User login
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
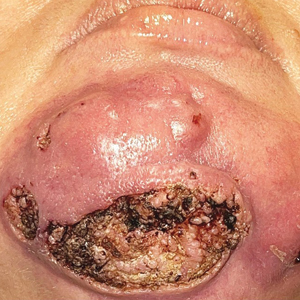
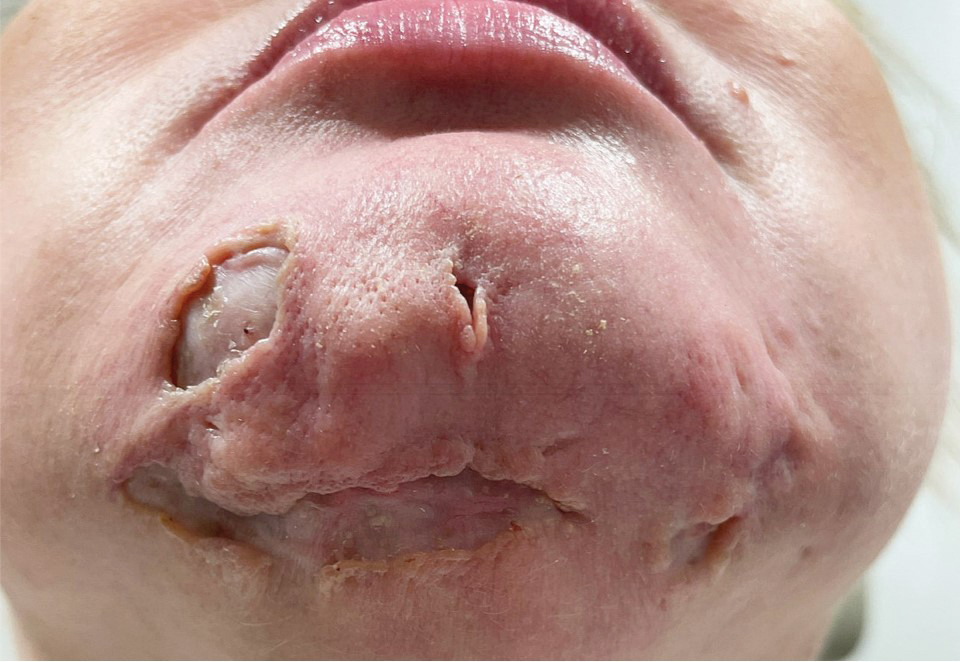
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.
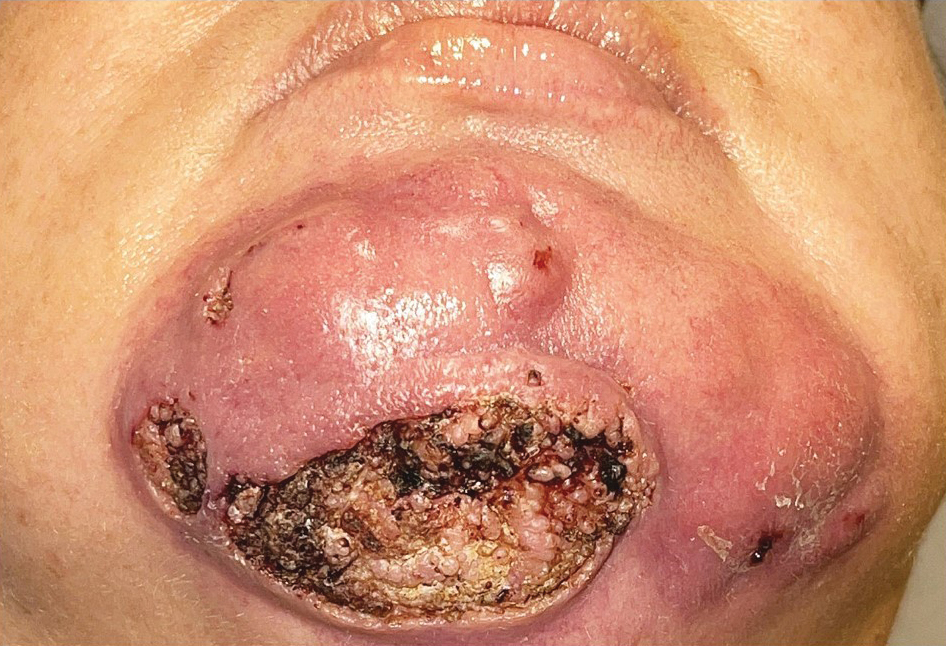
The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
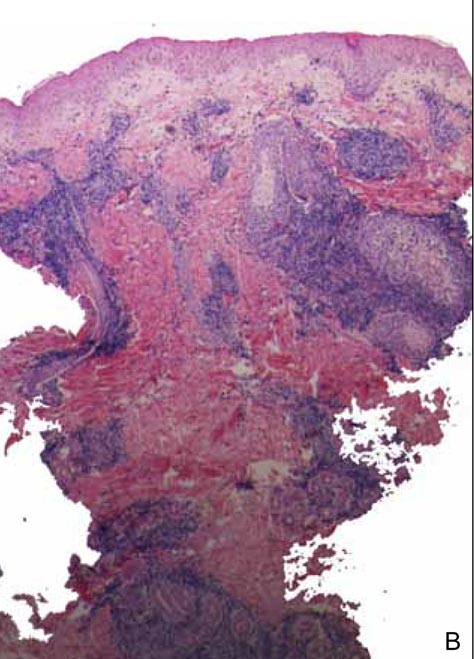
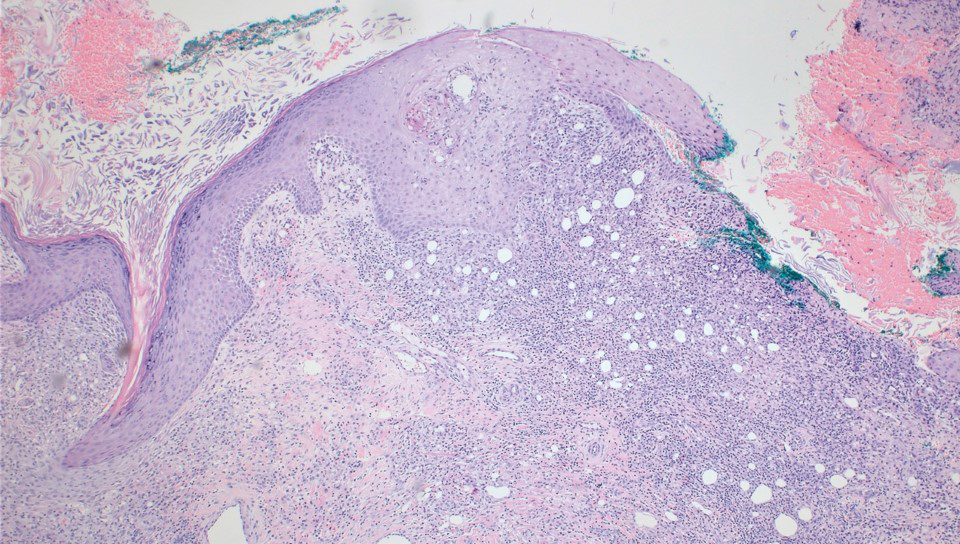
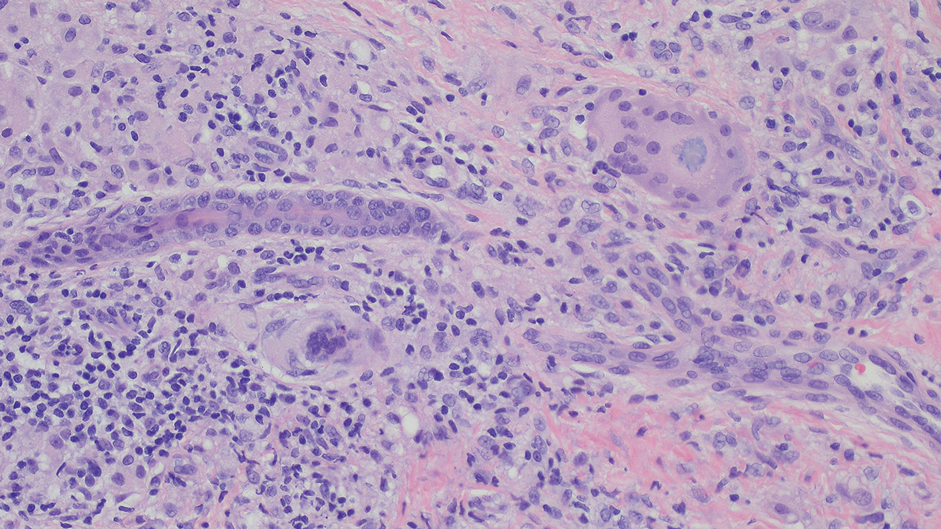
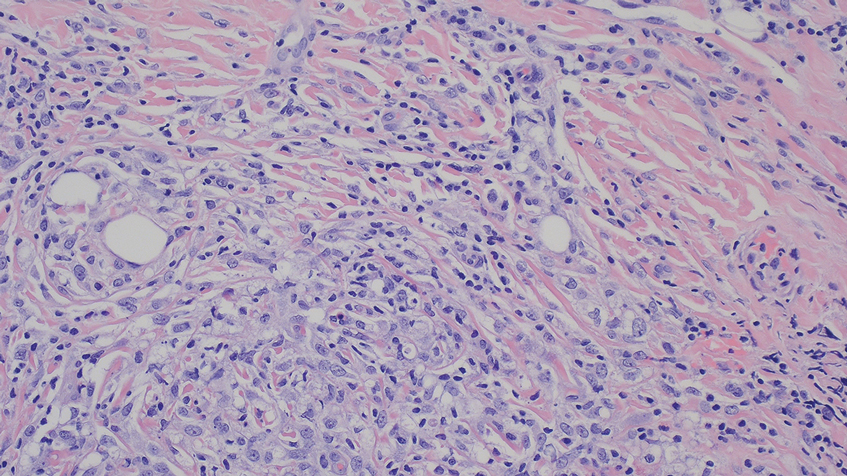
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.
Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Practice Points
- Severe complications can potentially arise from at-home microneedling procedures when combined with cosmeceuticals such as vitamin E oil.
- Clinicopathologic correlation with cosmetic procedures is imperative to prompt diagnosis and treatment of these skin reactions.
- Microneedling procedures should be performed under the supervision of a board-certified dermatologist to avoid complications, and clinicians should inquire specifically about skin care routines and cosmetic procedures when patients present with unusual lesions on the face.
Idiopathic Follicular Mucinosis or Mycosis Fungoides? Classification and Diagnostic Challenges
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
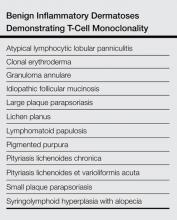
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
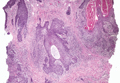
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
When follicular mucinosis (FM) is defined as an epithelial reaction pattern characterized by intrafollicular and perifollicular mucin accumulation, it cannot be considered a distinct disease entity, as this pattern is ubiquitously present in various inflammatory and neoplastic skin conditions.1,2 The distinction between idiopathic FM and lymphoma-associated follicular mucinosis (LAFM) was made several years ago by authors who evaluated the differences in the clinical presentation of these entities, including patient age at onset, number of lesions, pattern of distribution, and most importantly clinical progression.1 In this article, we discuss the importance of close clinical follow-up in patients with FM or patch-stage mycosis fungoides (MF) in whom histopathologic evaluation is ambiguous or nondiagnostic. We also highlight the value of ancillary testing, including T-cell receptor gene rearrangement, flow cytometry, and immunohistochemistry, as a component in the diagnostic process rather than the sole diagnostic moiety. A review of the pertinent literature also is performed.
History of FM and MF
Pinkus3 first described an entity he termed alopecia mucinosa in 1957. Pinkus noted 3 distinct patterns: an idiopathic form of alopecia mucinosa, lymphoblastoma with associated FM, and alopecia mucinosa that later transformed into lymphoblastoma.4 In 1983, however, Pinkus4 described uncertainty if alopecia mucinosa represented the first stage of MF or if patients with alopecia mucinosa were simply at an increased risk for developing lymphoma. He believed there were too many cases of lymphoma following a diagnosis of alopecia mucinosa for the relation to be coincidental, yet he noted that many of the cases resolved either spontaneously or following treatment with x-rays or topical steroids. He concluded his report with a sentiment that is echoed in many current studies regarding this entity: “Many questions surrounding this entity are as unanswerable today as they were 25 years ago.”4
Jablonska et al5 were the first to coin the term mucinosis follicularis, now known as FM, to replace alopecia mucinosa because they felt the description was more accurate, as lesions also arise on non–hair-bearing skin. Although there is general agreement that there is a form of MF that has associated FM, this is where the agreement ends with regard to the diagnosis of MF versus FM. Böer et al6 discussed the historic evolution of these terms, mostly to highlight the origins of the confusion. The investigators proposed that FM should only be used as a descriptive term and that all cases of alopecia mucinosa represent MF. They also concluded that many benign dermatoses associated with a risk for evolution to MF (eg, small and large plaque psoriasis [LPP]) should simply be diagnosed as MF.6 Subsequently, the proposal that idiopathic FM and LAFM are not 2 distinct entities but rather a clinicopathologic continuum and that idiopathic FM is simply a variant of MF along this spectrum has gained some approval.6,7 However, this belief is not shared among all authorities in the field, and attempts to define diagnostic criteria that distinguish between a benign clinical course and a course that is more progressive and fatal continue. Currently, it is agreed upon that when distinguishing between these 2 clinical courses, primary (idiopathic) follicular mucinosis refers to a benign course with no overt sign of malignancy, and lymphoma-associated follicular mucinosis refers to a diagnostic malignant condition. Lymphoma-associated follicular mucinosis refers to FM associated with cutaneous T-cell lymphoma, the most common form of which is FM. Many authors8-15 have sought ancillary methodologies in addition to clinical parameters to assist in the evaluation between both disease courses. Methodologies have included assessment of T-cell receptor gene rearrangements, flow cytometry, and immunohistochemical staining, mostly as an effort to establish monoclonality as a defining characteristic of LAFM; however, monoclonality in cutaneous T-cell infiltrates should be interpreted with caution and should not be considered as a confirmation of malignancy due to recent findings of monoclonality in benign inflammatory dermatoses such as lichen planus. The Table outlines several of the most common benign inflammatory dermatoses that demonstrate monoclonality, but this list should not be considered exhaustive, as there are many others in which monoclonality is sometimes seen.8-15 The lack of definitive criteria to distinguish between the 2 groups has led to confusion and consternation regarding the diagnosis of idiopathic FM versus LAFM and has led many in the field to consider the 2 conditions to be one and the same.
Diagnosis of FM and MF: Clinicopathologic Features
The World Health Organization (WHO) defined MF as an epidermotropic primary cutaneous T-cell lymphoma (CTCL) characterized by infiltrates of small- to medium-sized T lymphocytes with cerebriform nuclei. Further, the WHO stated that the term mycosis fungoides should be exclusively reserved for classical cases typified by the evolution of cutaneous patches, plaques, and tumors, or for variants that show a similar clinical course.16 Mycosis fungoides is divided into 3 stages—patch, plaque, and tumor—which are solely clinical descriptors.17 The WHO also described a clinical staging system with pathologic emphasis placed only on lymph node involvement and identification of Sézary cells.16 It lists folliculotropic MF as a variant, with only some cases presenting with mucinous degeneration of hair follicles. A lack of consensus among pathologists regarding criteria for diagnosis in patch-stage MF remains, but diagnosis of plaque-stage disease is not regularly debated due to its more reliably present, well-developed histologic features (eg, haloed lymphocytes, epidermotropism of lymphocytes, lymphocytes with convoluted nuceli, Pautrier microabscesses).18 Although there have been specific histologic findings reported to be associated with patch-stage MF, they have only been present in a few cases and are therefore of limited usefulness in practice.1,19 The categorization of patients with subtle histologic features common to both MF and inflammatory conditions such as parapsoriasis en plaques (the term plaque in this case is a misnomer because the word plaque means patch in French) continues to be elusive. A lack of agreement regarding LPP persists in the current literature in the same manner as FM. Some researchers have contended for many years that LPP is a type of MF, while others remain unconvinced, mainly due to the lack of evidence that lumping a benign condition (LPP) with an increased risk for malignant transformation and a known malignancy (MF) together is of any benefit to the patient. Assessment of clinicopathologic correlation, immunohistochemistry, clonality, and T-cell gene rearrangement have failed to positively identify patients who are at risk for disease progression, whether the diagnosis is called LPP or early patch-stage MF.20
Mycosis fungoides is more common in males and its incidence increases with age; however, diagnosis should not be ruled out based on age or gender. Typical presentation of early-stage disease includes erythematous patches or plaques, often with light scaling.19 Lesions routinely are of long-standing duration (months to years), are located in areas that are infrequently exposed to sunlight, and often are 5 cm in diameter or larger with irregular borders.21 Associated poikiloderma is relatively specific to MF but rarely is seen in other CTCLs, connective-tissue diseases, and some genodermatoses. Poikiloderma commonly is identified in LPP, which shows the same telangiectasia, mottled pigmentation, and epidermal atrophy as MF-associated poikiloderma, leading some to believe that there is no separation between the 2 conditions. In all stages of MF, lesions frequently are numerous and occur on multiple sites. Plaques and tumors can show spontaneous ulceration. When lesions are folliculotropic, they can cause localized alopecia, follicular-based papules, and fungating pseudotumors in more advanced stages.1 The clinical presentation of FM substantially overlaps with folliculotropic MF, and although FM lesions often are solitary and are located on the face or scalp, they also can present as multiple lesions located elsewhere on the body. It also has been proposed that folliculotropic MF should not be separated from FM-associated MF (or LAFM).22
The characteristic histologic picture of LAFM in patch or plaque stage shows mucin deposition within hair follicles, similar to idiopathic FM. On histology, both conditions demonstrate dense lymphoid infiltrates around and within hair follicles as well as in the dermis (Figure). Most cases of LAFM show epidermotropism of lymphocytes between follicles, but this finding is not present in every case and often disappears when the disease advances to the tumor stage.1,19 Although Pautrier microabscesses (collections of lymphocytes within the superficial epidermis) are considered to be somewhat specific to MF, they are only present in a minority of cases.20 In a study by the International Society for Cutaneous Lymphomas,21 the only histopathologic criteria that showed any appreciable sensitivity or specificity in the diagnosis of MF were the presence of lymphoid cells with variable nuclear and cytoplasmic features and/or strikingly irregular nuclear contours with the presence of lymphocytes larger than those usually seen in inflammatory dermatoses. Despite these criteria, the study reported a high misclassification rate. A complicated scoring system for diagnosis of MF in patch- or early plaque-stage disease was proposed by the International Society for Cutaneous Lymphomas,21 which integrates clinical, histopathologic, molecular, and immunophenotypic criteria. However, these criteria have been continually debated in the literature and are only discussed in this article in relation to the association between MF and FM. Diagnosis of tumor-stage MF is not addressed in this article, as it is readily identified as lymphoma and is not easily confused with idiopathic FM.
|
Clinical assessment of a patient’s medical history to identify persistent and progressive disease is paramount to the diagnosis of MF. Although MF lesions tend to increase in size and number over time, this presentation is not without exception.21 In early patch-stage disease, eliminating some of the patient’s current medications may be sufficient in clearing cutaneous patches that cannot be conclusively identified as either MF or a benign inflammatory lymphoid infiltrate, which further emphasizes the importance of clinical assessment of the patient’s medical history in the diagnosis of MF. The shape of the lesions also is helpful in distinguishing between MF and other skin disorders, such as digitate dermatosis or LPP; unlike the latter, the waxing and waning nature of MF lesions often produces irregularly shaped patches with little coalescence. Again, there are some investigators who believe that these lesions represent varying presentations of MF.6
In a study by Cerroni et al,1 44 patients with FM were divided into 2 groups: (1) a cohort of 16 patients with no history or clinical evidence of MF or Sézary syndrome (ie, LAFM), and (2) a cohort of 28 patients with clinicopathologic evidence of CTCL. Patients in both groups were followed for a maximum of 20 years. Results indicated that that the presence of perifollicular or intrafollicular mucin, epidermotropism of lymphocytes, monoclonality, and epidemiologic characteristics (eg, age, sex, race) cannot reliably distinguish the 2 disease forms. Furthermore, it was suggested that these conditions are not mutually exclusive entities and are actually variants of CTCL. The observation that the 2 diseases share prognostic overlap adds further credence to the already puzzling conundrum. Nineteen of 28 patients with MF were alive and well at follow-up, and all patients in the idiopathic FM group were alive, with only 9 of 16 patients showing residual disease and none with CTCL.1
Other clinical factors that may be helpful in the diagnosis of MF are the presentation of lesions in non–sun-exposed areas of the skin and multiple lesions, as unilesional MF is exceedingly uncommon.21 No histologic features have been proven to predict which early patch- or plaque-stage MFs will progress to full-blown CTCL versus benign idiopathic FM; thus, great caution should be taken in patients with early-stage disease to ensure they are not prematurely and/or incorrectly classified as CTCL. Such a diagnosis has medical, social, and economical ramifications that should not be overlooked.
If idiopathic FM and LAFM were considered distinct disease processes, the ambiguity in making a definitive diagnosis should give the physician pause, and a diagnosis of LAFM may only be appropriate when there is unequivocal clinicopathologic evidence. Otherwise, a lymphoma diagnosis is somewhat superfluous and potentially harmful. Definitive diagnosis of LAFM also is complicated by reports of other hematologic malignancies presenting with FM-like histopathologic findings, such as chronic myelogenous leukemia, leukemia-associated eosinophilic folliculitis, and acute myeloblastic leukemia.23,24 Although MF is the most common malignancy associated with FM, it is important to consider other less common malignancies that also may be present.
Diagnosis: Patient Consequences
Accurate diagnosis of idiopathic FM versus LAFM is critical, as the ramifications of a cancer diagnosis can have broad implications. For example, patients who receive cancer diagnoses often experience emotional trauma and social stigma, even when adequate patient education has been provided. The incidence of depression and anxiety also can increase following a cancer diagnosis and can be complicated by medical treatments (eg, systemic steroids, interferon),25 which are known to increase the frequency of these psychological disturbances. Health insurance premiums likely will be higher if a patient is diagnosed with cancer versus a benign inflammatory condition. Hesitation of the pathologist to assign a cancer diagnosis when unequivocal evidence is not present should not be regarded as trickery, malpractice, or deceit of the health care bylaws, as benign language with suggestion of close clinical follow-up in the setting of diagnostic uncertainty will “first, do no harm” and secondly, serve as a vehicle for patient advocacy.
If there is a definitive distinction between idiopathic FM and LAFM, it requires further research before it can be fully understood. Currently, the WHO does not recognize a diagnosis of FM-associated MF (or LAFM) and acknowledges that folliculotropic MF is not always associated with FM.16,26 Given uncertainty and repercussions associated with a cancer diagnosis, however indolent, it may be morally responsible and medically favorable for physicians to consider FM in the differential diagnosis when applicable rather than making a diagnosis of MF outright. Given the importance of both clinical and histologic factors, it may be beneficial for definitive diagnosis of FM versus MF to lie with the clinician, while the pathologist serves as an adjunct in the diagnostic process. Because the prognosis of idiopathic FM often is marred by possible transformation into MF or other CTCLs, therapeutic decisions should be dictated by close clinical follow-up. Additionally, stage of disease, patient age, treatment compliance, comorbidities, and possible side effects of medications should all be considered when evaluating potential therapeutic regimens.27
Conclusion
Research is underway to more accurately identify patients with FM who are at risk for progression to LAFM versus those with benign remitting FM. Once the required diagnostic criteria are established to accurately classify these patients, with an emphasis on prognosis and suggested treatments, it might be necessary to establish new, less debated terminology so pathologists and clinicians alike can improve patient care. Continued histopathologic scrutiny, use of sophisticated molecular techniques, and knowledge of other currently undiscovered modalities will shed light on this important disease process and aid in proper disease management, which may be advantageous to both patients and physicians.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
1. Cerroni L, Fink-Puches R, Bäck B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2002;138:182-189.
2. Parker SR, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline [published online ahead of print July 25, 2009]. J Am Acad Dermatol. 2010;62:139-141.
3. Pinkus H. Alopecia mucinosa; inflammatory plaques with alopecia characterized by root-sheath mucinosis. AMA Arch Dermatol. 1957;76:419-424, 424-426.
4. Pinkus H. Alopecia mucinosa. additional data in 1983. Arch Dermatol. 1983;119:698-699.
5. Jablonska S, Chorzelski T, Lancucki J. Mucinosis follicularis [in German]. Hautarzt. 1959;10:27-33.
6. Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004;26:33-52.
7. Brown HA, Gibson LE, Pujol RM, et al. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and withoutclonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002;47:856-862.
8. Schiller PI, Flaig MJ, Puchta U, et al. Detection of clonal T cells in lichen planus. Arch Dermatol Res. 2000;292:568-569.
9. Cerroni L, Kerl H. Primary follicular mucinosis and association with mycosis fungoides and other cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:146-147.
10. Dereure O, Levi E, Kadin ME. T-Cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
11. Haeffner AC, Smoller BR, Zepter K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131:321-324.
12. Schultz JC, Granados S, Vonderheid EC, et al. T-cell clonality of peripheral blood lymphocytes in patients with lymphomatoid papulosis. J Am Acad Dermatol. 2005;53:152-155.
13. Pfaltz K, Kerl K, Palmedo G, et al. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662.
14. Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
15. Guitart J, Magro C. Cutaneous T-cell lymphoid dyscrasia: a unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch Dermatol. 2007;143:921-932.
16. Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
17. Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides [published online ahead of print October 22, 2007]. Crit Rev Oncol Hematol. 2008;65:172-182.
18. Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
19. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957.
20. Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol. 2009;75:229-235.
21. Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
22. Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides. a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525-530.
23. Rashid R, Hymes S. Folliculitis, follicular mucinosis, and papular mucinosis as a presentation of chronic myelomonocytic leukemia. Dermatol Online J. 2009;15:16.
24. Wada T, Yoshinaga E, Oiso N, et al. Adult T-cell leukemia-lymphoma associated with follicular mucinosis. J Dermatol. 2009;36:638-642.
25. Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma [published online ahead of print December 16, 2008]. Br J Dermatol. 2009;160:815-822.
26. Boone SL, Guitart J, Gerami P. Follicular mycosis fungoides: a histopathologic, immunohistochemical, and genotypic review. G Ital Dermatol Venereol. 2008;143:409-414.
27. Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome [published online ahead of print August 20, 2009]. Blood. 2009;114:4337-4353.
Practice Points
- An isolated patch in the head or neck area is much more likely to be follicular mucinosis (FM) than mycosis fungoides (MF).
- Monoclonality does not reliably distinguish FM from MF.
- Younger patients are more likely to have FM with spontaneous remission, and older patients are more likely to develop MF.
- None of the clinicopathologic features of FM or MF are without overlap.