User login
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
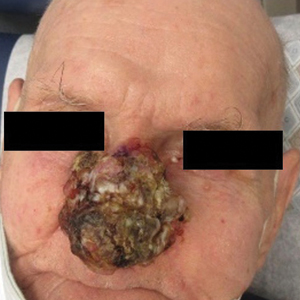
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
PRACTICE POINTS
- Dermatologists should consider using vismodegib for treatment of unresectable basal cell carcinoma.
- Vismodegib dosing regimens can vary; drug holidays can be used to mitigate adverse effects while maintaining desirable treatment outcomes.
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
A 26-year-old male with Fitzpatrick skin type II presented for evaluation in the dermatology clinic after being referred by his primary care practitioner (PCP) with a complaint of spider veins. The patient reported a lifelong history of blue subcutaneous nodules that initially appeared on his face during childhood but have since involved his trunk and upper and lower extremities. The patient reported that some of the nodules were painful and increased in size with exercise. His medical history was unremarkable with no other chronic conditions or daily medication use. The patient reported no gastrointestinal (GI) symptoms, melena, or hematochezia. The patient’s mother had similar nodules but his 7 siblings did not.
Upon physical examination, numerous blue subcutaneous nodules, 2 to 8 mm in size, were scattered across his trunk, and proximal and distal extremities were present (Figure 1). The physical examination was otherwise unremarkable. Upon discussing differential diagnosis of these lesions with the patient, he was amenable to a punch biopsy for further diagnostic clarity (Figure 2).
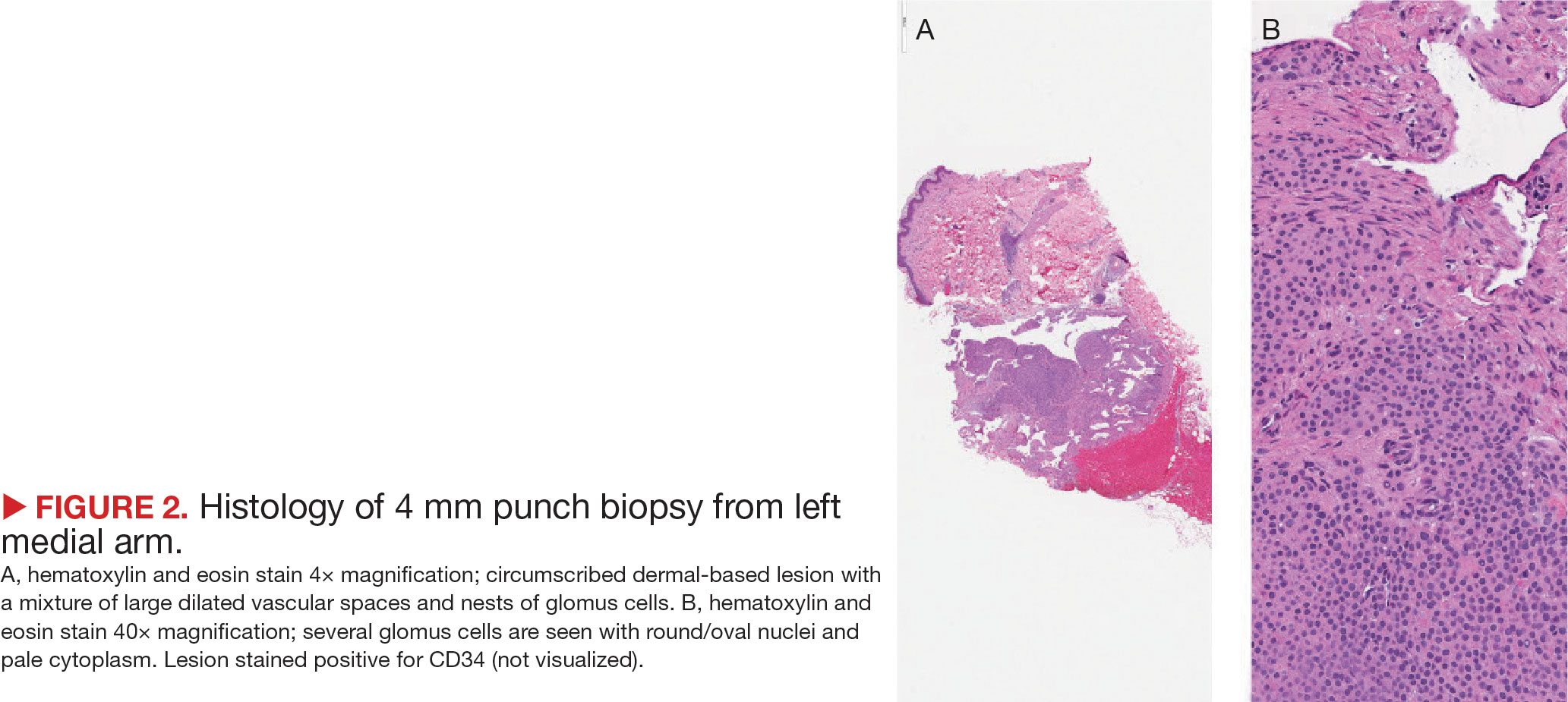
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.
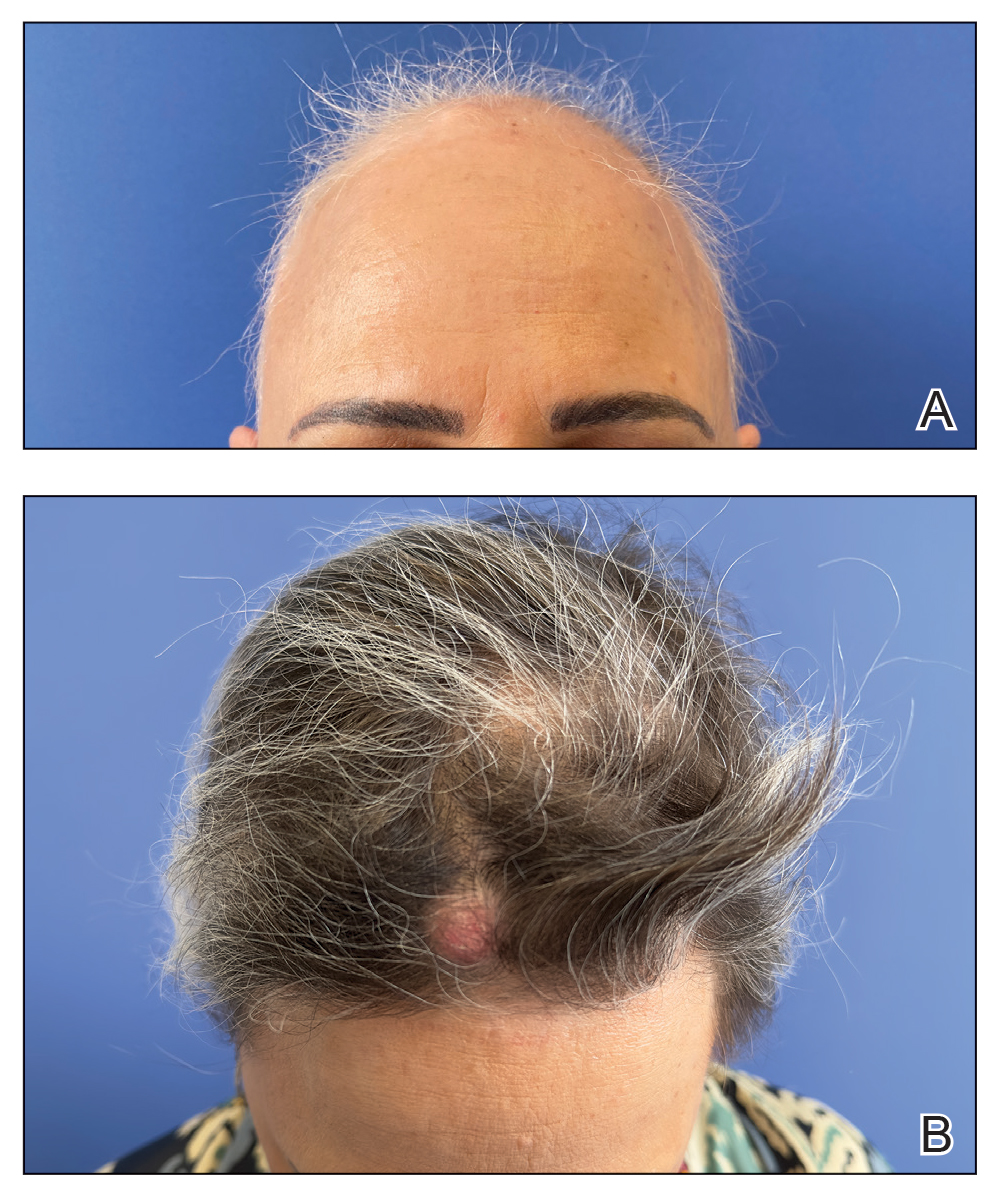
The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
PRACTICE POINTS
- The rapid growth of trichilemmal cysts may serve as an indicator of a quick-responder phenotype to baricitinib in cases of alopecia areata (AA), although more evidence is needed.
- It is imperative to consider personal and family history of trichilemmal cysts prior to initiating baricitinib treatment for AA.
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.
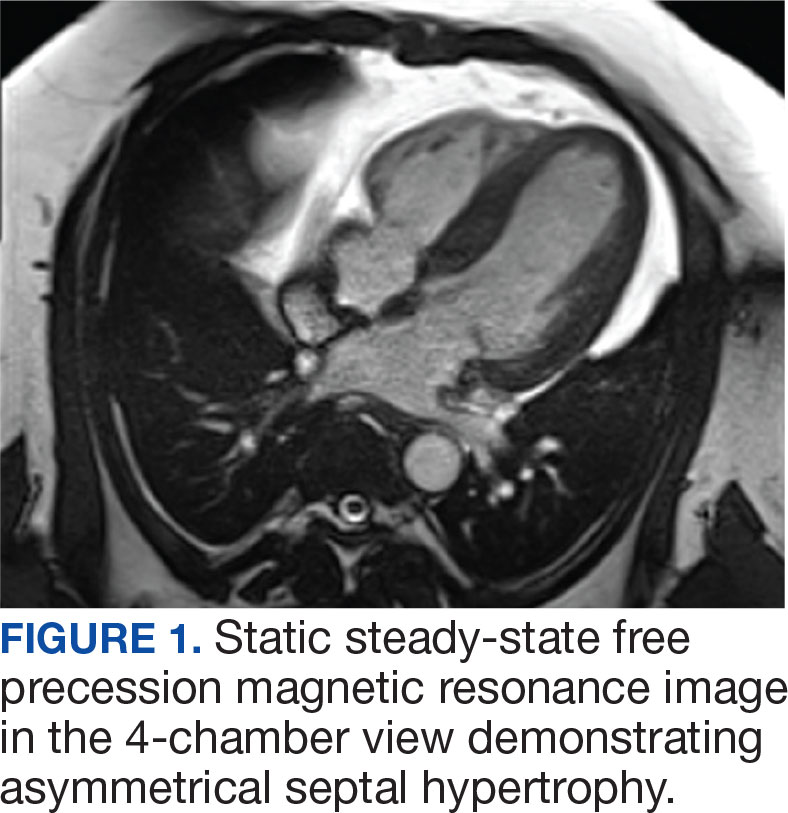
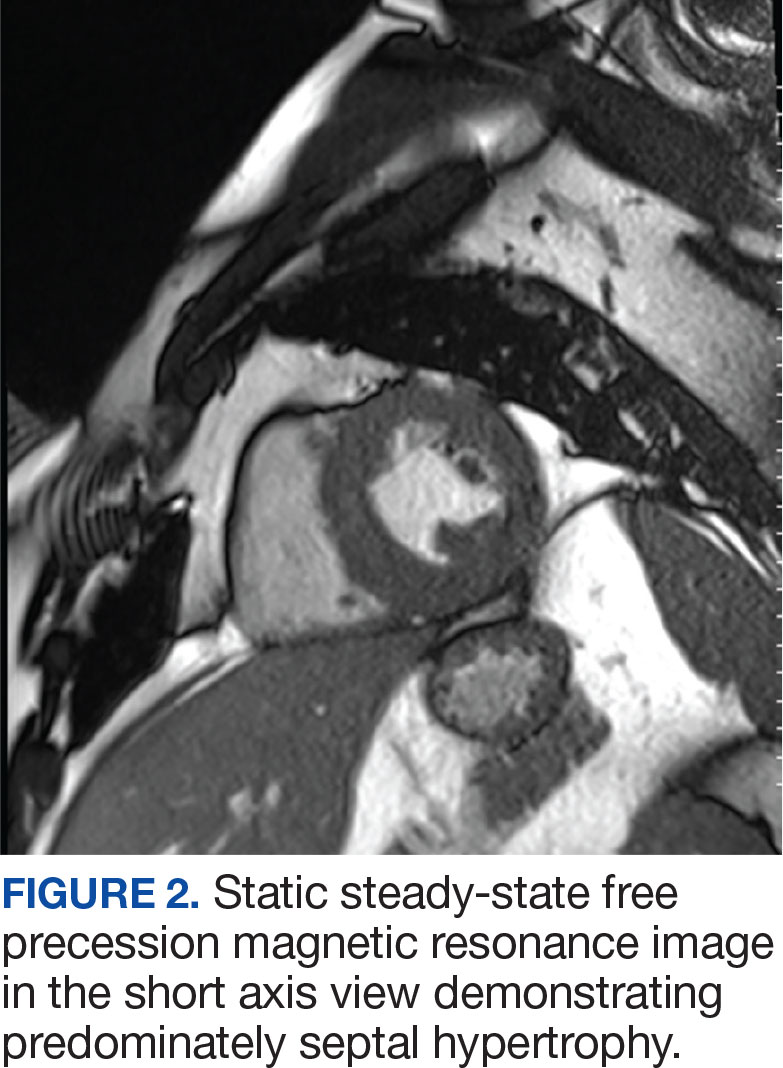
DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.
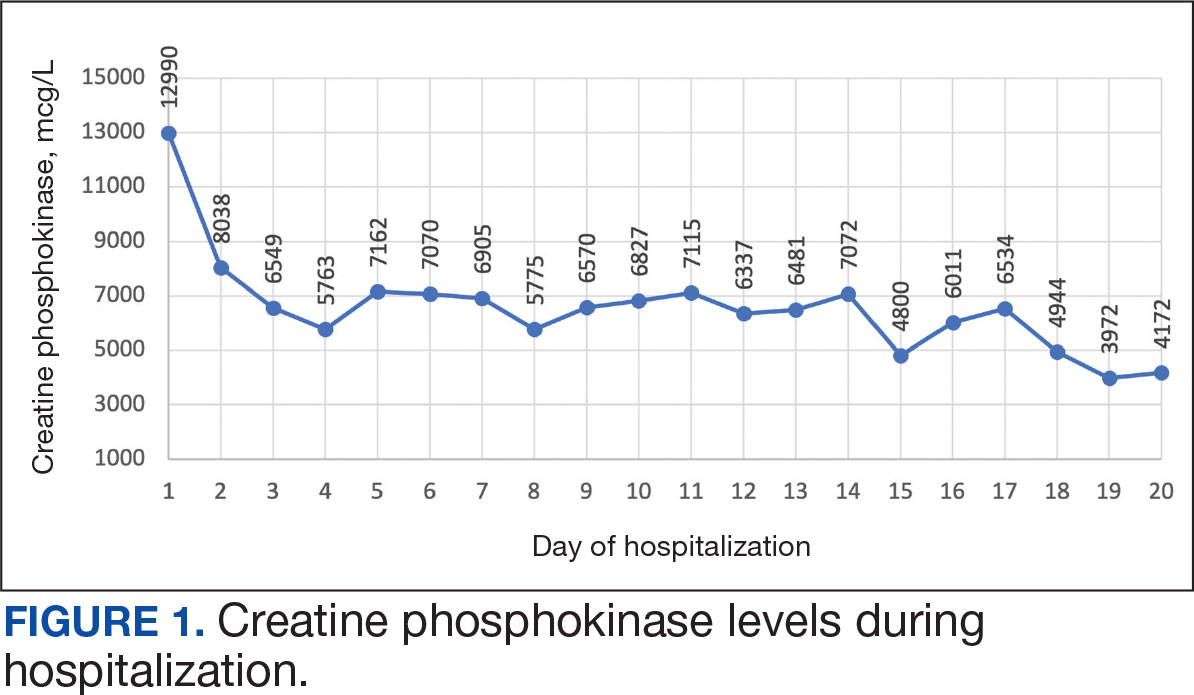
After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).
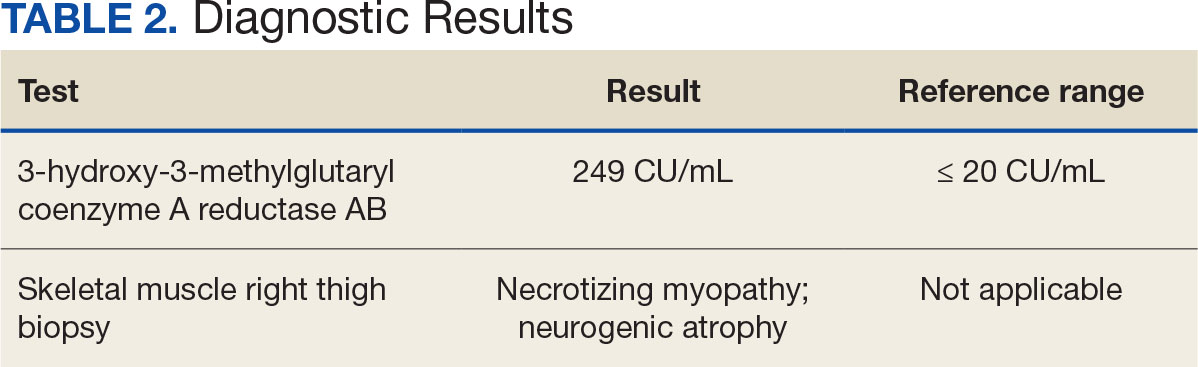
On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.
The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).
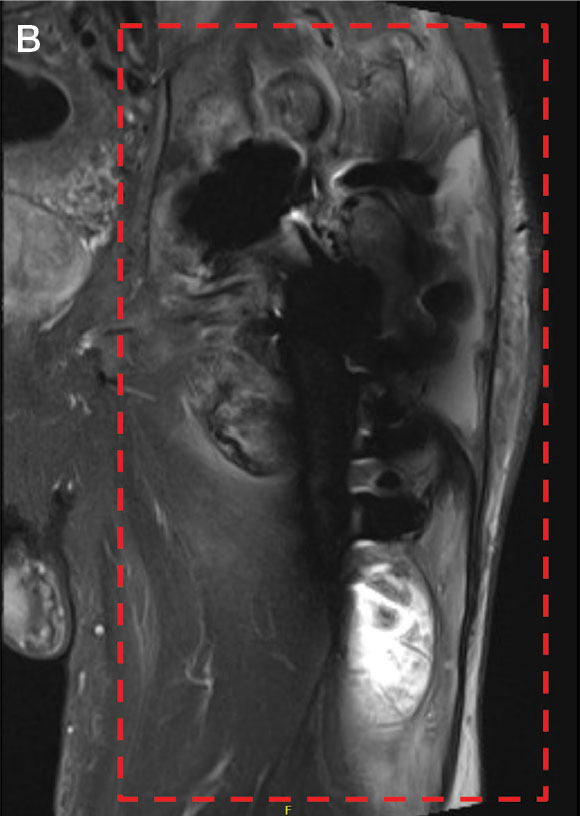
Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
Prosthetic joint infection (PJI) occurs in about 1% to 2% of joint replacements. 1 Risk factors include immunosuppression, diabetes, chronic illnesses, and prolonged operative time.2 Bacterial infections constitute most of these infections, while fungal pathogens account for about 1%. Candida (C.) species, predominantly C. albicans, are responsible for most PJIs.1,3 In contrast, C. glabrata is a rare cause of fungal PJI, with only 18 PJI cases currently reported in the literature.4C. glabrata PJI occurs more frequently among immunosuppressed patients and is associated with a higher treatment failure rate despite antifungal therapy.5 Treatment of fungal PJI is often complicated, involving multiple surgical debridements, prolonged antifungal therapy, and in some cases, prosthesis removal.6 However, given the rarity of C. glabrata as a PJI pathogen, no standardized treatment guidelines exist, leading to potential delays in diagnosis and tailored treatment.7,8
CASE PRESENTATION
A male Vietnam veteran aged 75 years presented to the emergency department in July 2023 with a fluid collection over his left hip surgical incision site. The patient had a complex medical history that included chronic kidney disease, well-controlled type 2 diabetes, hypertension, and osteoarthritis. His history was further complicated by nonalcoholic steatohepatitis with hepatocellular carcinoma that was treated with transarterial radioembolization and yttrium-90. The patient had undergone a left total hip arthroplasty in 1996 and subsequent open reduction and internal fixation about 9 months prior to his presentation. The patient reported the fluid had been present for about 6 weeks, while he received outpatient monitoring by the orthopedic surgery service. He sought emergency care after noting a moderate amount of purulent discharge on his clothing originating from his hip. In the week prior to admission, the patient observed progressive erythema, warmth, and tenderness over the incision site. Despite these symptoms, the patient remained ambulatory and able to walk long distances with the use of an assistive device.
Upon presentation, the patient was afebrile and normotensive. Laboratory testing revealed an elevated erythrocyte sedimentation rate of 77 mm/h (reference range, 0-20 mm/h) and a C-reactive protein of 9.8 mg/L (reference range, 0-2.5 mg/L), suggesting an underlying infectious process. A physical examination revealed a well-healed incision over the left hip with a poorly defined area of fluctuance and evidence of wound dehiscence. The left lower extremity was swollen with 2+ pitting edema, but tenderness was localized to the incision site. Magnetic resonance imaging of the left hip revealed a multiloculated fluid collection abutting the left greater trochanter with extension to the skin surface and inferior extension along the entire length of the surgical fixation hardware (Figure).


Upon admission, orthopedic surgery performed a bedside aspiration of the fluid collection. Samples were sent for analysis, including cell count and bacterial and fungal cultures. Initial blood cultures were sterile. Due to concerns for a bacterial infection, the patient was started on empiric intravenous (IV) ceftriaxone 2 g/day and IV vancomycin 1250 mg/day. Synovial fluid analysis revealed an elevated white blood cell count of 45,000/ìL, but bacterial cultures were negative. Five days after admission, the fungal culture from the left hip wound was notable for presence of C. glabrata, prompting an infectious diseases (ID) consultation. IV micafungin 100 mg/day was initiated as empiric antifungal therapy.
ID and orthopedic surgery teams determined that a combined medical and surgical approach would be best suited for infection control. They proposed 2 main approaches: complete hardware replacement with washout, which carried a higher morbidity risk but a better chance of infection resolution, or partial hardware replacement with washout, which was associated with a lower morbidity risk but a higher risk of infection persistence and recurrence. This decision was particularly challenging for the patient, who prioritized maintaining his functional status, including his ability to continue dancing for pleasure. The patient opted for a more conservative approach, electing to proceed with antifungal therapy and debridement while retaining the prosthetic joint.
After 11 days of hospitalization, the patient was discharged with a peripherally inserted central catheter for long-term antifungal infusions of micafungin 150 mg/day at home. Fungal sensitivity test results several days after discharge confirmed susceptibility to micafungin.
About 2 weeks after discharge, the patient underwent debridement and implant retention (DAIR). Wound cultures were positive for C. glabrata, Enterococcus faecalis, Staphylococcus epidermidis, and Corynebacterium tuberculostearicum. Based on susceptibilities, he completed a 2-month course of IV micafungin 150 mg daily and daptomycin 750 mg daily, followed by an oral suppressive regimen consisting of doxycycline 100 mg twice daily, amoxicillin-clavulanate 2 g twice daily, and fluconazole initially 800 mg daily adjusted to 400 mg daily. The patient continued wound management with twice-daily dressing changes.
Nine months after DAIR, the patient remained on suppressive antifungal and antibacterial therapy. He continued to experience serous drainage from the wound, which greatly affected his quality of life. After discussion with his family and the orthopedic surgery team, he agreed to proceed with a 2-staged revision arthroplasty involving prosthetic explant and antibiotic spacer placement. However, the surgery was postponed due to findings of anemia (hemoglobin, 8.9 g/dL) and thrombocytopenia (platelet count, 73 x 103/λL). At the time of this report, the patient was being monitored closely with his multidisciplinary care team for the planned orthopedic procedure.
DISCUSSION
PJI is the most common cause of primary hip arthroplasty failure; however, fungal species only make up about 1% of PJIs.3,9-11 Patients are typically immunocompromised, undergoing antineoplastic therapies for malignancy, or have other comorbid conditions such as diabetes.12,13C. glabrata presents a unique diagnostic and therapeutic challenge as it is not only rare but also notorious for its resistance to common antifungal agents. C. glabrata is known to develop multidrug resistance through the rapid accumulation of genomic mutations.14 Its propensity towards forming protective biofilm also arms it with intrinsic resistance to agents like fluconazole.15 Furthermore, based on a review of the available reports in the literature, C. glabrata PJIs are often insidious and present with symptoms closely mimicking those of bacterial PJIs, as it did in the patient in this case.16
Synovial fluid analysis, fungal cultures, and sensitivity testing are paramount for ensuring proper diagnosis for fungal PJI. The patient in this case was empirically treated with micafungin based on recommendations from the ID team. When the sensitivities results were reviewed, the same antifungal therapy was continued. Echinocandins have a favorable toxicity profile in long-term use, as well as efficacy against biofilm-producing organisms like C. glabrata.17,18
While there are a few cases citing DAIR as a feasible surgical strategy for treating fungal PJI, more recent studies have reported greater success with a 2-staged revision arthroplasty involving some combination of debridement, placement of antibiotic-loaded bone cement spacers, and partial or total exchange of the infected prosthetic joint.4,19-23 In this case, complete hardware replacement would have offered the patient the most favorable outlook for eliminating this fungal infection. However, given the patient’s advanced age, significant underlying comorbidities, and functional status, medical management with antifungal therapy and DAIR was favored.
Based on the discussion from the 6-month follow-up visit, the patient was experiencing progressive and persistent wound drainage and frequent dressing changes, highlighting the limitations of medical management for PJI in the setting of retained prosthesis. If the patient ultimately proceeds with a more invasive surgical intervention, another important consideration will be the likelihood of fungal PJI recurrence. At present, fungal PJI recurrence rates following antifungal and surgical treatment have been reported to range between 0% to 50%, which is too imprecise to be considered clinically useful.22-24
Given the ambiguity surrounding management guidelines and limited treatment options, it is crucial to emphasize the timeline of this patient’s clinical presentation and subsequent course of treatment. Upon presentation to the ED in late July, fungal PJI was considered less likely. Initial blood cultures from presentation were negative, which is common with PJIs. It was not until 5 days later that the left hip wound culture showed moderate growth of C. glabrata. Identifying a PJI is clinically challenging due to the lack of standardized diagnostic criteria. However, timely identification and diagnosis of fungal PJI with appropriate antifungal therapy, in patients with limited curative options due to comorbidities, can significantly improve quality of life and overall outcomes.25 Routine fungal and mycobacterial cultures are not currently recommended in PJI guidelines, but this case illustrates it is imperative in immunocompromised hosts.26
This case and the current paucity of similar cases in the literature stress the importance of clinicians publishing their experience in the management of fungal PJI. We strongly recommend that clinicians approach each suspected PJI with careful consideration of the patient’s unique risk factors, comorbidities, and goals of care, when deciding on a curative vs suppressive approach to therapy.
CONCLUSIONS
This case report highlights the importance of considering fungal pathogens for PJIs, especially in high-risk patients, the value of obtaining fungal cultures, the necessity of a multidisciplinary approach, the role of antifungal susceptibility testing, and consideration for the feasibility of a surgical intervention. It underscores the challenges in diagnosis and treatment of C. glabrata-associated PJI, emphasizing the importance of clinician experience-sharing in developing evidence-based management strategies. As the understanding of fungal PJI evolves, continued research and clinical data collection remain crucial for improving patient outcomes in the management of these complex cases.
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
- Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1-10. doi:10.1093/cid/cis966
- Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. doi:10.3978/j.issn.2305-5839.2015.09.26
- Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11(1):89-96. doi:10.1093/clinids/11.1.89
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430- 1443. doi:10.12998/wjcc.v7.i12.1430
- Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80-96. doi:10.1128/CMR.12.1.80
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Lee YR, Kim HJ, Lee EJ, Sohn JW, Kim MJ, Yoon YK. Prosthetic joint infections caused by candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23-33. doi:10.1007/s11046-018-0286-1
- Herndon CL, Rowe TM, Metcalf RW, et al. Treatment outcomes of fungal periprosthetic joint infection. J Arthroplasty. 2023;38(11):2436-2440.e1. doi:10.1016/j.arth.2023.05.009
- Delaunay C, Hamadouche M, Girard J, Duhamel A; SoFCOT. What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res. 2013;471(12): 3863-3869. doi:10.1007/s11999-013-2935-5
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1): 128-133. doi:10.2106/JBJS.H.00155
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586. doi:10.1302/0301-620x.83b4.11223
- Gonzalez MR, Bedi ADS, Karczewski D, Lozano-Calderon SA. Treatment and outcomes of fungal prosthetic joint infections: a systematic review of 225 cases. J Arthroplasty. 2023;38(11):2464-2471.e1. doi:10.1016/j.arth.2023.05.003
- Gonzalez MR, Pretell-Mazzini J, Lozano-Calderon SA. Risk factors and management of prosthetic joint infections in megaprostheses-a review of the literature. Antibiotics (Basel). 2023;13(1):25. doi:10.3390/antibiotics13010025
- Biswas C, Chen SC, Halliday C, et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin Microbiol Infect. 2017;23(9):676.e7-676.e10. doi:10.1016/j.cmi.2017.03.014
- Hassan Y, Chew SY, Than LTL. Candida glabrata: pathogenicity and resistance mechanisms for adaptation and survival. J Fungi (Basel). 2021;7(8):667. doi:10.3390/jof7080667
- Aboltins C, Daffy J, Choong P, Stanley P. Current concepts in the management of prosthetic joint infection. Intern Med J. 2014;44(9):834-840. doi:10.1111/imj.12510
- Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494-1500. doi:10.1038/nport.2008.141
- Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou- Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: a case report and review of the literature. Mycoses. 2018;61(4):266-269. doi:10.1111/myc.12736
- Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13(6):707-712. doi:10.1016/s0883-5403(98)80017-x
- Merrer J, Dupont B, Nieszkowska A, De Jonghe B, Outin H. Candida albicans prosthetic arthritis treated with fluconazole alone. J Infect. 2001;42(3):208-209. doi:10.1053/jinf.2001.0819
- Koutserimpas C, Naoum S, Alpantaki K, et al. Fungal prosthetic joint infection in revised knee arthroplasty: an orthopaedic surgeon’s nightmare. Diagnostics (Basel). 2022;12(7):1606. doi:10.3390/diagnostics12071606
- Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549-5557. doi:10.12659/MSM.909168
- Hwang BH, Yoon JY, Nam CH, et al. Fungal periprosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656-659. doi:10.1302/0301-620X.94B5.28125
- Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002-3009. doi:10.1007/s11999-013-3007-6
- Nodzo, Scott R. MD; Bauer, Thomas MD, PhD; Pottinger, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23 Suppl:S18-S25. doi:10.5435/JAAOS-D-14-00385
- American Academy of Orthopaedic Surgeons. Diagnosis and prevention of periprosthetic joint infections. March 11, 2019. Accessed February 5, 2025. https://www.aaos.org/pjicpg
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
A Candida Glabrata-Associated Prosthetic Joint Infection: Case Report and Literature Review
Violaceous Papules on Face
Violaceous Papules on Face
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Violaceous Papules on Face
Violaceous Papules on Face
A 40-year-old man with no significant medical history or comorbidities presented with a violaceous papule involving his nasal tip and scaly, violaceous plaques with associated alopecia involving his beard (Figure). Skin biopsy confirmed granulomatous dermatitis. Additional workup was notable for hypercalcemia (10.5 mg/dL; reference range, 8.4-10.2 mg/dL), elevated chitotriosidase (317 nmol/h/mL; reference range, < 150 nmol/h/mL), and bibasilar opacities with left perihilar consolidation on chest X-ray. The patient had a prolonged PR interval (207 ms; reference range, 120-200 ms) on electrocardiogram. A cardiac positron emission tomography revealed low level fluorodeoxyglucose uptake in the left ventricle. No ocular involvement was noted on evaluation by ophthalmology. The patient’s pharmacotherapy included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and hydroxychloroquine 200 mg daily.
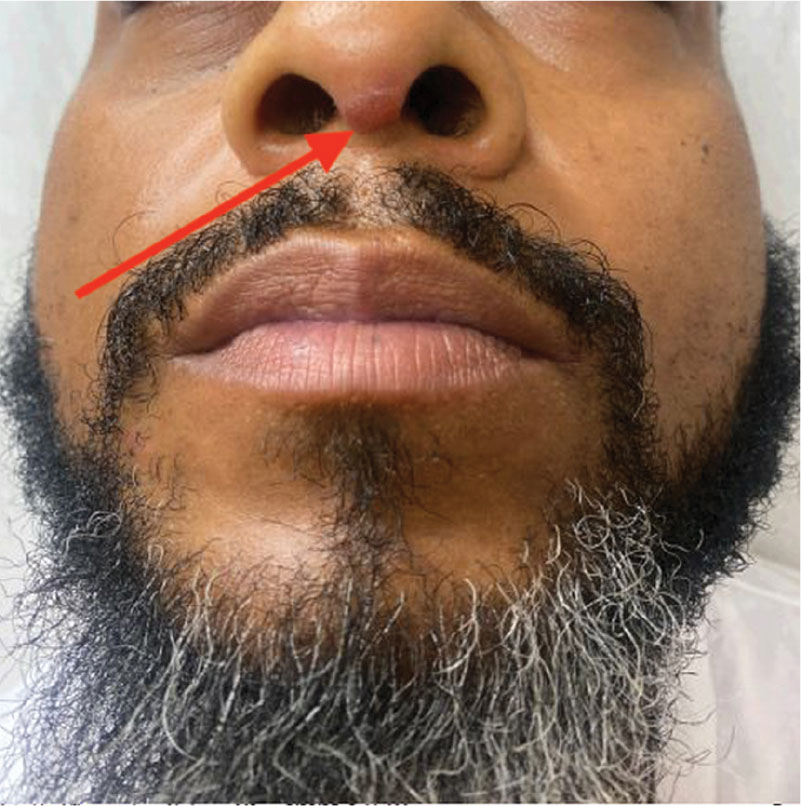
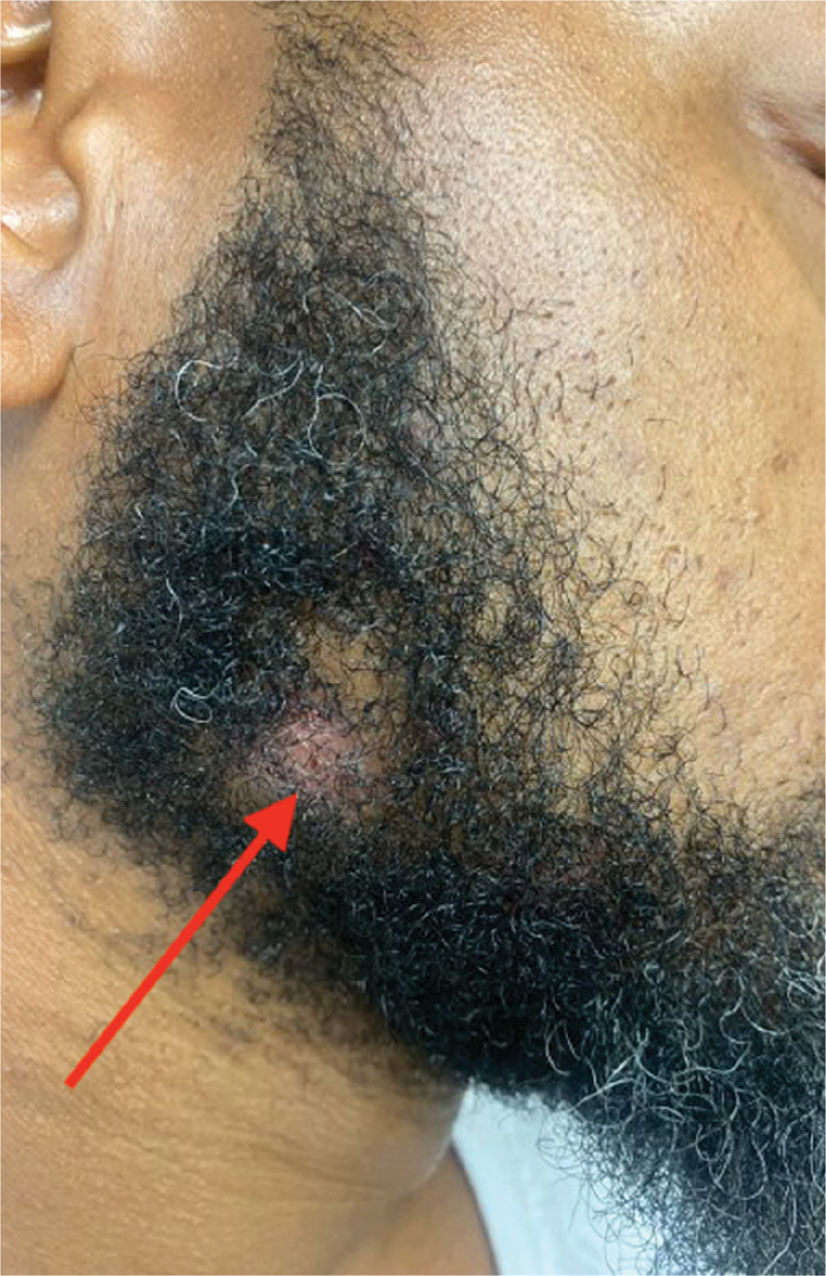
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.
At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.
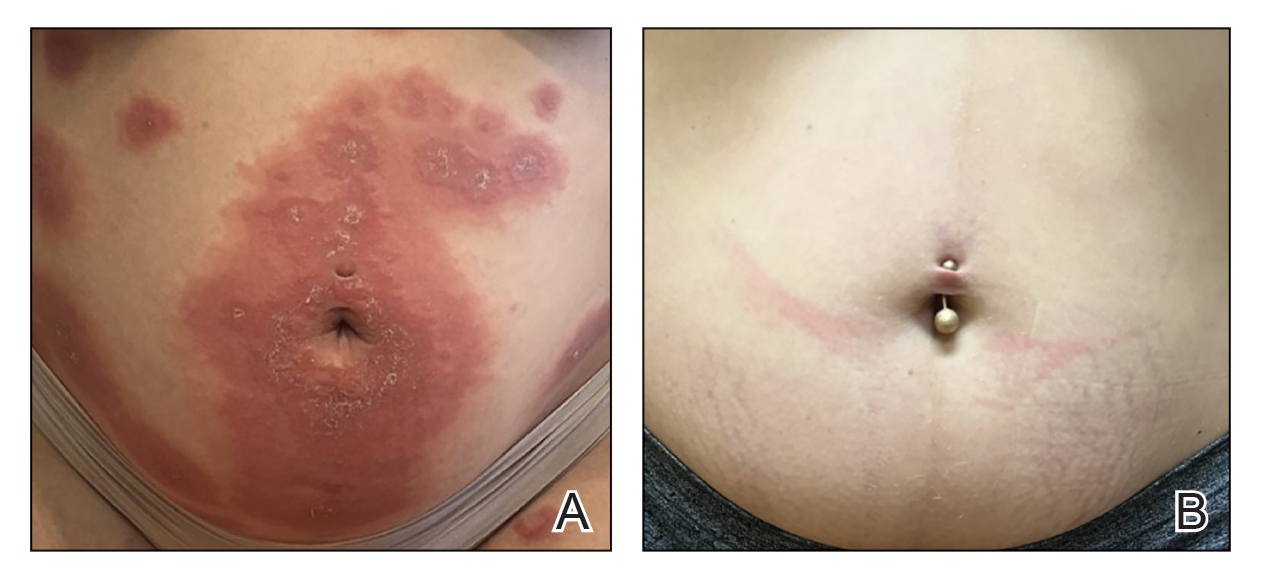
In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
PRACTICE POINTS
- Dupilumab inhibits the IL-4Rα subunit, which is bound by IL‐4 and IL‐13, thereby reducing type 2 inflammation associated with pemphigoid gestationis (PG).
- Dupilumab may reduce the dose and duration of systemic corticosteroid therapy for PG, and its use in the second and third trimesters of pregnancy has been supported by emerging safety data.
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.
The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).
The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1
The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.
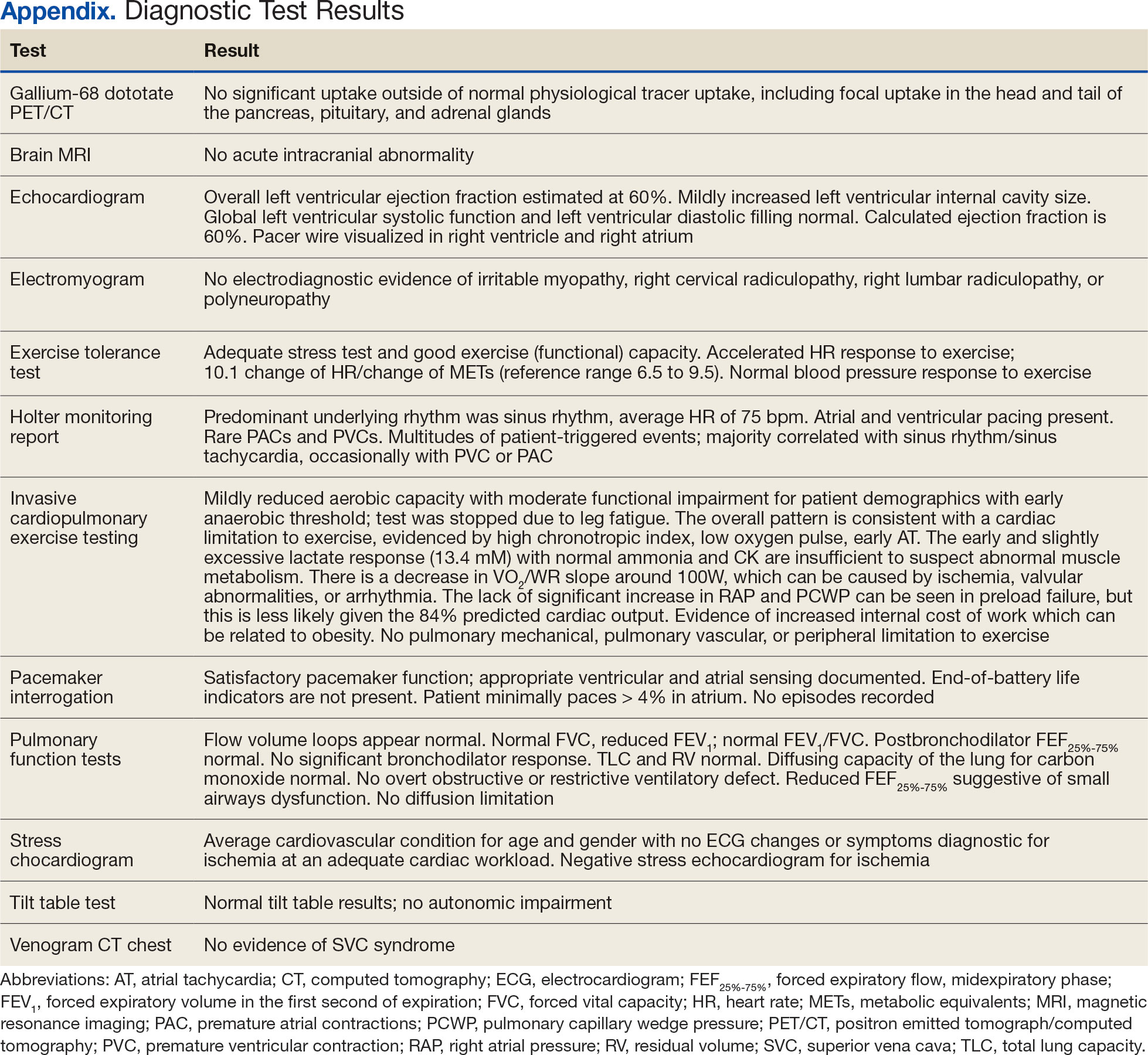
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Idiopathic postprandial syndrome (IPP), initially termed reactive hypoglycemia, presents with hypoglycemic-like symptoms in the absence of biochemical hypoglycemia and remains a diagnosis of exclusion. Its pathophysiology is poorly understood. The diagnosis requires thorough evaluation of cardiac, metabolic, neurologic, and gastrointestinal causes, as well as Whipple triad criteria. Dietary modifications, including reduced carbohydrate intake, increased protein and fiber, and frequent small meals, remain the cornerstone of IPP management. Continuous glucose monitoring (CGM) may be a useful adjunct in correlating symptoms with glucose trends, but its role is still evolving.
In the evaluation of patients with symptoms suggestive of hypoglycemia (Figure 1), patients should first be assessed for Whipple triad: symptoms consistent with hypoglycemia, blood glucose level < 55 mg/dL, and reversal of symptoms with glucose.1 Patients who meet Whipple triad criteria should be investigated to identify further etiologies of hypoglycemia. They may include insulinoma, medication-induced (insulin, sulfonylurea, meglitinide, or β blocker use), postbariatric surgery complications, noninsulinoma pancreatogenous hypoglycemia syndrome, ackee fruit consumption, or familial conditions.2 The presence of hypoglycemic symptoms in the postprandial or fasting state can provide valuable insights into underlying etiology.
Patients who do not meet Whipple triad criteria, but exhibit postprandial symptoms consistent with hypoglycemia, as in this case, present a diagnostic dilemma. IPP is defined as hypoglycemic symptoms occuring after carbohydrate ingestion without biochemical hypoglycemia. Initially termed reactive hypoglycemia, it was renamed in 1981to reflect the absence of low blood glucose levels.3
The understanding of this diagnosis has not significantly progressed since the 1980s. Its prevalence, incidence, risk factors, and societal burden remain unclear. IPP is a challenging diagnosis due to nonspecific symptoms that overlap with a myriad of conditions. These symptoms may include adrenergic symptoms such as diaphoresis, tremulousness, palpitations, anxiety, and hunger. Potentially severe neuroglycopenic symptoms, including weakness, dizziness, behavior changes, confusion, and coma, are not typically observed.4 Given that objective criteria are not well established, IPP remains a diagnosis of exclusion. It is imperative to rule out alternative etiologies, particularly cardiac, gastrointestinal, and neurologic causes.
CASE PRESENTATION
A male aged 41 years presented to primary care for evaluation of acute on chronic symptomatic postprandial episodes. He reported a history of symptomatic sinus bradycardia in the setting of sick sinus syndrome following dual-chamber pacemaker placement, posttraumatic stress disorder, and gastroesophageal reflux disease. He was a retired Navy sailor without any known occupational exposures who worked in the real estate industry. The patient reported feeling lightheaded, tremulous, and anxious most afternoons after lunch for several years. He also reported that meals heavy in carbohydrates exacerbated his symptoms, whereas skipping meals or lying down alleviated his symptoms. The patient also reported concomitant arm numbness, shortness of breath, palpitations, and nausea during these episodes. Review of systems was otherwise negative, including no weight changes, fever, chills, night sweats, chest pain, or syncope.

The patient’s medications included ferrous sulfate 325 mg once every other day, bupropion 200 mg once daily, metoprolol succinate 25 mg once daily, and as-needed lorazepam 1 mg once daily. The patient reported no current substance use but reported previous tobacco use 3 years prior (maximum 1 pack/week) and alcohol use 5 years prior (750 ml/day for 15 years). The patient did not exercise and typically ate oatmeal for breakfast, a sandwich or salad for lunch, and taquitos or salad for dinner, with snacks throughout the day. Notable family history included a maternal grandmother with colon cancer. The patient’s vital signs included a 36.8 °C temperature, heart rate 87 beats/min, 118/71 mm Hg blood pressure, oxygen saturation 98% on room air, 125.2 kg weight, and 38.5 body mass index. There were no orthostatic vital sign changes. A physical examination demonstrated obesity with an unremarkable cardiopulmonary and volume examination.
Additional testing included Gallium-68 dototate positron emission tomography/computed tomography, brain magnetic resonance imaging, echocardiogram, electromyogram, exercise tolerance test, Holter monitoring, invasive cardiopulmonary exercise testing, pacemaker interrogation, pulmonary function testing, stress echocardiogram, tilt table test, and venogram computed tomography of the chest, but the results were unremarkable (Appendix). His afternoon nonfasting glucose level was 138 mg/dL with a concurrent hemoglobin A1c of 5.2%. The patient had a fasting C-peptide level of 3.7 ng/mL (reference range 0.5-2.0 ng/mL), fasting insulin level 19.1 mIU/L (reference range < 25 mIU/L), and a fasting glucose level of 93 mg/dL (reference range 70-99 mg/dL). The patient’s urine 5-HIAA, plasma metanephrines, urine metanephrines, insulin-like growth factor 1, prolactin, corticotropin, fasting cortisol, and thyrotropin yielded results within reference ranges (Table). The veteran was prescribed a CGM, which demonstrated normal glucose levels (≥ 55 mg/dL) during symptomatic episodes (Figure 2).

The patient was diagnosed with IPP given normoglycemia, exclusion of alternative diagnoses, and symptomatic improvement with dietary changes. He was referred to a nutritionist for a high-protein, high-fiber, and low-carbohydrate diet.
DISCUSSION
Seemingly simple diagnostic tools can lead to diagnostic pitfalls. Home glucose monitoring with the use of a standard glucometer during an episode is the typical first step in identifying hypoglycemia, as it is both pragmatic and accurate, with a mean absolute relative difference (MARD) of about 10% in hypoglycemic ranges.5 While the advent of CGM provides real-time data and can reveal clinically relevant fluctuations, it reveals mild hypoglycemia (54 to 70 mg/dL) of no clinical significance in a large proportion of individuals.
Additionally, CGM is less accurate than glucometers with a MARD of about 20% in hypoglycemia ranges.6 CGM technology, however, is rapidly evolving and undergoing further investigation for hypoglycemia detection. Therefore, CGM may be considered in select patients as prospective study results are established; the newest CGMs have MARDs very similar to fingerstick blood glucose data.7,8 In the patient described in this case, CGM helped corroborate the diagnosis, given that symptomatic episodes correlated with lower glucose levels. Provocative testing with oral glucose tolerance testing can frequently result in false positive hypoglycemic readings and is not recommended.9 Supervised mixed meal testing can also be used, which entails monitoring after consuming a mixed macronutrient meal. The test concludes after hypoglycemic symptoms develop or 5 hours elapse, whichever occurs first.1

The pathophysiology of IPP is poorly understood. Proposed mechanisms include increased insulin sensitivity, increased adrenergic sensitivity, impaired glucagon regulation, emotional distress, insulin resistance, and increased glucagon-like peptide-1 production.10-13 Research suggests this may occur as pancreatic β cells fail in early type 2 diabetes mellitus, with diminished first-phase insulin release leading to an initial exuberant rise in blood glucose, an overshooting of the second phase of insulin secretion, and the feeling of the postprandial blood glucose falling, even though the final glucose level achieved is not truly low.13 There are contradictory studies in the literature demonstrating no association between insulin resistance and hypoglycemic symptoms.14 In 2022, Kosuda and colleagues looked at homeostatic model assessment for insulin resistance in patients with postprandial syndrome. They found that the patients were slightly insulin resistant but had normal or exaggerated insulin secretory capacity compared to an oral glucose load, whereas glucagon levels were robustly suppressed by a glucose load. The observed hormonal responses may result in the glycemic patterns and symptoms observed; further study is warranted to elucidate the mechanism.15
Dietary modification is the cornerstone treatment for postprandial syndrome, including reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals. There is also evidence that a Mediterranean diet may be beneficial for managing hypoglycemic symptoms.16 Furthermore, α-glucosidase inhibitors, whose mechanism of action delays the digestion of carbohydrates, have demonstrated promise. This medication class has demonstrated significance in raising postprandial glucose levels and alleviating hypoglycemic symptoms in patients with true postprandial hypoglycemia.17
CONCLUSIONS
IPP is a benign diagnosis encompassing hypoglycemic symptoms without biochemical hypoglycemia. It is not a true hypoglycemic disorder. IPP is challenging to diagnose, given that it is an interpretation of exclusion, supported by symptom improvement with dietary changes (ie, reduced carbohydrate intake, increased protein and fiber intake, and more frequent and smaller meals). Supervised mixed meal testing or CGM can be used to assist with diagnosis. Even though CGM is undergoing further study in this patient population, it corroborated the diagnosis in the patient described in this case.
For hypoglycemic symptoms, physicians should first assess for evidence of Whipple triad to evaluate for true biochemical hypoglycemia. For true hypoglycemia (< 55 mg/dL), physicians may conduct an examination in conjunction with an endocrinologist. For normoglycemia (≥ 55 mg/dL), physicians should first exclude alternative etiologies (including cardiac and neurologic), and subsequently consider IPP.

Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. MDText.com, Inc.; 2000.
Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332(17):1144-1152.doi:10.1056/NEJM199504273321707
Charles MA, Hofeldt F, Shackelford A, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycemia: absence of hypoglycemia after meals. Diabetes. 1981;30(6):465-470.
Douillard C, Jannin A, Vantyghem MC. Rare causes of hypoglycemia in adults. Ann Endocrinol (Paris). 2020;81(2-3):110-117. doi:10.1016/j.ando.2020.04.003
Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558-566. doi:10.1177/1932296816672237
Alitta Q, Grino M, Adjemout L, Langar A, Retornaz F, Oliver C. Overestimation of hypoglycemia diagnosis by FreeStyle Libre continuous glucose monitoring in long-term care home residents with diabetes. J Diabetes Sci Technol. 2018;12(3):727-728. doi:10.1177/1932296817747887
Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. doi:10.1016/j.jcte.2019.100202
Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi:10.1210/jc.2018-02763
Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. doi:10.1210/jc.2008-1410
Galati SJ, Rayfield EJ. Approach to the patient with postprandial hypoglycemia. Endocr Pract. 2014;20(4):331-340. doi:10.4158/EP13132.RA
Altuntas Y. Postprandial reactive hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53(3):215-220.doi:10.14744/SEMB.2019.59455
HARRIS S. HYPERINSULINISM AND DYSINSULINISM. JAMA. 1924;83(10):729-733.doi:10.1001/jama.1924.02660100003002
Harris S. HYPERINSULINISM AND DYSINSULINISM (INSULOGENIC HYPOGLYCBMIA). Endocrinology. 1932;16(1):29-42. doi:10.1210/endo-16-1-29
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):276. doi:10.3390/jpm11040276
Kosuda M, Watanabe K, Koike M, et al. Glucagon response to glucose challenge in patients with idiopathic postprandial syndrome. J Nippon Med Sch. 2022;89(1):102-107. doi:10.1272/jnms.JNMS.2022_89-205
Hall M, Walicka M, Panczyk M, Traczyk I. Assessing long-term impact of dietary interventions on occurrence of symptoms consistent with hypoglycemia in patients without diabetes: a one-year follow-up study. Nutrients. 2022;14(3):497. doi:10.3390/nu14030497
Ozgen AG, Hamulu F, Bayraktar F, et al. Long-term treatment with acarbose for the treatment of reactive hypoglycemia. Eat Weight Disord. 1998;3(3):136-140. doi:10.1007/BF03340001
A Veteran Presenting With Symptomatic Postprandial Episodes
A Veteran Presenting With Symptomatic Postprandial Episodes