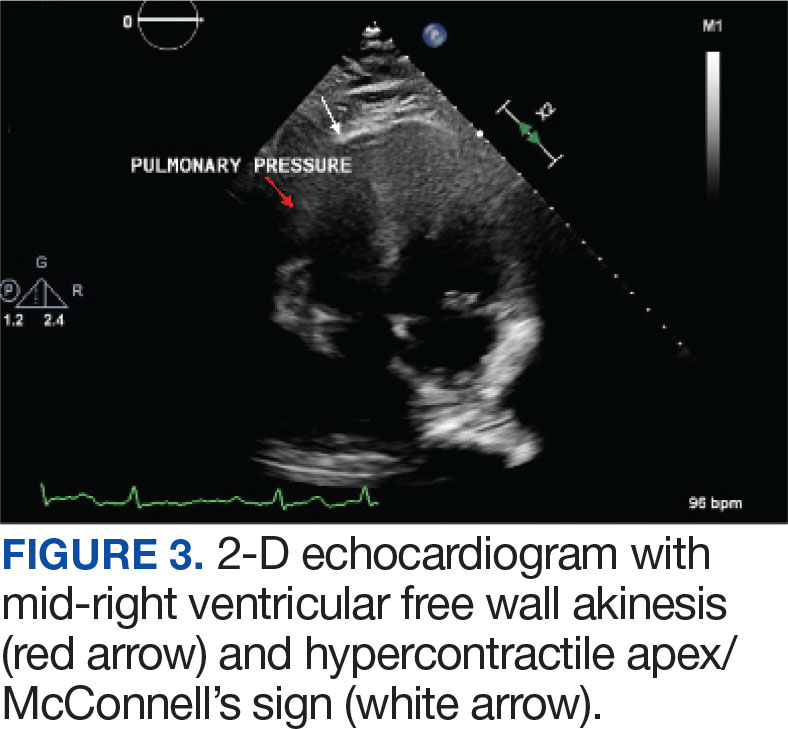
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.
Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
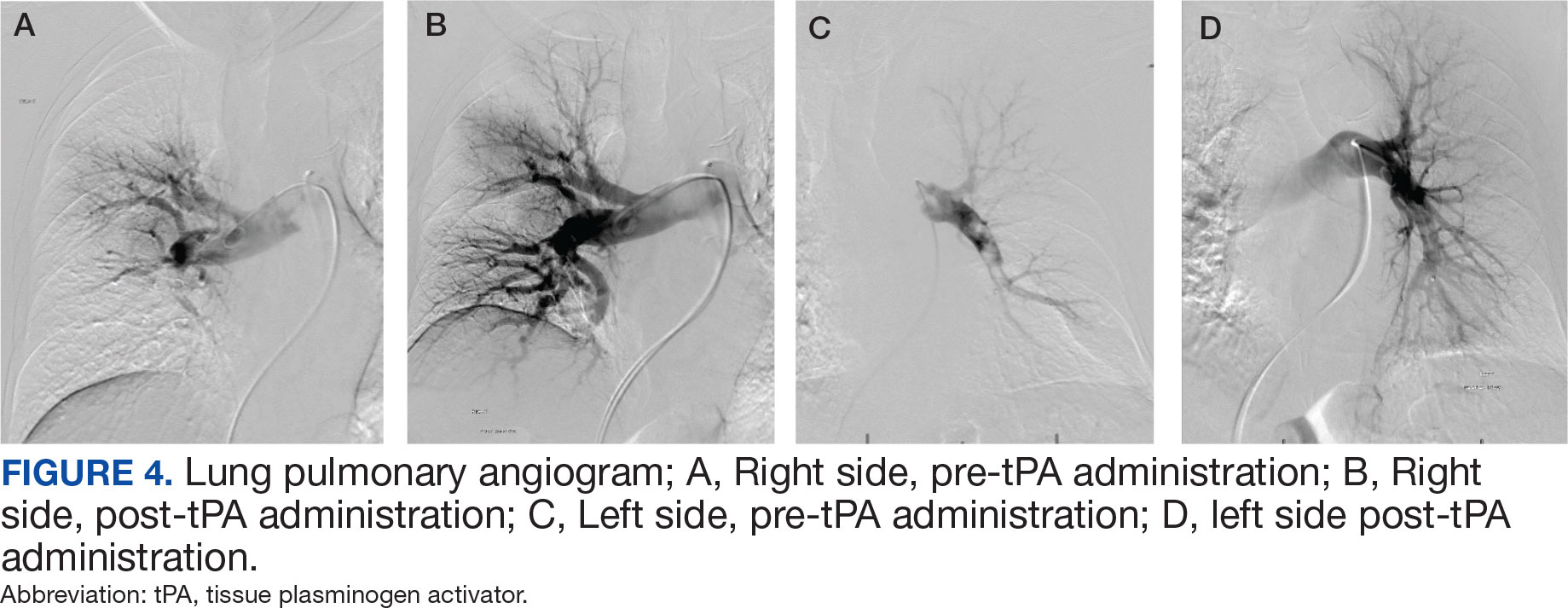
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.
DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.

Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.

DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.

Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.

DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.
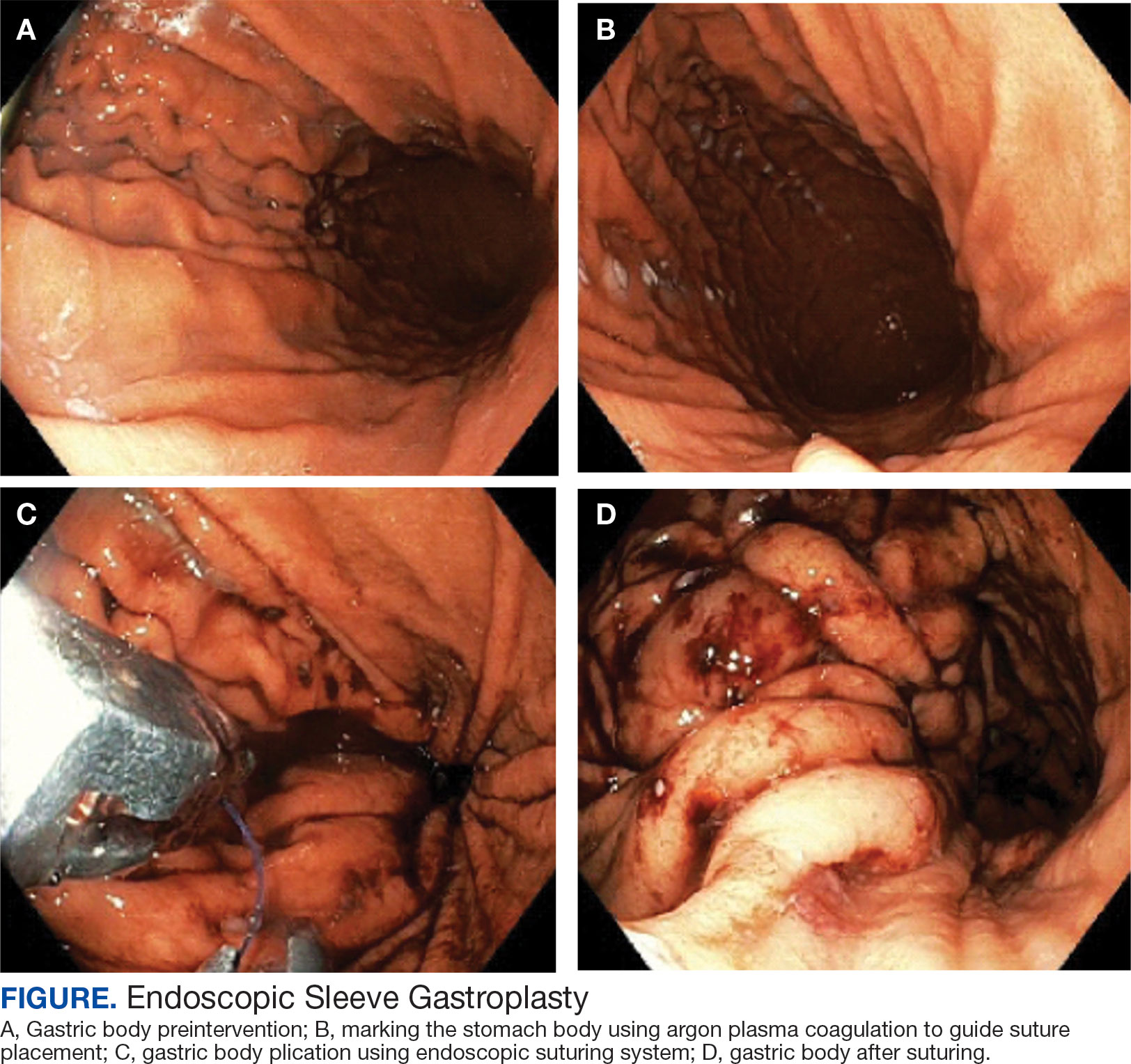
Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
Obesity is a growing worldwide epidemic with significant implications for individual health and public health care costs. It is also associated with several medical conditions, including diabetes, cardiovascular disease, cancer, and mental health disorders.1 Comprehensive lifestyle intervention is a first-line therapy for obesity consisting of dietary and exercise interventions. Despite initial success, long-term results and durability of weight loss with lifestyle modifications are limited. 2 Bariatric surgery, including sleeve gastrectomy and gastric bypass surgery, is a more invasive approach that is highly effective in weight loss. However, these operations are not reversible, and patients may not be eligible for or may not desire surgery. Overall, bariatric surgery is widely underutilized, with < 1% of eligible patients ultimately undergoing surgery.3,4
Endoscopic bariatric therapies are increasingly popular procedures that address the need for additional treatments for obesity among individuals who have not had success with lifestyle changes and are not surgical candidates. The most common procedure is the endoscopic sleeve gastroplasty (ESG), which applies full-thickness sutures in the stomach to reduce gastric volume, delay gastric emptying, and limit food intake while keeping the fundus intact compared with sleeve gastrectomy. This procedure is typically considered in patients with body mass index (BMI) ≥ 30, who do not qualify for or do not want traditional bariatric surgery. The literature supports robust outcomes after ESG, with studies demonstrating significant and sustained total body weight loss of up to 14% to 16% at 5 years and significant improvement in ≥ 1 metabolic comorbidities in 80% of patients.5,6 ESG adverse events (AEs) include abdominal pain, nausea, and vomiting that are typically self-limited to 1 week. Rarer but more serious AEs include bleeding, perforation, or infection, and occur in 2% of cases based on large trial data.5,7
Although the weight loss benefits of ESG are well established, to date, there are limited data on the effects of endoscopic bariatric therapies like ESG on mental health conditions. Here, we describe a case of a veteran with a history of mental health disorders that prevented him from completing bariatric surgery. The patient underwent ESG and had a successful clinical course.
CASE PRESENTATION
A 59-year-old male veteran with a medical history of class III obesity (42.4 BMI), obstructive sleep apnea, hypothyroidism, hypertension, type 2 diabetes mellitus, and a large ventral hernia was referred to the MOVE! (Management of Overweight/ Obese Veterans Everywhere!) multidisciplinary high-intensity weight loss program at the US Department of Veterans Affairs (VA) West Los Angeles VA Medical Center (WLAVAMC). His psychiatric history included generalized anxiety disorder, posttraumatic stress disorder (PTSD), and panic disorder, managed by the Psychiatry Service and treated with sertraline 25 mg daily, lorazepam 0.5 mg twice daily, and hydroxyzine 20 mg nightly. He had previously implemented lifestyle changes and attended MOVE! classes and nutrition coaching for 1 year but was unsuccessful in losing weight. He had also tried liraglutide 3 mg daily for weight loss but was unable to tolerate it and reported worsening medication-related anxiety.
The patient declined further weight loss pharmacotherapy and was referred to bariatric surgery. He was scheduled for a surgical sleeve gastrectomy. However, on the day he arrived at the hospital for surgery, he developed severe anxiety and had a panic attack, and it was canceled. Due to his mental health issues, he was no longer comfortable proceeding with surgery and was left without other options for obesity treatment. The veteran was extremely disappointed because the ventral hernia caused significant quality of life impairment, limited his ability to exercise, and caused him embarrassment in public settings. The hernia could not be surgically repaired until there was significant weight loss.
A bariatric endoscopy program within the Division of Gastroenterology was developed and implemented at the WLAVAMC in February 2023 in conjunction with MOVE! The patient was referred for consideration of an endoscopic weight loss procedure. He was determined to be a suitable candidate for ESG based on his BMI being > 40 and personal preference not to proceed with surgery to lose enough weight to qualify for hernia repair. The veteran underwent an endoscopy, which showed normal anatomy and gastric mucosa. ESG was performed in standard fashion (Figure).8 Three vertical lines were made using argon plasma coagulation from the incisura to 2 cm below the gastroesophageal junction along the anterior, posterior, and greater curvature of the stomach to mark the area for endoscopic suture placement. Starting at the incisura, 7 full-thickness sutures were placed to create a volume reduction plication, with preservation of the fundus. The patient did well postprocedure with no immediate or delayed AEs and was discharged home the same day.

Follow-up
The veteran followed a gradual dietary advancement from a clear liquid diet to pureed and soft texture food. The patient’s weight dropped from 359 lbs preprocedure to 304 lbs 6 months postprocedure, a total body weight loss (TWBL) of 15.3%. At 12 months the veteran weighed 299 lbs (16.7% TBWL). He also had notable improvements in metabolic parameters. His systolic blood pressure decreased from ≥ 140 mm Hg to 120 to 130 mm Hg and hemoglobin A1c dropped from 7.0% to 6.3%. Remarkably, his psychiatrist noted significant improvement in his overall mental health. The veteran reported complete cessation of panic attacks since the ESG, improvements in PTSD and anxiety, and was able to discontinue lorazepam and decrease his dose of sertraline to 12.5 mg daily. He reported feeling more energetic and goal-oriented with increased clarity of thought. Perhaps the most significant outcome was that after the 55-lb weight loss at 6 months, the patient was eligible to undergo ventral hernia surgical repair, which had previously contributed to shame and social isolation. This, in turn, improved his quality of life, allowed him to start walking again, up to 8 miles daily, and to feel comfortable again going out in public settings.
DISCUSSION
Bariatric surgeries are an effective method of achieving weight loss and improving obesity-related comorbidities. However, only a small percentage of individuals with obesity are candidates for bariatric surgery. Given the dramatic increase in the prevalence of obesity, other options are needed. Specifically, within the VA, an estimated 80% of veterans are overweight or obese, but only about 500 bariatric surgeries are performed annually.9 With the need for additional weight loss therapies, VA programs are starting to offer endoscopic bariatric procedures as an alternative option. This may be a desirable choice for patients with obesity (BMI > 30), with or without associated metabolic comorbidities, who need more aggressive intervention beyond dietary and lifestyle changes and are either not interested in or not eligible for bariatric surgery or weight loss medications.
Although there is evidence that metabolic comorbidities are associated with obesity, there has been less research on obesity and mental health comorbidities such as depression and anxiety. These psychiatric conditions may even be more common among patients seeking weight loss procedures and more prominent in certain groups such as veterans, which may ultimately exclude these patients from bariatric surgery.10 Prior studies suggest that bariatric surgery can reduce the severity of depression and, to a lesser extent, anxiety symptoms at 2 years following the initial surgery; however, there is limited literature describing the impact of weight loss procedure on panic disorders.11-14 We suspect that a weight loss procedure such as ESG may have indirectly improved the veteran’s mood disorder due to the weight loss it induced, increasing the ability to exercise, quality of sleep, and participation in public settings.
This case highlights a veteran who did not tolerate weight loss medication and had severe anxiety and PTSD that prevented him from going through with bariatric surgery. He then underwent an endoscopic weight loss procedure. The ESG helped him successfully achieve significant weight loss, increase his physical activity, reduce his anxiety and panic disorder, and overall, significantly improve his quality of life. More than 1 year after the procedure, the patient has sustained improvements in his psychiatric and emotional health along with durable weight loss, maintaining > 15% of his total weight lost. Additional studies are needed to further understand the prevalence and long-term outcomes of mental health comorbidities, as well as weight loss outcomes in this group of patients who undergo endoscopic bariatric procedures.
CONCLUSIONS
We describe a case of a veteran with severe obesity and significant psychiatric comorbidities that prevented him from undergoing bariatric surgery, who underwent an ESG. This procedure led to significant weight loss, improvement of metabolic parameters, reduction in anxiety and PTSD, and enhancement of his quality of life. This case emphasizes the unique advantages of ESG and supports the expansion of endoscopic bariatric programs in the VA.
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319-326. doi:10.1016/j.numecd.2006.07.005
- Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723. doi:10.1111/obr.12551
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using andersen’s model of health services use. Surg Obes Relat Dis. 2018;14(3):404-412. doi:10.1016/j.soard.2017.11.012
- Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150- 163. doi:10.1001/jama.2015.18118
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al.. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441-451. doi:10.1016/S0140-6736(22)01280-6
- Matteo MV, Bove V, Ciasca G, et al. Success predictors of endoscopic sleeve gastroplasty. Obes Surg. 2024;34(5):1496-1504. doi:10.1007/s11695-024-07109-4
- Maselli DB, Hoff AC, Kucera A, et al. Endoscopic sleeve gastroplasty in class III obesity: efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc. 2023;15(6):469-479. doi:10.4253/wjge.v15.i6.469
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32(4):2159-2164. doi:10.1007/s00464-017-5869-2
- Maggard-Gibbons M, Shekelle PG, Girgis MD, et al. Endoscopic Bariatric Interventions versus lifestyle interventions or surgery for weight loss in patients with obesity: a systematic review and meta-analysis. Department of Veterans Affairs (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK587943/
- Maggard Gibbons MA, Maher AM, Dawes AJ, et al. Psychological clearance for bariatric surgery: a systematic review. VA-ESP project #05-2262014.
- van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15(4):552-560. doi:10.1381/0960892053723484
- Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61(3):348-358. doi:10.1016/j.biopsych.2006.03.040
- Aylward L, Lilly C, Konsor M, et al. How soon do depression and anxiety symptoms improve after bariatric surgery?. Healthcare (Basel). 2023;11(6):862. doi:10.3390/healthcare11060862
- Law S, Dong S, Zhou F, Zheng D, Wang C, Dong Z. Bariatric surgery and mental health outcomes: an umbrella review. Front Endocrinol (Lausanne). 2023;14:1283621. doi:10.3389/fendo.2023.1283621
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Endoscopic Sleeve Gastroplasty is an Effective Treatment for Obesity in a Veteran With Metabolic and Psychiatric Comorbidities
Anti-Tumor Necrosis Factor Treatment for Glomerulopathy: Case Report and Review of Literature
Podocytes are terminally differentiated, highly specialized cells located in juxtaposition to the basement membrane over the abluminal surfaces of endothelial cells within the glomerular tuft. This triad structure is the site of the filtration barrier, which forms highly delicate and tightly regulated architecture to carry out the ultrafiltration function of the kidney.1 The filtration barrier is characterized by foot processes that are connected by specialized junctions called slit diaphragms.
Insults to components of the filtration barrier can initiate cascading events and perpetuate structural alterations that may eventually result in sclerotic changes.2 Common causes among children include minimal change disease (MCD) with the collapse of foot processes resulting in proteinuria, Alport syndrome due to mutation of collagen fibers within the basement membrane leading to hematuria and proteinuria, immune complex mediated nephropathy following common infections or autoimmune diseases, and focal segmental glomerulosclerosis (FSGS) that can show variable histopathology toward eventual glomerular scarring.3,4 These children often clinically have minimal, if any, signs of systemic inflammation.3-5 This has been a limiting factor for the commitment to immunomodulatory treatment, except for steroids for the treatment of MCD.6 Although prolonged steroid treatment may be efficacious, adverse effects are significant in a growing child. Alternative treatments, such as tacrolimus and rituximab have been suggested as second-line steroid-sparing agents.7,8 Not uncommonly, however, these cases are managed by supportive measures only during the progression of the natural course of the disease, which may eventually lead to renal failure, requiring transplant for survival.8,9
This case report highlights a child with a variant of uncertain significance (VUS) in genes involved in Alport syndrome and FSGS who developed an abrupt onset of proteinuria and hematuria after a respiratory illness. To our knowledge, he represents the youngest case demonstrating the benefit of targeted treatment against tumor necrosis factor-α (TNF-α) for glomerulopathy using biologic response modifiers.
Case Description
This is currently a 7-year-old male patient who was born at 39 weeks gestation to gravida 3 para 3 following induced labor due to elevated maternal blood pressure. During the first 2 years of life, his growth and development were normal and his immunizations were up to date. The patient's medical history included upper respiratory tract infections (URIs), respiratory syncytial virus, as well as 3 bouts of pneumonia and multiple otitis media that resulted in 18 rounds of antibiotics. The child was also allergic to nuts and milk protein. The patient’s parents are of Northern European and Native American descent. There is no known family history of eye, ear, or kidney diseases.
Renal concerns were first noted at the age of 2 years and 6 months when he presented to an emergency department in Fall 2019 (week 0) for several weeks of intermittent dark-colored urine. His mother reported that the discoloration recently progressed in intensity to cola-colored, along with the onset of persistent vomiting without any fever or diarrhea. On physical examination, the patient had normal vitals: weight 14.8 kg (68th percentile), height 91 cm (24th percentile), and body surface area 0.6 m2. There was no edema, rash, or lymphadenopathy, but he appeared pale.
The patient’s initial laboratory results included: complete blood count with white blood cells (WBC) 10 x 103/L (reference range, 4.5-13.5 x 103/L); differential lymphocytes 69%; neutrophils 21%; hemoglobin 10 g/dL (reference range, 12-16 g/dL); hematocrit, 30%; (reference range, 37%-45%); platelets 437 103/L (reference range, 150-450 x 103/L); serum creatinine 0.46 mg/dL (reference range, 0.5-0.9 mg/dL); and albumin 3.1 g/dL (reference range, 3.5-5.2 g/dL). Serum electrolyte levels and liver enzymes were normal. A urine analysis revealed 3+ protein and 3+ blood with dysmorphic red blood cells (RBC) and RBC casts without WBC. The patient's spot urine protein-to-creatinine ratio was 4.3 and his renal ultrasound was normal. The patient was referred to Nephrology.
During the next 2 weeks, his protein-to-creatinine ratio progressed to 5.9 and serum albumin fell to 2.7 g/dL. His urine remained red colored, and a microscopic examination with RBC > 500 and WBC up to 10 on a high powered field. His workup was negative for antinuclear antibodies, antineutrophil cytoplasmic antibody, antistreptolysin-O (ASO) and anti-DNase B. Serum C3 was low at 81 mg/dL (reference range, 90-180 mg/dL), C4 was 13.3 mg/dL (reference range, 10-40 mg/dL), and immunoglobulin G was low at 452 mg/dL (reference range 719-1475 mg/dL). A baseline audiology test revealed normal hearing.
Percutaneous renal biopsy yielded about 12 glomeruli, all exhibiting mild mesangial matrix expansion and hypercellularity (Figure 1). One glomerulus had prominent parietal epithelial cells without endocapillary hypercellularity or crescent formation. There was no interstitial fibrosis or tubular atrophy. Immunofluorescence studies showed no evidence of immune complex deposition with negative staining for immunoglobulin heavy and light chains, C3 and C1q. Staining for α 2 and α 5 units of collagen was normal. Electron microscopy showed patchy areas of severe basement membrane thinning with frequent foci of mild to moderate lamina densa splitting and associated visceral epithelial cell foot process effacement (Figure 2).
These were reported as concerning findings for possible Alport syndrome by 3 independent pathology teams. The genetic testing was submitted at a commercial laboratory to screen 17 mutations, including COL4A3, COL4A4, and COL4A5. Results showed the presence of a heterozygous VUS in the COL4A4 gene (c.1055C > T; p.Pro352Leu; dbSNP ID: rs371717486; PolyPhen-2: Probably Damaging; SIFT: Deleterious) as well as the presence of a heterozygous VUS in TRPC6 gene (c2463A>T; p.Lys821Asn; dbSNP ID: rs199948731; PolyPhen-2: Benign; SIFT: Tolerated). Further genetic investigation by whole exome sequencing on approximately 20,000 genes through MNG Laboratories showed a new heterozygous VUS in the OSGEP gene [c.328T>C; p.Cys110Arg]. Additional studies ruled out mitochondrial disease, CoQ10 deficiency, and metabolic disorders upon normal findings for mitochondrial DNA, urine amino acids, plasma acylcarnitine profile, orotic acid, ammonia, and homocysteine levels.
Figure 3 summarizes the patient’s treatment response during 170 weeks of follow-up (Fall 2019 to Summer 2023). The patient was started on enalapril 0.6 mg/kg daily at week 3, which continued throughout treatment. Following a rheumatology consult at week 30, the patient was started on prednisolone 3 mg/mL to assess the role of inflammation through the treatment response. An initial dose of 2 mg/kg daily (9 mL) for 1 month was followed by every other day treatment that was tapered off by week 48. To control mild but noticeably increasing proteinuria in the interim, subcutaneous anakinra 50 mg (3 mg/kg daily) was added as a steroid
DISCUSSION
This case describes a child with rapidly progressive proteinuria and hematuria following a URI who was found to have VUS mutations in 3 different genes associated with chronic kidney disease. Serology tests on the patient were negative for streptococcal antibodies and antinuclear antibodies, ruling out poststreptococcal glomerulonephritis, or systemic lupus erythematosus. His renal biopsy findings were concerning for altered podocytes, mesangial cells, and basement membrane without inflammatory infiltrate, immune complex, complements, immunoglobulin A, or vasculopathy. His blood inflammatory markers, erythrocyte sedimentation rate, C-reactive protein, and ferritin were normal when his care team initiated daily steroids.
Overall, the patient’s clinical presentation and histopathology findings were suggestive of Alport syndrome or thin basement membrane nephropathy with a high potential to progress into FSGS.10-12 Alport syndrome affects 1 in 5000 to 10,000 children annually due to S-linked inheritance of COL4A5, or autosomal recessive inheritance of COL4A3 or COL4A4 genes. It presents with hematuria and hearing loss.10 Our patient had a single copy COL4A4 gene mutation that was classified as VUS. He also had 2 additional VUS affecting the TRPC6 and OSGEP genes. TRPC6 gene mutation can be associated with FSGS through autosomal dominant inheritance. Both COL4A4 and TRPC6 gene mutations were paternally inherited. Although the patient’s father not having renal disease argues against the clinical significance of these findings, there is literature on the potential role of heterozygous COL4A4 variant mimicking thin basement membrane nephropathy that can lead to renal impairment upon copresence of superimposed conditions.13 The patient’s rapidly progressing hematuria and changes in the basement membrane were worrisome for emerging FSGS. Furthermore, VUS of TRPC6 has been reported in late onset autosomal dominant FSGS and can be associated with early onset steroid-resistant nephrotic syndrome (NS) in children.14 This concern was voiced by 3 nephrology consultants during the initial evaluation, leading to the consensus that steroid treatment for podocytopathy would not alter the patient’s long-term outcomes (ie, progression to FSGS).
Immunomodulation
Our rationale for immunomodulatory treatment was based on the abrupt onset of renal concerns following a URI, suggesting the importance of an inflammatory trigger causing altered homeostasis in a genetically susceptible host. Preclinical models show that microbial products such as lipopolysaccharides can lead to podocytopathy by several mechanisms through activation of toll-like receptor signaling. It can directly cause apoptosis by downregulation of the intracellular Akt survival pathway.15 Lipopolysaccharide can also activate the NF-αB pathway and upregulate the production of interleukin-1 (IL-1) and TNF-α in mesangial cells.16,17
Both cytokines can promote mesangial cell proliferation.18 Through autocrine and paracrine mechanisms, proinflammatory cytokines can further perpetuate somatic tissue changes and contribute to the development of podocytopathy. For instance, TNF-α can promote podocyte injury and proteinuria by downregulation of the slit diaphragm protein expression (ie, nephrin, ezrin, or podocin), and disruption of podocyte cytoskeleton.19,20 TNF-α promotes the influx and activation of macrophages and inflammatory cells. It is actively involved in chronic alterations within the glomeruli by the upregulation of matrix metalloproteases by integrins, as well as activation of myofibroblast progenitors and extracellular matrix deposition in crosstalk with transforming growth factor and other key mediators.17,21,22
For the patient described in this case report, initial improvement on steroids encouraged the pursuit of additional treatment to downregulate inflammatory pathways within the glomerular milieu. However, within the COVID-19 environment, escalating the patient’s treatment using traditional immunomodulators (ie, calcineurin inhibitors or mycophenolate mofetil) was not favored due to the risk of infection. Initially, anakinra, a recombinant IL-1 receptor antagonist, was preferred as a steroid-sparing agent for its short life and safety profile during the pandemic. At first, the patient responded well to anakinra and was allowed a steroid wean when the dose was titrated up to 6 mg/kg daily. However, anakinra did not prevent the escalation of proteinuria following a URI. After the treatment was changed to adalimumab, a fully humanized monoclonal antibody to TNF-α, the patient continued to improve and reach full remission despite experiencing a cold and the flu in the following months.
Literature Review
There is a paucity of literature on applications of biological response modifiers for idiopathic NS and FSGS.23,24 Angeletti and colleagues reported that 3 patients with severe long-standing FSGS benefited from anakinra 4 mg/kg daily to reduce proteinuria and improve kidney function. All the patients had positive C3 staining in renal biopsy and treatment response, which supported the role of C3a in inducing podocyte injury through upregulated expression of IL-1 and IL-1R.23 Trachtman and colleagues reported on the phase II FONT trial that included 14 of 21 patients aged < 18 years with advanced FSGS who were treated with adalimumab 24 mg/m2, or ≤ 40 mg every other week.24 Although, during a 6-month period, none of the 7 patients met the endpoint of reduced proteinuria by ≥ 50%, and the authors suggested that careful patient selection may improve the treatment response in future trials.24
A recent study involving transcriptomics on renal tissue samples combined with available pathology (fibrosis), urinary markers, and clinical characteristics on 285 patients with MCD or FSGS from 3 different continents identified 3 distinct clusters. Patients with evidence of activated kidney TNF pathway (n = 72, aged > 18 years) were found to have poor clinical outcomes.25 The study identified 2 urine markers associated with the TNF pathway (ie, tissue inhibitor of metalloproteinases-1 and monocyte chemoattractant protein-1), which aligns with the preclinical findings previously mentioned.25
Conclusions
The patient’s condition in this case illustrates the complex nature of biologically predetermined cascading events in the emergence of glomerular disease upon environmental triggers under the influence of genetic factors.
Chronic kidney disease affects 7.7% of veterans annually, illustrating the need for new therapeutics.26 Based on our experience and literature review, upregulation of TNF-α is a root cause of glomerulopathy; further studies are warranted to evaluate the efficacy of anti-TNF biologic response modifiers for the treatment of these patients. Long-term postmarketing safety profile and steroid-sparing properties of adalimumab should allow inclusion of pediatric cases in future trials. Results may also contribute to identifying new predictive biomarkers related to the basement membrane when combined with precision nephrology to further advance patient selection and targeted treatment.25,27
Acknowledgments
The authors thank the patient’s mother for providing consent to allow publication of this case report.
1. Arif E, Nihalani D. Glomerular filtration barrier assembly: an insight. Postdoc J. 2013;1(4):33-45.
2. Garg PA. Review of podocyte biology. Am J Nephrol. 2018;47(suppl 1):3-13. doi:10.1159/000481633SUPPL
3. Warady BA, Agarwal R, Bangalore S, et al. Alport syndrome classification and management. Kidney Med. 2020;2(5):639-649. doi:10.1016/j.xkme.2020.05.014
4. Angioi A, Pani A. FSGS: from pathogenesis to the histological lesion. J Nephrol. 2016;29(4):517-523. doi:10.1007/s40620-016-0333-2
5. Roca N, Martinez C, Jatem E, Madrid A, Lopez M, Segarra A. Activation of the acute inflammatory phase response in idiopathic nephrotic syndrome: association with clinicopathological phenotypes and with response to corticosteroids. Clin Kidney J. 2021;14(4):1207-1215. doi:10.1093/ckj/sfaa247
6. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332-345.
7. Medjeral-Thomas NR, Lawrence C, Condon M, et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with De Novo minimal change disease: a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15(2):209-218. doi:10.2215/CJN.06290420
8. Ye Q, Lan B, Liu H, Persson PB, Lai EY, Mao J. A critical role of the podocyte cytoskeleton in the pathogenesis of glomerular proteinuria and autoimmune podocytopathies. Acta Physiol (Oxf). 2022;235(4):e13850. doi:10.1111/apha.13850
9. Trautmann A, Schnaidt S, Lipska-Ziμtkiewicz BS, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055-3065. doi:10.1681/ASN.2016101121
10. Kashtan CE, Gross O. Clinical practice recommendations for the diagnosis and management of Alport syndrome in children, adolescents, and young adults-an update for 2020. Pediatr Nephrol. 2021;36(3):711-719. doi:10.1007/s00467-020-04819-6
11. Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY. Thin basement membrane nephropathy. Kidney Int. 2003;64(4):1169-78. doi:10.1046/j.1523-1755.2003.00234.x
12. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017; 12(3):502-517. doi:10.2215/CJN.05960616
13. Savige J. Should we diagnose autosomal dominant Alport syndrome when there is a pathogenic heterozygous COL4A3 or COL4A4 variant? Kidney Int Rep. 2018;3(6):1239-1241. doi:10.1016/j.ekir.2018.08.002
14. Gigante M, Caridi G, Montemurno E, et al. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol. 2011;6(7):1626-1634. doi:10.2215/CJN.07830910
15. Saurus P, Kuusela S, Lehtonen E, et al. Podocyte apoptosis is prevented by blocking the toll-like receptor pathway. Cell Death Dis. 2015;6(5):e1752. doi:10.1038/cddis.2015.125
16. Baud L, Oudinet JP, Bens M, et al. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35(5):1111-1118. doi:10.1038/ki.1989.98
17. White S, Lin L, Hu K. NF-κB and tPA signaling in kidney and other diseases. Cells. 2020;9(6):1348. doi:10.3390/cells9061348
18. Tesch GH, Lan HY, Atkins RC, Nikolic-Paterson DJ. Role of interleukin-1 in mesangial cell proliferation and matrix deposition in experimental mesangioproliferative nephritis. Am J Pathol. 1997;151(1):141-150.
19. Lai KN, Leung JCK, Chan LYY, et al. Podocyte injury induced by mesangial-derived cytokines in IgA Nephropathy. Nephrol Dial Transplant. 2009;24(1):62-72. doi:10.1093/ndt/gfn441
20. Saleem MA, Kobayashi Y. Cell biology and genetics of minimal change disease. F1000 Res. 2016;5: F1000 Faculty Rev-412. doi:10.12688/f1000research.7300.1
21. Kim KP, Williams CE, Lemmon CA. Cell-matrix interactions in renal fibrosis. Kidney Dial. 2022;2(4):607-624. doi:10.3390/kidneydial2040055
22. Zvaifler NJ. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther. 2006;8(3):210. doi:10.1186/ar1963
23. Angeletti A, Magnasco A, Trivelli A, et al. Refractory minimal change disease and focal segmental glomerular sclerosis treated with Anakinra. Kidney Int Rep. 2021;7(1):121-124. doi:10.1016/j.ekir.2021.10.018
24. Trachtman H, Vento S, Herreshoff E, et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 2015;16:111. doi:10.1186/s12882-015-0094-5
25. Mariani LH, Eddy S, AlAkwaa FM, et al. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023;103(3):565-579. doi:10.1016/j.kint.2022.10.023
26. Korshak L, Washington DL, Powell J, Nylen E, Kokkinos P. Kidney Disease in Veterans. US Dept of Veterans Affairs, Office of Health Equity. Updated May 13, 2020. Accessed June 28, 2024. https://www.va.gov/HEALTHEQUITY/Kidney_Disease_In_Veterans.asp
27. Malone AF, Phelan PJ, Hall G, et al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 2014;86(6):1253-1259. doi:10.1038/ki.2014.305
Podocytes are terminally differentiated, highly specialized cells located in juxtaposition to the basement membrane over the abluminal surfaces of endothelial cells within the glomerular tuft. This triad structure is the site of the filtration barrier, which forms highly delicate and tightly regulated architecture to carry out the ultrafiltration function of the kidney.1 The filtration barrier is characterized by foot processes that are connected by specialized junctions called slit diaphragms.
Insults to components of the filtration barrier can initiate cascading events and perpetuate structural alterations that may eventually result in sclerotic changes.2 Common causes among children include minimal change disease (MCD) with the collapse of foot processes resulting in proteinuria, Alport syndrome due to mutation of collagen fibers within the basement membrane leading to hematuria and proteinuria, immune complex mediated nephropathy following common infections or autoimmune diseases, and focal segmental glomerulosclerosis (FSGS) that can show variable histopathology toward eventual glomerular scarring.3,4 These children often clinically have minimal, if any, signs of systemic inflammation.3-5 This has been a limiting factor for the commitment to immunomodulatory treatment, except for steroids for the treatment of MCD.6 Although prolonged steroid treatment may be efficacious, adverse effects are significant in a growing child. Alternative treatments, such as tacrolimus and rituximab have been suggested as second-line steroid-sparing agents.7,8 Not uncommonly, however, these cases are managed by supportive measures only during the progression of the natural course of the disease, which may eventually lead to renal failure, requiring transplant for survival.8,9
This case report highlights a child with a variant of uncertain significance (VUS) in genes involved in Alport syndrome and FSGS who developed an abrupt onset of proteinuria and hematuria after a respiratory illness. To our knowledge, he represents the youngest case demonstrating the benefit of targeted treatment against tumor necrosis factor-α (TNF-α) for glomerulopathy using biologic response modifiers.
Case Description
This is currently a 7-year-old male patient who was born at 39 weeks gestation to gravida 3 para 3 following induced labor due to elevated maternal blood pressure. During the first 2 years of life, his growth and development were normal and his immunizations were up to date. The patient's medical history included upper respiratory tract infections (URIs), respiratory syncytial virus, as well as 3 bouts of pneumonia and multiple otitis media that resulted in 18 rounds of antibiotics. The child was also allergic to nuts and milk protein. The patient’s parents are of Northern European and Native American descent. There is no known family history of eye, ear, or kidney diseases.
Renal concerns were first noted at the age of 2 years and 6 months when he presented to an emergency department in Fall 2019 (week 0) for several weeks of intermittent dark-colored urine. His mother reported that the discoloration recently progressed in intensity to cola-colored, along with the onset of persistent vomiting without any fever or diarrhea. On physical examination, the patient had normal vitals: weight 14.8 kg (68th percentile), height 91 cm (24th percentile), and body surface area 0.6 m2. There was no edema, rash, or lymphadenopathy, but he appeared pale.
The patient’s initial laboratory results included: complete blood count with white blood cells (WBC) 10 x 103/L (reference range, 4.5-13.5 x 103/L); differential lymphocytes 69%; neutrophils 21%; hemoglobin 10 g/dL (reference range, 12-16 g/dL); hematocrit, 30%; (reference range, 37%-45%); platelets 437 103/L (reference range, 150-450 x 103/L); serum creatinine 0.46 mg/dL (reference range, 0.5-0.9 mg/dL); and albumin 3.1 g/dL (reference range, 3.5-5.2 g/dL). Serum electrolyte levels and liver enzymes were normal. A urine analysis revealed 3+ protein and 3+ blood with dysmorphic red blood cells (RBC) and RBC casts without WBC. The patient's spot urine protein-to-creatinine ratio was 4.3 and his renal ultrasound was normal. The patient was referred to Nephrology.
During the next 2 weeks, his protein-to-creatinine ratio progressed to 5.9 and serum albumin fell to 2.7 g/dL. His urine remained red colored, and a microscopic examination with RBC > 500 and WBC up to 10 on a high powered field. His workup was negative for antinuclear antibodies, antineutrophil cytoplasmic antibody, antistreptolysin-O (ASO) and anti-DNase B. Serum C3 was low at 81 mg/dL (reference range, 90-180 mg/dL), C4 was 13.3 mg/dL (reference range, 10-40 mg/dL), and immunoglobulin G was low at 452 mg/dL (reference range 719-1475 mg/dL). A baseline audiology test revealed normal hearing.
Percutaneous renal biopsy yielded about 12 glomeruli, all exhibiting mild mesangial matrix expansion and hypercellularity (Figure 1). One glomerulus had prominent parietal epithelial cells without endocapillary hypercellularity or crescent formation. There was no interstitial fibrosis or tubular atrophy. Immunofluorescence studies showed no evidence of immune complex deposition with negative staining for immunoglobulin heavy and light chains, C3 and C1q. Staining for α 2 and α 5 units of collagen was normal. Electron microscopy showed patchy areas of severe basement membrane thinning with frequent foci of mild to moderate lamina densa splitting and associated visceral epithelial cell foot process effacement (Figure 2).
These were reported as concerning findings for possible Alport syndrome by 3 independent pathology teams. The genetic testing was submitted at a commercial laboratory to screen 17 mutations, including COL4A3, COL4A4, and COL4A5. Results showed the presence of a heterozygous VUS in the COL4A4 gene (c.1055C > T; p.Pro352Leu; dbSNP ID: rs371717486; PolyPhen-2: Probably Damaging; SIFT: Deleterious) as well as the presence of a heterozygous VUS in TRPC6 gene (c2463A>T; p.Lys821Asn; dbSNP ID: rs199948731; PolyPhen-2: Benign; SIFT: Tolerated). Further genetic investigation by whole exome sequencing on approximately 20,000 genes through MNG Laboratories showed a new heterozygous VUS in the OSGEP gene [c.328T>C; p.Cys110Arg]. Additional studies ruled out mitochondrial disease, CoQ10 deficiency, and metabolic disorders upon normal findings for mitochondrial DNA, urine amino acids, plasma acylcarnitine profile, orotic acid, ammonia, and homocysteine levels.
Figure 3 summarizes the patient’s treatment response during 170 weeks of follow-up (Fall 2019 to Summer 2023). The patient was started on enalapril 0.6 mg/kg daily at week 3, which continued throughout treatment. Following a rheumatology consult at week 30, the patient was started on prednisolone 3 mg/mL to assess the role of inflammation through the treatment response. An initial dose of 2 mg/kg daily (9 mL) for 1 month was followed by every other day treatment that was tapered off by week 48. To control mild but noticeably increasing proteinuria in the interim, subcutaneous anakinra 50 mg (3 mg/kg daily) was added as a steroid
DISCUSSION
This case describes a child with rapidly progressive proteinuria and hematuria following a URI who was found to have VUS mutations in 3 different genes associated with chronic kidney disease. Serology tests on the patient were negative for streptococcal antibodies and antinuclear antibodies, ruling out poststreptococcal glomerulonephritis, or systemic lupus erythematosus. His renal biopsy findings were concerning for altered podocytes, mesangial cells, and basement membrane without inflammatory infiltrate, immune complex, complements, immunoglobulin A, or vasculopathy. His blood inflammatory markers, erythrocyte sedimentation rate, C-reactive protein, and ferritin were normal when his care team initiated daily steroids.
Overall, the patient’s clinical presentation and histopathology findings were suggestive of Alport syndrome or thin basement membrane nephropathy with a high potential to progress into FSGS.10-12 Alport syndrome affects 1 in 5000 to 10,000 children annually due to S-linked inheritance of COL4A5, or autosomal recessive inheritance of COL4A3 or COL4A4 genes. It presents with hematuria and hearing loss.10 Our patient had a single copy COL4A4 gene mutation that was classified as VUS. He also had 2 additional VUS affecting the TRPC6 and OSGEP genes. TRPC6 gene mutation can be associated with FSGS through autosomal dominant inheritance. Both COL4A4 and TRPC6 gene mutations were paternally inherited. Although the patient’s father not having renal disease argues against the clinical significance of these findings, there is literature on the potential role of heterozygous COL4A4 variant mimicking thin basement membrane nephropathy that can lead to renal impairment upon copresence of superimposed conditions.13 The patient’s rapidly progressing hematuria and changes in the basement membrane were worrisome for emerging FSGS. Furthermore, VUS of TRPC6 has been reported in late onset autosomal dominant FSGS and can be associated with early onset steroid-resistant nephrotic syndrome (NS) in children.14 This concern was voiced by 3 nephrology consultants during the initial evaluation, leading to the consensus that steroid treatment for podocytopathy would not alter the patient’s long-term outcomes (ie, progression to FSGS).
Immunomodulation
Our rationale for immunomodulatory treatment was based on the abrupt onset of renal concerns following a URI, suggesting the importance of an inflammatory trigger causing altered homeostasis in a genetically susceptible host. Preclinical models show that microbial products such as lipopolysaccharides can lead to podocytopathy by several mechanisms through activation of toll-like receptor signaling. It can directly cause apoptosis by downregulation of the intracellular Akt survival pathway.15 Lipopolysaccharide can also activate the NF-αB pathway and upregulate the production of interleukin-1 (IL-1) and TNF-α in mesangial cells.16,17
Both cytokines can promote mesangial cell proliferation.18 Through autocrine and paracrine mechanisms, proinflammatory cytokines can further perpetuate somatic tissue changes and contribute to the development of podocytopathy. For instance, TNF-α can promote podocyte injury and proteinuria by downregulation of the slit diaphragm protein expression (ie, nephrin, ezrin, or podocin), and disruption of podocyte cytoskeleton.19,20 TNF-α promotes the influx and activation of macrophages and inflammatory cells. It is actively involved in chronic alterations within the glomeruli by the upregulation of matrix metalloproteases by integrins, as well as activation of myofibroblast progenitors and extracellular matrix deposition in crosstalk with transforming growth factor and other key mediators.17,21,22
For the patient described in this case report, initial improvement on steroids encouraged the pursuit of additional treatment to downregulate inflammatory pathways within the glomerular milieu. However, within the COVID-19 environment, escalating the patient’s treatment using traditional immunomodulators (ie, calcineurin inhibitors or mycophenolate mofetil) was not favored due to the risk of infection. Initially, anakinra, a recombinant IL-1 receptor antagonist, was preferred as a steroid-sparing agent for its short life and safety profile during the pandemic. At first, the patient responded well to anakinra and was allowed a steroid wean when the dose was titrated up to 6 mg/kg daily. However, anakinra did not prevent the escalation of proteinuria following a URI. After the treatment was changed to adalimumab, a fully humanized monoclonal antibody to TNF-α, the patient continued to improve and reach full remission despite experiencing a cold and the flu in the following months.
Literature Review
There is a paucity of literature on applications of biological response modifiers for idiopathic NS and FSGS.23,24 Angeletti and colleagues reported that 3 patients with severe long-standing FSGS benefited from anakinra 4 mg/kg daily to reduce proteinuria and improve kidney function. All the patients had positive C3 staining in renal biopsy and treatment response, which supported the role of C3a in inducing podocyte injury through upregulated expression of IL-1 and IL-1R.23 Trachtman and colleagues reported on the phase II FONT trial that included 14 of 21 patients aged < 18 years with advanced FSGS who were treated with adalimumab 24 mg/m2, or ≤ 40 mg every other week.24 Although, during a 6-month period, none of the 7 patients met the endpoint of reduced proteinuria by ≥ 50%, and the authors suggested that careful patient selection may improve the treatment response in future trials.24
A recent study involving transcriptomics on renal tissue samples combined with available pathology (fibrosis), urinary markers, and clinical characteristics on 285 patients with MCD or FSGS from 3 different continents identified 3 distinct clusters. Patients with evidence of activated kidney TNF pathway (n = 72, aged > 18 years) were found to have poor clinical outcomes.25 The study identified 2 urine markers associated with the TNF pathway (ie, tissue inhibitor of metalloproteinases-1 and monocyte chemoattractant protein-1), which aligns with the preclinical findings previously mentioned.25
Conclusions
The patient’s condition in this case illustrates the complex nature of biologically predetermined cascading events in the emergence of glomerular disease upon environmental triggers under the influence of genetic factors.
Chronic kidney disease affects 7.7% of veterans annually, illustrating the need for new therapeutics.26 Based on our experience and literature review, upregulation of TNF-α is a root cause of glomerulopathy; further studies are warranted to evaluate the efficacy of anti-TNF biologic response modifiers for the treatment of these patients. Long-term postmarketing safety profile and steroid-sparing properties of adalimumab should allow inclusion of pediatric cases in future trials. Results may also contribute to identifying new predictive biomarkers related to the basement membrane when combined with precision nephrology to further advance patient selection and targeted treatment.25,27
Acknowledgments
The authors thank the patient’s mother for providing consent to allow publication of this case report.
Podocytes are terminally differentiated, highly specialized cells located in juxtaposition to the basement membrane over the abluminal surfaces of endothelial cells within the glomerular tuft. This triad structure is the site of the filtration barrier, which forms highly delicate and tightly regulated architecture to carry out the ultrafiltration function of the kidney.1 The filtration barrier is characterized by foot processes that are connected by specialized junctions called slit diaphragms.
Insults to components of the filtration barrier can initiate cascading events and perpetuate structural alterations that may eventually result in sclerotic changes.2 Common causes among children include minimal change disease (MCD) with the collapse of foot processes resulting in proteinuria, Alport syndrome due to mutation of collagen fibers within the basement membrane leading to hematuria and proteinuria, immune complex mediated nephropathy following common infections or autoimmune diseases, and focal segmental glomerulosclerosis (FSGS) that can show variable histopathology toward eventual glomerular scarring.3,4 These children often clinically have minimal, if any, signs of systemic inflammation.3-5 This has been a limiting factor for the commitment to immunomodulatory treatment, except for steroids for the treatment of MCD.6 Although prolonged steroid treatment may be efficacious, adverse effects are significant in a growing child. Alternative treatments, such as tacrolimus and rituximab have been suggested as second-line steroid-sparing agents.7,8 Not uncommonly, however, these cases are managed by supportive measures only during the progression of the natural course of the disease, which may eventually lead to renal failure, requiring transplant for survival.8,9
This case report highlights a child with a variant of uncertain significance (VUS) in genes involved in Alport syndrome and FSGS who developed an abrupt onset of proteinuria and hematuria after a respiratory illness. To our knowledge, he represents the youngest case demonstrating the benefit of targeted treatment against tumor necrosis factor-α (TNF-α) for glomerulopathy using biologic response modifiers.
Case Description
This is currently a 7-year-old male patient who was born at 39 weeks gestation to gravida 3 para 3 following induced labor due to elevated maternal blood pressure. During the first 2 years of life, his growth and development were normal and his immunizations were up to date. The patient's medical history included upper respiratory tract infections (URIs), respiratory syncytial virus, as well as 3 bouts of pneumonia and multiple otitis media that resulted in 18 rounds of antibiotics. The child was also allergic to nuts and milk protein. The patient’s parents are of Northern European and Native American descent. There is no known family history of eye, ear, or kidney diseases.
Renal concerns were first noted at the age of 2 years and 6 months when he presented to an emergency department in Fall 2019 (week 0) for several weeks of intermittent dark-colored urine. His mother reported that the discoloration recently progressed in intensity to cola-colored, along with the onset of persistent vomiting without any fever or diarrhea. On physical examination, the patient had normal vitals: weight 14.8 kg (68th percentile), height 91 cm (24th percentile), and body surface area 0.6 m2. There was no edema, rash, or lymphadenopathy, but he appeared pale.
The patient’s initial laboratory results included: complete blood count with white blood cells (WBC) 10 x 103/L (reference range, 4.5-13.5 x 103/L); differential lymphocytes 69%; neutrophils 21%; hemoglobin 10 g/dL (reference range, 12-16 g/dL); hematocrit, 30%; (reference range, 37%-45%); platelets 437 103/L (reference range, 150-450 x 103/L); serum creatinine 0.46 mg/dL (reference range, 0.5-0.9 mg/dL); and albumin 3.1 g/dL (reference range, 3.5-5.2 g/dL). Serum electrolyte levels and liver enzymes were normal. A urine analysis revealed 3+ protein and 3+ blood with dysmorphic red blood cells (RBC) and RBC casts without WBC. The patient's spot urine protein-to-creatinine ratio was 4.3 and his renal ultrasound was normal. The patient was referred to Nephrology.
During the next 2 weeks, his protein-to-creatinine ratio progressed to 5.9 and serum albumin fell to 2.7 g/dL. His urine remained red colored, and a microscopic examination with RBC > 500 and WBC up to 10 on a high powered field. His workup was negative for antinuclear antibodies, antineutrophil cytoplasmic antibody, antistreptolysin-O (ASO) and anti-DNase B. Serum C3 was low at 81 mg/dL (reference range, 90-180 mg/dL), C4 was 13.3 mg/dL (reference range, 10-40 mg/dL), and immunoglobulin G was low at 452 mg/dL (reference range 719-1475 mg/dL). A baseline audiology test revealed normal hearing.
Percutaneous renal biopsy yielded about 12 glomeruli, all exhibiting mild mesangial matrix expansion and hypercellularity (Figure 1). One glomerulus had prominent parietal epithelial cells without endocapillary hypercellularity or crescent formation. There was no interstitial fibrosis or tubular atrophy. Immunofluorescence studies showed no evidence of immune complex deposition with negative staining for immunoglobulin heavy and light chains, C3 and C1q. Staining for α 2 and α 5 units of collagen was normal. Electron microscopy showed patchy areas of severe basement membrane thinning with frequent foci of mild to moderate lamina densa splitting and associated visceral epithelial cell foot process effacement (Figure 2).
These were reported as concerning findings for possible Alport syndrome by 3 independent pathology teams. The genetic testing was submitted at a commercial laboratory to screen 17 mutations, including COL4A3, COL4A4, and COL4A5. Results showed the presence of a heterozygous VUS in the COL4A4 gene (c.1055C > T; p.Pro352Leu; dbSNP ID: rs371717486; PolyPhen-2: Probably Damaging; SIFT: Deleterious) as well as the presence of a heterozygous VUS in TRPC6 gene (c2463A>T; p.Lys821Asn; dbSNP ID: rs199948731; PolyPhen-2: Benign; SIFT: Tolerated). Further genetic investigation by whole exome sequencing on approximately 20,000 genes through MNG Laboratories showed a new heterozygous VUS in the OSGEP gene [c.328T>C; p.Cys110Arg]. Additional studies ruled out mitochondrial disease, CoQ10 deficiency, and metabolic disorders upon normal findings for mitochondrial DNA, urine amino acids, plasma acylcarnitine profile, orotic acid, ammonia, and homocysteine levels.
Figure 3 summarizes the patient’s treatment response during 170 weeks of follow-up (Fall 2019 to Summer 2023). The patient was started on enalapril 0.6 mg/kg daily at week 3, which continued throughout treatment. Following a rheumatology consult at week 30, the patient was started on prednisolone 3 mg/mL to assess the role of inflammation through the treatment response. An initial dose of 2 mg/kg daily (9 mL) for 1 month was followed by every other day treatment that was tapered off by week 48. To control mild but noticeably increasing proteinuria in the interim, subcutaneous anakinra 50 mg (3 mg/kg daily) was added as a steroid
DISCUSSION
This case describes a child with rapidly progressive proteinuria and hematuria following a URI who was found to have VUS mutations in 3 different genes associated with chronic kidney disease. Serology tests on the patient were negative for streptococcal antibodies and antinuclear antibodies, ruling out poststreptococcal glomerulonephritis, or systemic lupus erythematosus. His renal biopsy findings were concerning for altered podocytes, mesangial cells, and basement membrane without inflammatory infiltrate, immune complex, complements, immunoglobulin A, or vasculopathy. His blood inflammatory markers, erythrocyte sedimentation rate, C-reactive protein, and ferritin were normal when his care team initiated daily steroids.
Overall, the patient’s clinical presentation and histopathology findings were suggestive of Alport syndrome or thin basement membrane nephropathy with a high potential to progress into FSGS.10-12 Alport syndrome affects 1 in 5000 to 10,000 children annually due to S-linked inheritance of COL4A5, or autosomal recessive inheritance of COL4A3 or COL4A4 genes. It presents with hematuria and hearing loss.10 Our patient had a single copy COL4A4 gene mutation that was classified as VUS. He also had 2 additional VUS affecting the TRPC6 and OSGEP genes. TRPC6 gene mutation can be associated with FSGS through autosomal dominant inheritance. Both COL4A4 and TRPC6 gene mutations were paternally inherited. Although the patient’s father not having renal disease argues against the clinical significance of these findings, there is literature on the potential role of heterozygous COL4A4 variant mimicking thin basement membrane nephropathy that can lead to renal impairment upon copresence of superimposed conditions.13 The patient’s rapidly progressing hematuria and changes in the basement membrane were worrisome for emerging FSGS. Furthermore, VUS of TRPC6 has been reported in late onset autosomal dominant FSGS and can be associated with early onset steroid-resistant nephrotic syndrome (NS) in children.14 This concern was voiced by 3 nephrology consultants during the initial evaluation, leading to the consensus that steroid treatment for podocytopathy would not alter the patient’s long-term outcomes (ie, progression to FSGS).
Immunomodulation
Our rationale for immunomodulatory treatment was based on the abrupt onset of renal concerns following a URI, suggesting the importance of an inflammatory trigger causing altered homeostasis in a genetically susceptible host. Preclinical models show that microbial products such as lipopolysaccharides can lead to podocytopathy by several mechanisms through activation of toll-like receptor signaling. It can directly cause apoptosis by downregulation of the intracellular Akt survival pathway.15 Lipopolysaccharide can also activate the NF-αB pathway and upregulate the production of interleukin-1 (IL-1) and TNF-α in mesangial cells.16,17
Both cytokines can promote mesangial cell proliferation.18 Through autocrine and paracrine mechanisms, proinflammatory cytokines can further perpetuate somatic tissue changes and contribute to the development of podocytopathy. For instance, TNF-α can promote podocyte injury and proteinuria by downregulation of the slit diaphragm protein expression (ie, nephrin, ezrin, or podocin), and disruption of podocyte cytoskeleton.19,20 TNF-α promotes the influx and activation of macrophages and inflammatory cells. It is actively involved in chronic alterations within the glomeruli by the upregulation of matrix metalloproteases by integrins, as well as activation of myofibroblast progenitors and extracellular matrix deposition in crosstalk with transforming growth factor and other key mediators.17,21,22
For the patient described in this case report, initial improvement on steroids encouraged the pursuit of additional treatment to downregulate inflammatory pathways within the glomerular milieu. However, within the COVID-19 environment, escalating the patient’s treatment using traditional immunomodulators (ie, calcineurin inhibitors or mycophenolate mofetil) was not favored due to the risk of infection. Initially, anakinra, a recombinant IL-1 receptor antagonist, was preferred as a steroid-sparing agent for its short life and safety profile during the pandemic. At first, the patient responded well to anakinra and was allowed a steroid wean when the dose was titrated up to 6 mg/kg daily. However, anakinra did not prevent the escalation of proteinuria following a URI. After the treatment was changed to adalimumab, a fully humanized monoclonal antibody to TNF-α, the patient continued to improve and reach full remission despite experiencing a cold and the flu in the following months.
Literature Review
There is a paucity of literature on applications of biological response modifiers for idiopathic NS and FSGS.23,24 Angeletti and colleagues reported that 3 patients with severe long-standing FSGS benefited from anakinra 4 mg/kg daily to reduce proteinuria and improve kidney function. All the patients had positive C3 staining in renal biopsy and treatment response, which supported the role of C3a in inducing podocyte injury through upregulated expression of IL-1 and IL-1R.23 Trachtman and colleagues reported on the phase II FONT trial that included 14 of 21 patients aged < 18 years with advanced FSGS who were treated with adalimumab 24 mg/m2, or ≤ 40 mg every other week.24 Although, during a 6-month period, none of the 7 patients met the endpoint of reduced proteinuria by ≥ 50%, and the authors suggested that careful patient selection may improve the treatment response in future trials.24
A recent study involving transcriptomics on renal tissue samples combined with available pathology (fibrosis), urinary markers, and clinical characteristics on 285 patients with MCD or FSGS from 3 different continents identified 3 distinct clusters. Patients with evidence of activated kidney TNF pathway (n = 72, aged > 18 years) were found to have poor clinical outcomes.25 The study identified 2 urine markers associated with the TNF pathway (ie, tissue inhibitor of metalloproteinases-1 and monocyte chemoattractant protein-1), which aligns with the preclinical findings previously mentioned.25
Conclusions
The patient’s condition in this case illustrates the complex nature of biologically predetermined cascading events in the emergence of glomerular disease upon environmental triggers under the influence of genetic factors.
Chronic kidney disease affects 7.7% of veterans annually, illustrating the need for new therapeutics.26 Based on our experience and literature review, upregulation of TNF-α is a root cause of glomerulopathy; further studies are warranted to evaluate the efficacy of anti-TNF biologic response modifiers for the treatment of these patients. Long-term postmarketing safety profile and steroid-sparing properties of adalimumab should allow inclusion of pediatric cases in future trials. Results may also contribute to identifying new predictive biomarkers related to the basement membrane when combined with precision nephrology to further advance patient selection and targeted treatment.25,27
Acknowledgments
The authors thank the patient’s mother for providing consent to allow publication of this case report.
1. Arif E, Nihalani D. Glomerular filtration barrier assembly: an insight. Postdoc J. 2013;1(4):33-45.
2. Garg PA. Review of podocyte biology. Am J Nephrol. 2018;47(suppl 1):3-13. doi:10.1159/000481633SUPPL
3. Warady BA, Agarwal R, Bangalore S, et al. Alport syndrome classification and management. Kidney Med. 2020;2(5):639-649. doi:10.1016/j.xkme.2020.05.014
4. Angioi A, Pani A. FSGS: from pathogenesis to the histological lesion. J Nephrol. 2016;29(4):517-523. doi:10.1007/s40620-016-0333-2
5. Roca N, Martinez C, Jatem E, Madrid A, Lopez M, Segarra A. Activation of the acute inflammatory phase response in idiopathic nephrotic syndrome: association with clinicopathological phenotypes and with response to corticosteroids. Clin Kidney J. 2021;14(4):1207-1215. doi:10.1093/ckj/sfaa247
6. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332-345.
7. Medjeral-Thomas NR, Lawrence C, Condon M, et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with De Novo minimal change disease: a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15(2):209-218. doi:10.2215/CJN.06290420
8. Ye Q, Lan B, Liu H, Persson PB, Lai EY, Mao J. A critical role of the podocyte cytoskeleton in the pathogenesis of glomerular proteinuria and autoimmune podocytopathies. Acta Physiol (Oxf). 2022;235(4):e13850. doi:10.1111/apha.13850
9. Trautmann A, Schnaidt S, Lipska-Ziμtkiewicz BS, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055-3065. doi:10.1681/ASN.2016101121
10. Kashtan CE, Gross O. Clinical practice recommendations for the diagnosis and management of Alport syndrome in children, adolescents, and young adults-an update for 2020. Pediatr Nephrol. 2021;36(3):711-719. doi:10.1007/s00467-020-04819-6
11. Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY. Thin basement membrane nephropathy. Kidney Int. 2003;64(4):1169-78. doi:10.1046/j.1523-1755.2003.00234.x
12. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017; 12(3):502-517. doi:10.2215/CJN.05960616
13. Savige J. Should we diagnose autosomal dominant Alport syndrome when there is a pathogenic heterozygous COL4A3 or COL4A4 variant? Kidney Int Rep. 2018;3(6):1239-1241. doi:10.1016/j.ekir.2018.08.002
14. Gigante M, Caridi G, Montemurno E, et al. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol. 2011;6(7):1626-1634. doi:10.2215/CJN.07830910
15. Saurus P, Kuusela S, Lehtonen E, et al. Podocyte apoptosis is prevented by blocking the toll-like receptor pathway. Cell Death Dis. 2015;6(5):e1752. doi:10.1038/cddis.2015.125
16. Baud L, Oudinet JP, Bens M, et al. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35(5):1111-1118. doi:10.1038/ki.1989.98
17. White S, Lin L, Hu K. NF-κB and tPA signaling in kidney and other diseases. Cells. 2020;9(6):1348. doi:10.3390/cells9061348
18. Tesch GH, Lan HY, Atkins RC, Nikolic-Paterson DJ. Role of interleukin-1 in mesangial cell proliferation and matrix deposition in experimental mesangioproliferative nephritis. Am J Pathol. 1997;151(1):141-150.
19. Lai KN, Leung JCK, Chan LYY, et al. Podocyte injury induced by mesangial-derived cytokines in IgA Nephropathy. Nephrol Dial Transplant. 2009;24(1):62-72. doi:10.1093/ndt/gfn441
20. Saleem MA, Kobayashi Y. Cell biology and genetics of minimal change disease. F1000 Res. 2016;5: F1000 Faculty Rev-412. doi:10.12688/f1000research.7300.1
21. Kim KP, Williams CE, Lemmon CA. Cell-matrix interactions in renal fibrosis. Kidney Dial. 2022;2(4):607-624. doi:10.3390/kidneydial2040055
22. Zvaifler NJ. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther. 2006;8(3):210. doi:10.1186/ar1963
23. Angeletti A, Magnasco A, Trivelli A, et al. Refractory minimal change disease and focal segmental glomerular sclerosis treated with Anakinra. Kidney Int Rep. 2021;7(1):121-124. doi:10.1016/j.ekir.2021.10.018
24. Trachtman H, Vento S, Herreshoff E, et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 2015;16:111. doi:10.1186/s12882-015-0094-5
25. Mariani LH, Eddy S, AlAkwaa FM, et al. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023;103(3):565-579. doi:10.1016/j.kint.2022.10.023
26. Korshak L, Washington DL, Powell J, Nylen E, Kokkinos P. Kidney Disease in Veterans. US Dept of Veterans Affairs, Office of Health Equity. Updated May 13, 2020. Accessed June 28, 2024. https://www.va.gov/HEALTHEQUITY/Kidney_Disease_In_Veterans.asp
27. Malone AF, Phelan PJ, Hall G, et al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 2014;86(6):1253-1259. doi:10.1038/ki.2014.305
1. Arif E, Nihalani D. Glomerular filtration barrier assembly: an insight. Postdoc J. 2013;1(4):33-45.
2. Garg PA. Review of podocyte biology. Am J Nephrol. 2018;47(suppl 1):3-13. doi:10.1159/000481633SUPPL
3. Warady BA, Agarwal R, Bangalore S, et al. Alport syndrome classification and management. Kidney Med. 2020;2(5):639-649. doi:10.1016/j.xkme.2020.05.014
4. Angioi A, Pani A. FSGS: from pathogenesis to the histological lesion. J Nephrol. 2016;29(4):517-523. doi:10.1007/s40620-016-0333-2
5. Roca N, Martinez C, Jatem E, Madrid A, Lopez M, Segarra A. Activation of the acute inflammatory phase response in idiopathic nephrotic syndrome: association with clinicopathological phenotypes and with response to corticosteroids. Clin Kidney J. 2021;14(4):1207-1215. doi:10.1093/ckj/sfaa247
6. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332-345.
7. Medjeral-Thomas NR, Lawrence C, Condon M, et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with De Novo minimal change disease: a multicenter, randomized, controlled trial. Clin J Am Soc Nephrol. 2020;15(2):209-218. doi:10.2215/CJN.06290420
8. Ye Q, Lan B, Liu H, Persson PB, Lai EY, Mao J. A critical role of the podocyte cytoskeleton in the pathogenesis of glomerular proteinuria and autoimmune podocytopathies. Acta Physiol (Oxf). 2022;235(4):e13850. doi:10.1111/apha.13850
9. Trautmann A, Schnaidt S, Lipska-Ziμtkiewicz BS, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055-3065. doi:10.1681/ASN.2016101121
10. Kashtan CE, Gross O. Clinical practice recommendations for the diagnosis and management of Alport syndrome in children, adolescents, and young adults-an update for 2020. Pediatr Nephrol. 2021;36(3):711-719. doi:10.1007/s00467-020-04819-6
11. Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY. Thin basement membrane nephropathy. Kidney Int. 2003;64(4):1169-78. doi:10.1046/j.1523-1755.2003.00234.x
12. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017; 12(3):502-517. doi:10.2215/CJN.05960616
13. Savige J. Should we diagnose autosomal dominant Alport syndrome when there is a pathogenic heterozygous COL4A3 or COL4A4 variant? Kidney Int Rep. 2018;3(6):1239-1241. doi:10.1016/j.ekir.2018.08.002
14. Gigante M, Caridi G, Montemurno E, et al. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol. 2011;6(7):1626-1634. doi:10.2215/CJN.07830910
15. Saurus P, Kuusela S, Lehtonen E, et al. Podocyte apoptosis is prevented by blocking the toll-like receptor pathway. Cell Death Dis. 2015;6(5):e1752. doi:10.1038/cddis.2015.125
16. Baud L, Oudinet JP, Bens M, et al. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35(5):1111-1118. doi:10.1038/ki.1989.98
17. White S, Lin L, Hu K. NF-κB and tPA signaling in kidney and other diseases. Cells. 2020;9(6):1348. doi:10.3390/cells9061348
18. Tesch GH, Lan HY, Atkins RC, Nikolic-Paterson DJ. Role of interleukin-1 in mesangial cell proliferation and matrix deposition in experimental mesangioproliferative nephritis. Am J Pathol. 1997;151(1):141-150.
19. Lai KN, Leung JCK, Chan LYY, et al. Podocyte injury induced by mesangial-derived cytokines in IgA Nephropathy. Nephrol Dial Transplant. 2009;24(1):62-72. doi:10.1093/ndt/gfn441
20. Saleem MA, Kobayashi Y. Cell biology and genetics of minimal change disease. F1000 Res. 2016;5: F1000 Faculty Rev-412. doi:10.12688/f1000research.7300.1
21. Kim KP, Williams CE, Lemmon CA. Cell-matrix interactions in renal fibrosis. Kidney Dial. 2022;2(4):607-624. doi:10.3390/kidneydial2040055
22. Zvaifler NJ. Relevance of the stroma and epithelial-mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther. 2006;8(3):210. doi:10.1186/ar1963
23. Angeletti A, Magnasco A, Trivelli A, et al. Refractory minimal change disease and focal segmental glomerular sclerosis treated with Anakinra. Kidney Int Rep. 2021;7(1):121-124. doi:10.1016/j.ekir.2021.10.018
24. Trachtman H, Vento S, Herreshoff E, et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 2015;16:111. doi:10.1186/s12882-015-0094-5
25. Mariani LH, Eddy S, AlAkwaa FM, et al. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023;103(3):565-579. doi:10.1016/j.kint.2022.10.023
26. Korshak L, Washington DL, Powell J, Nylen E, Kokkinos P. Kidney Disease in Veterans. US Dept of Veterans Affairs, Office of Health Equity. Updated May 13, 2020. Accessed June 28, 2024. https://www.va.gov/HEALTHEQUITY/Kidney_Disease_In_Veterans.asp
27. Malone AF, Phelan PJ, Hall G, et al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 2014;86(6):1253-1259. doi:10.1038/ki.2014.305
Three Anomalies and a Complication: Ruptured Noncoronary Sinus of Valsalva Aneurysm, Atrial Septal Aneurysm, and Patent Foramen Ovale
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.
The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.
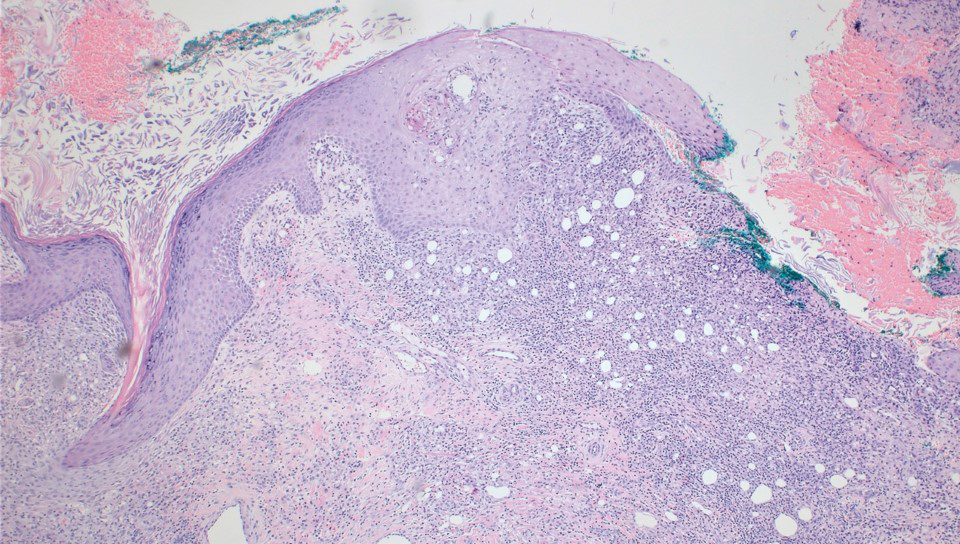
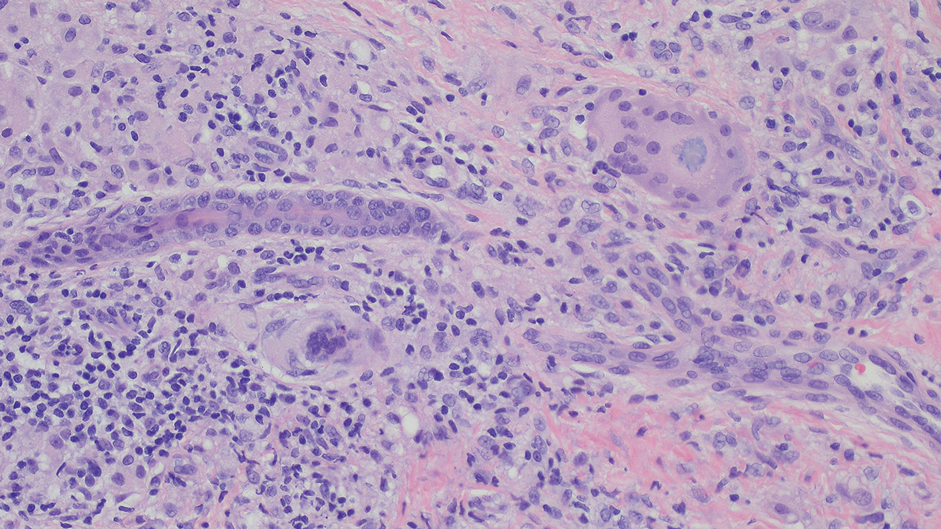
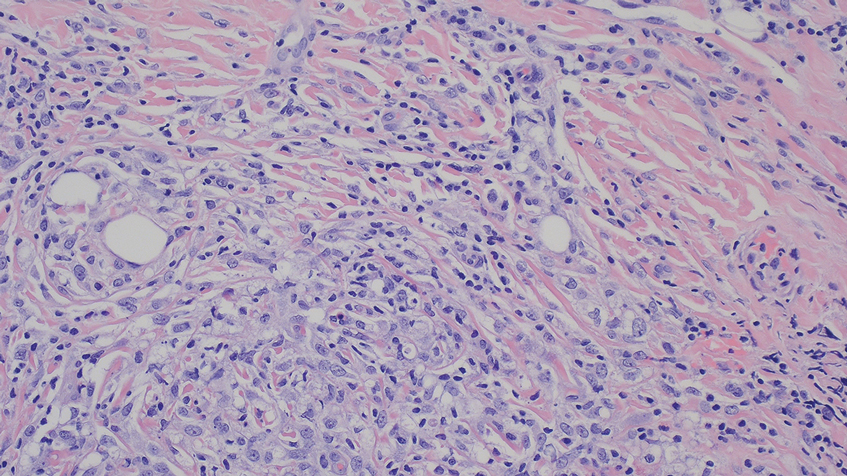
Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Practice Points
- Severe complications can potentially arise from at-home microneedling procedures when combined with cosmeceuticals such as vitamin E oil.
- Clinicopathologic correlation with cosmetic procedures is imperative to prompt diagnosis and treatment of these skin reactions.
- Microneedling procedures should be performed under the supervision of a board-certified dermatologist to avoid complications, and clinicians should inquire specifically about skin care routines and cosmetic procedures when patients present with unusual lesions on the face.
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Case Presentation: First Ever VA "Bloodless" Autologous Stem Cell Transplant Was a Success
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
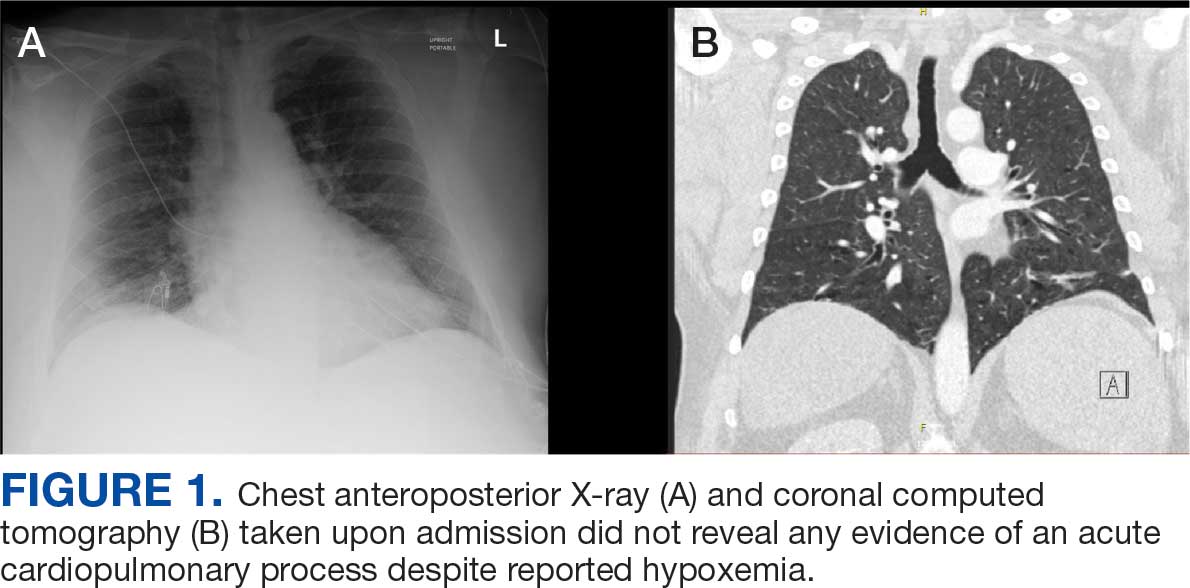
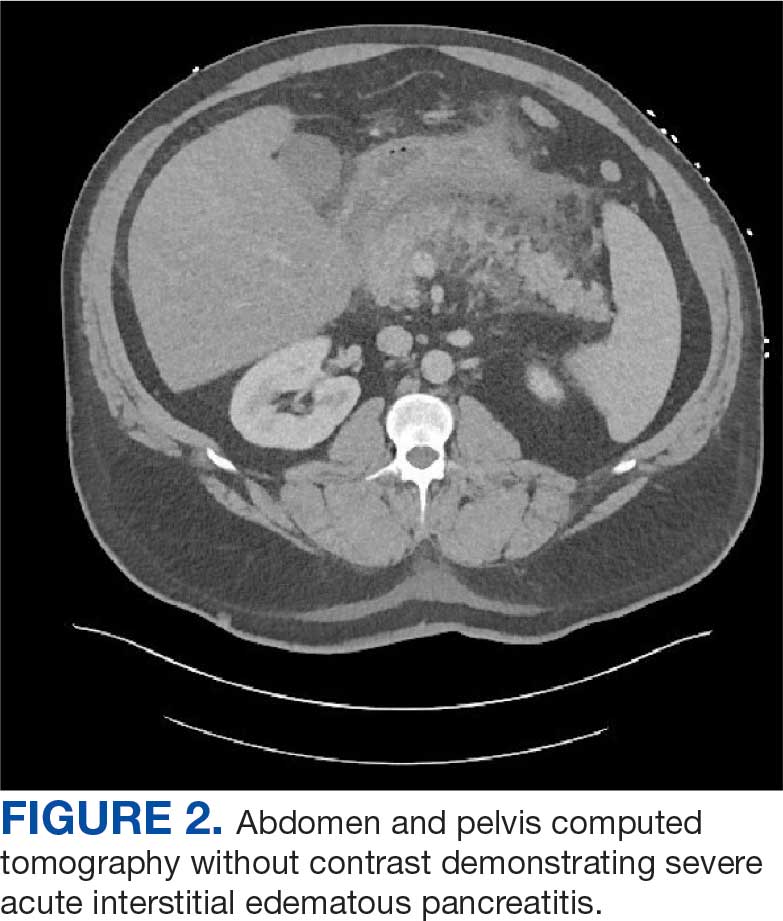
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
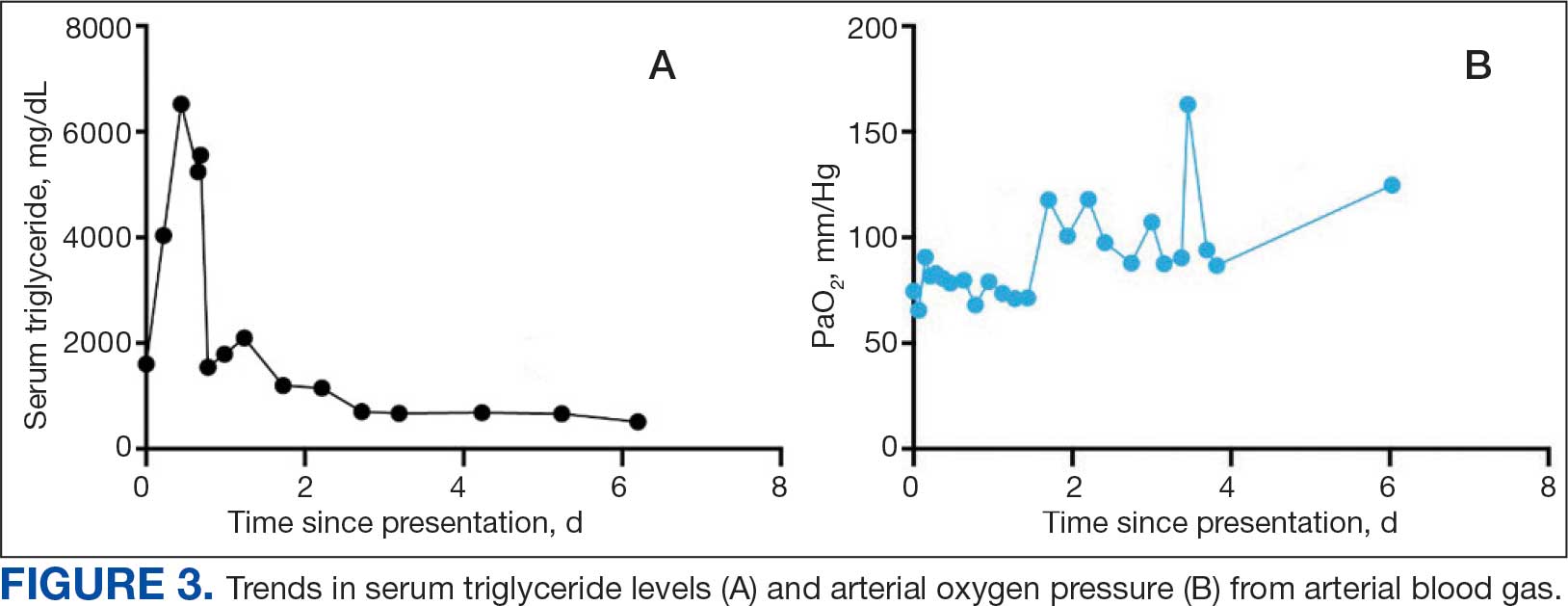
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
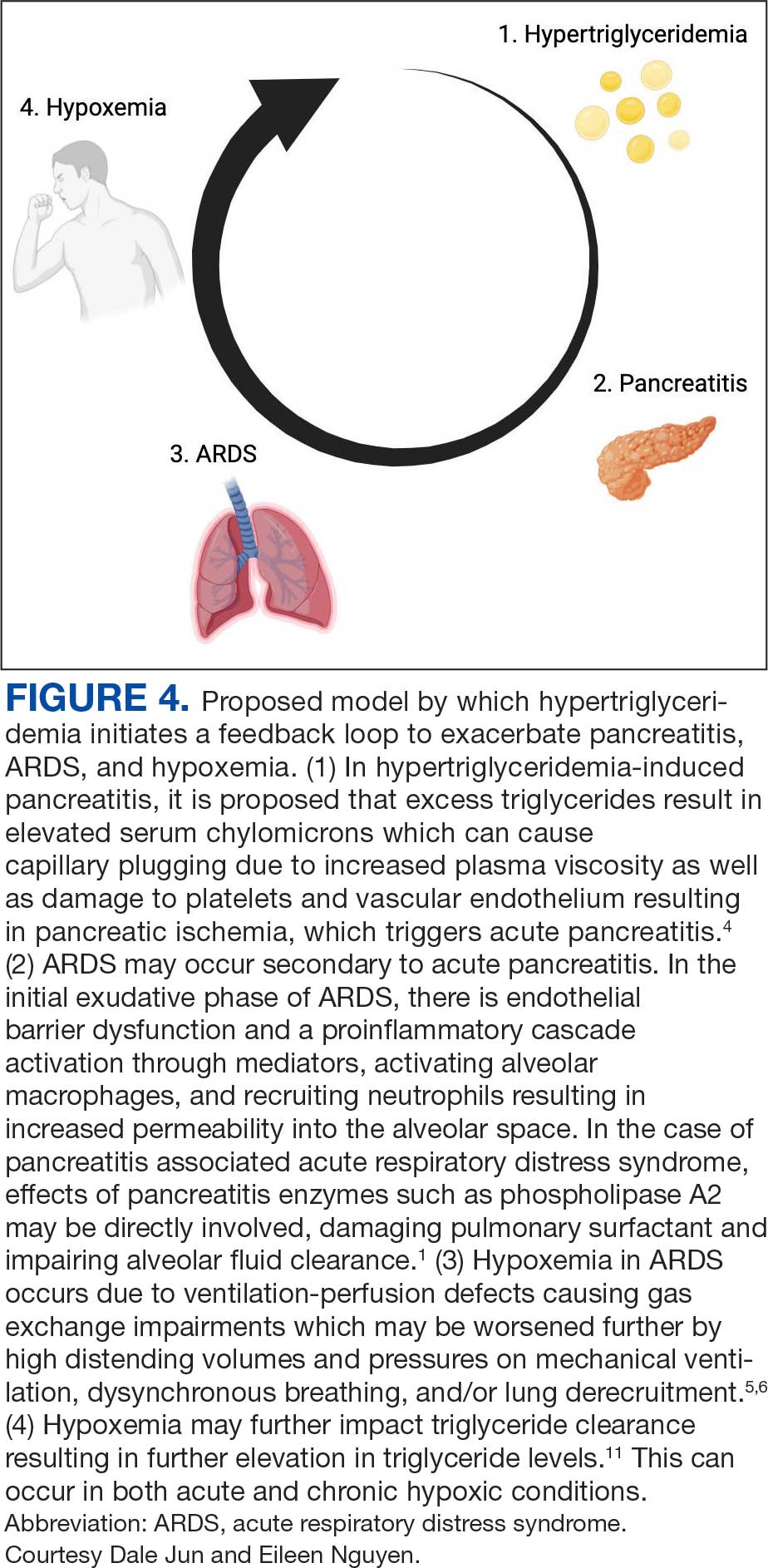
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.
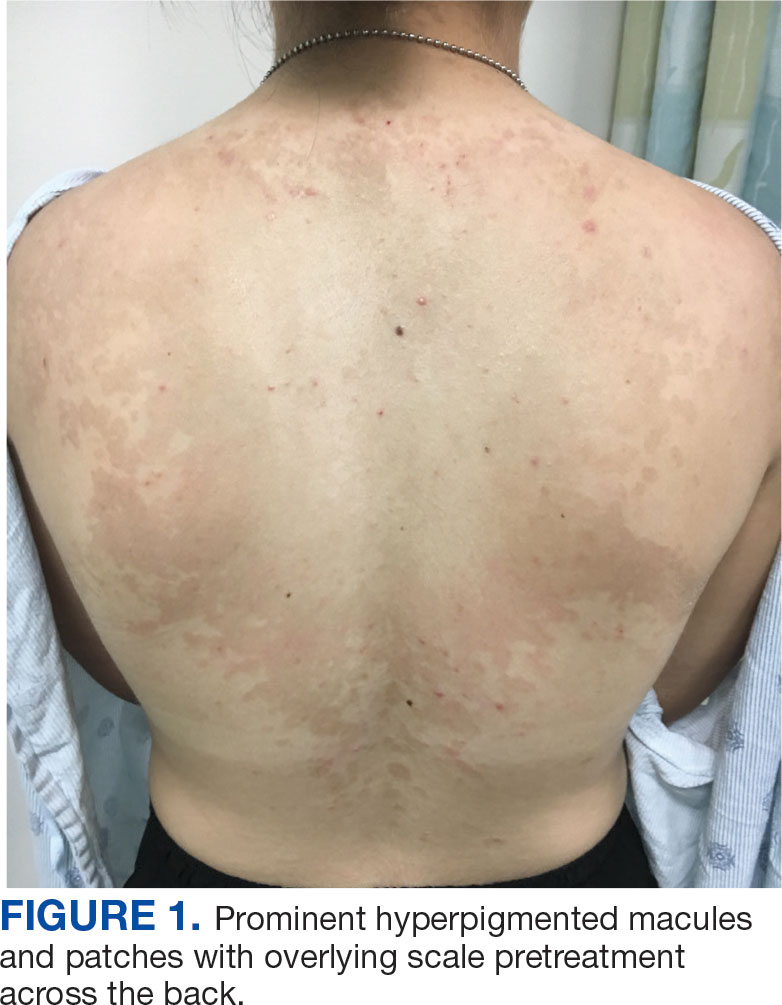
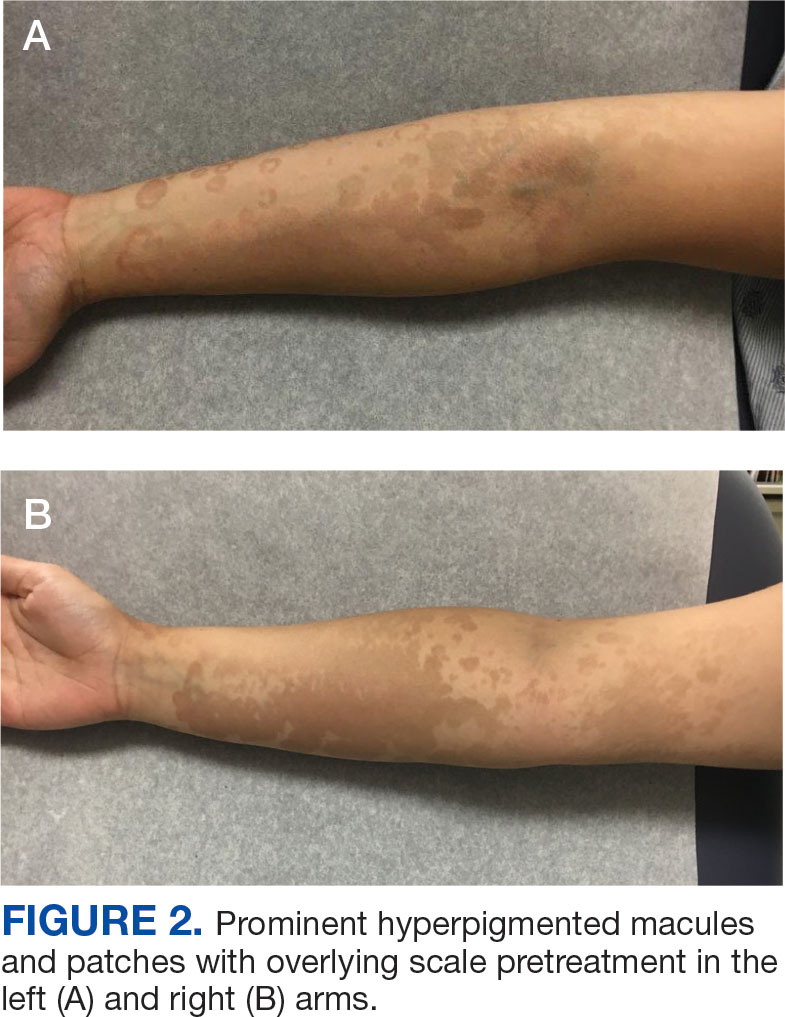
The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.
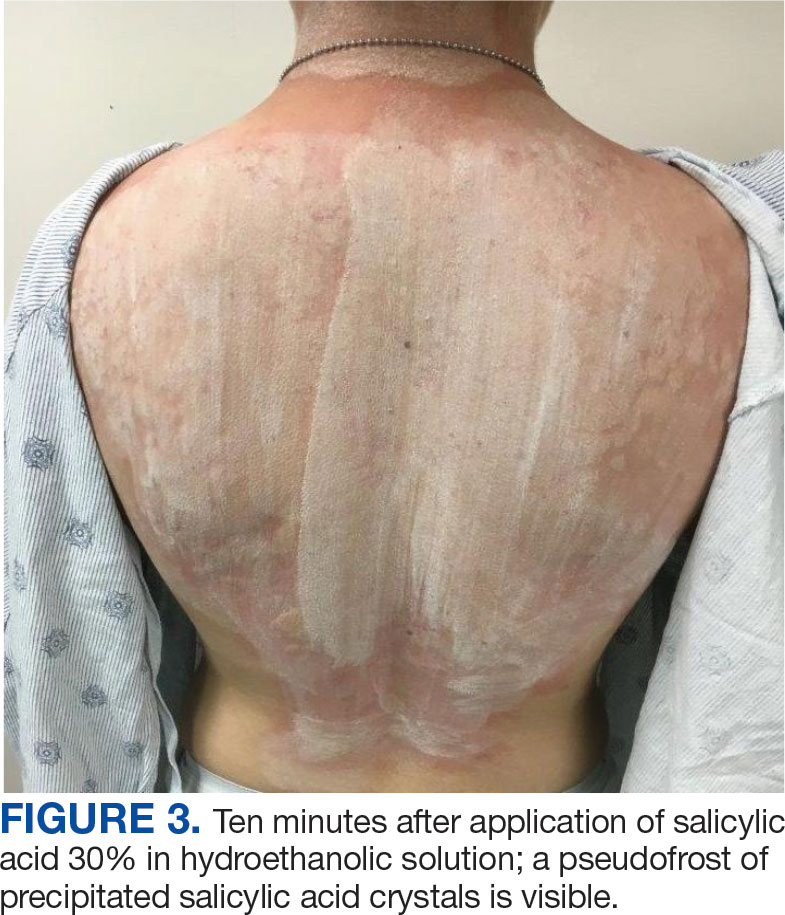
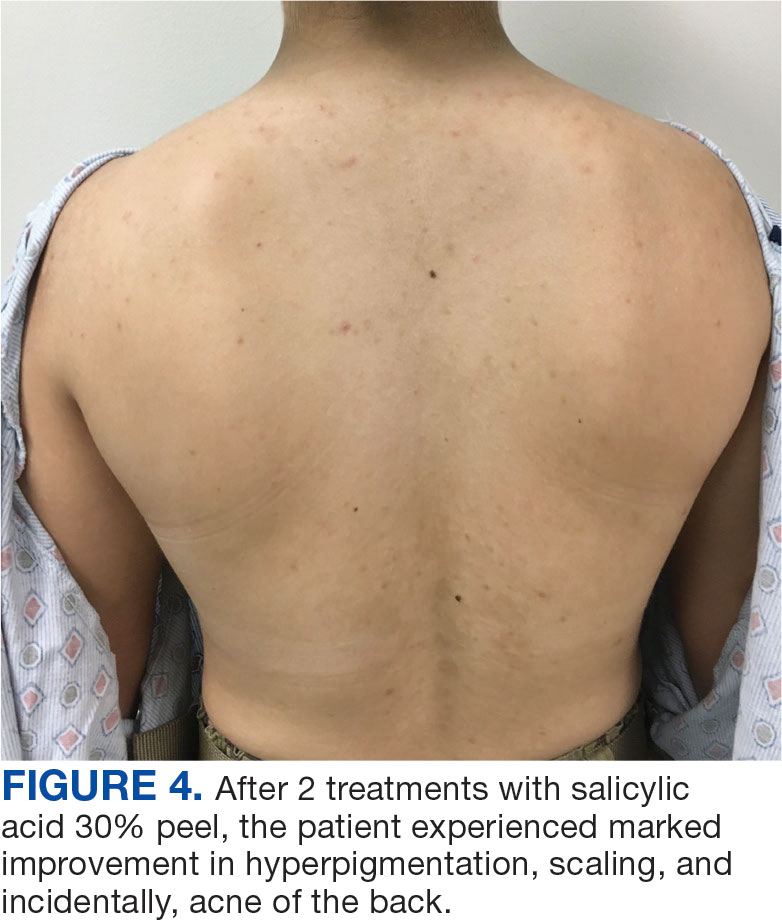
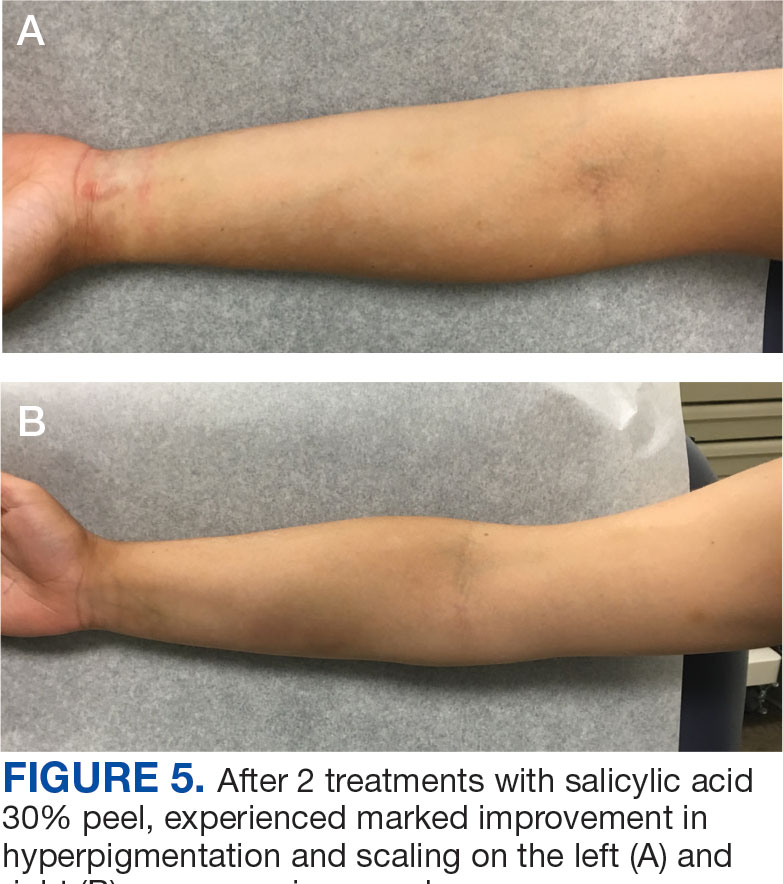
Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel