User login
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
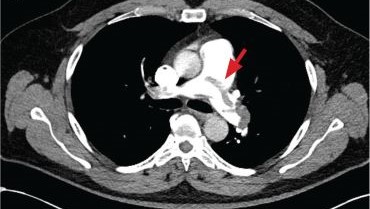
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.
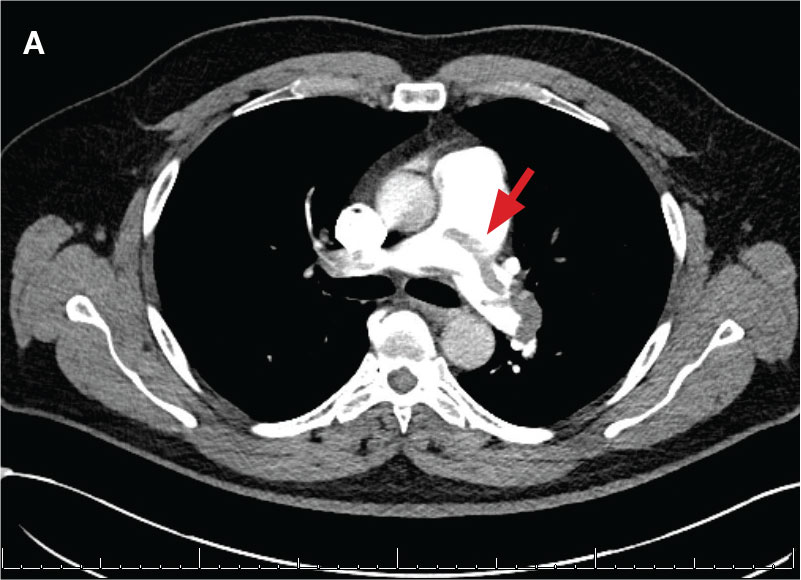
indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
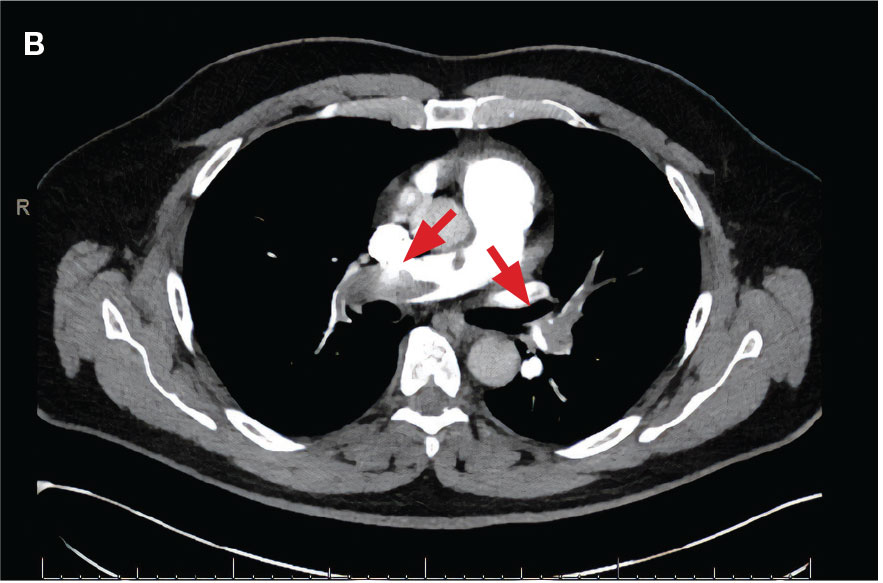
indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.
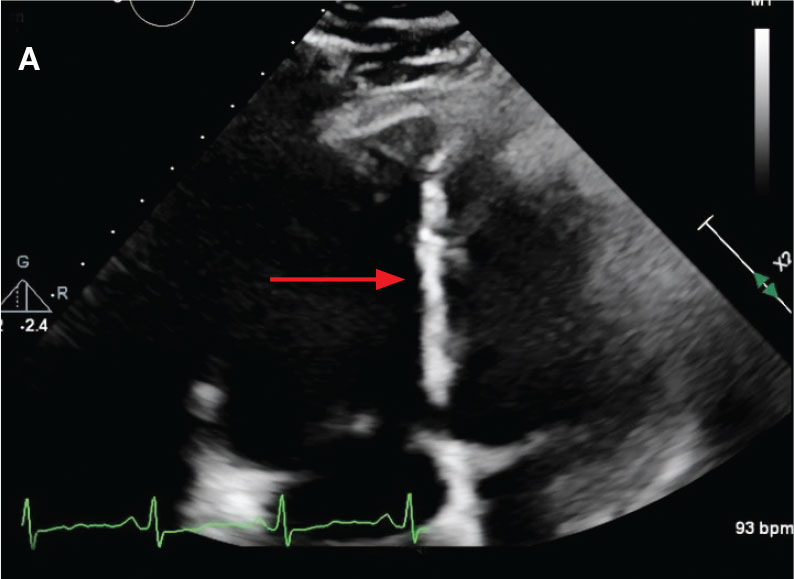
flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
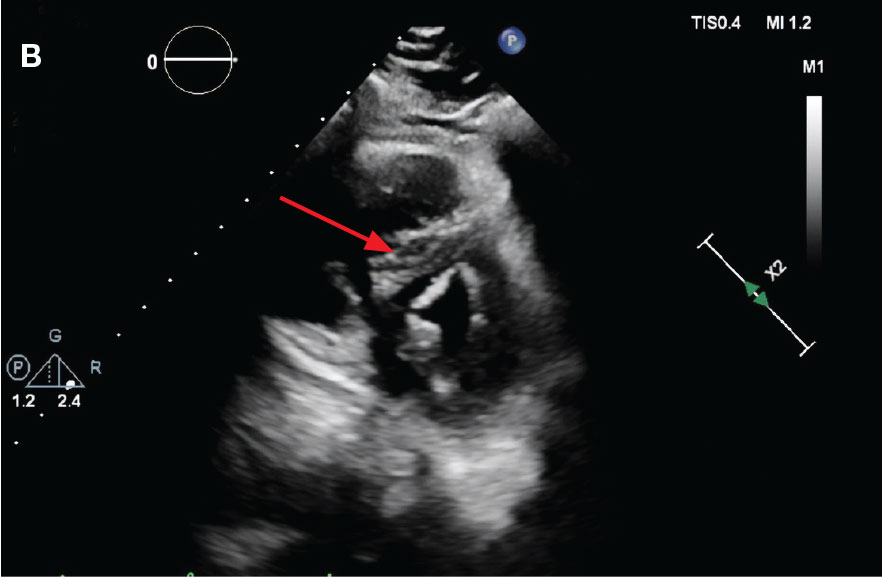
flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
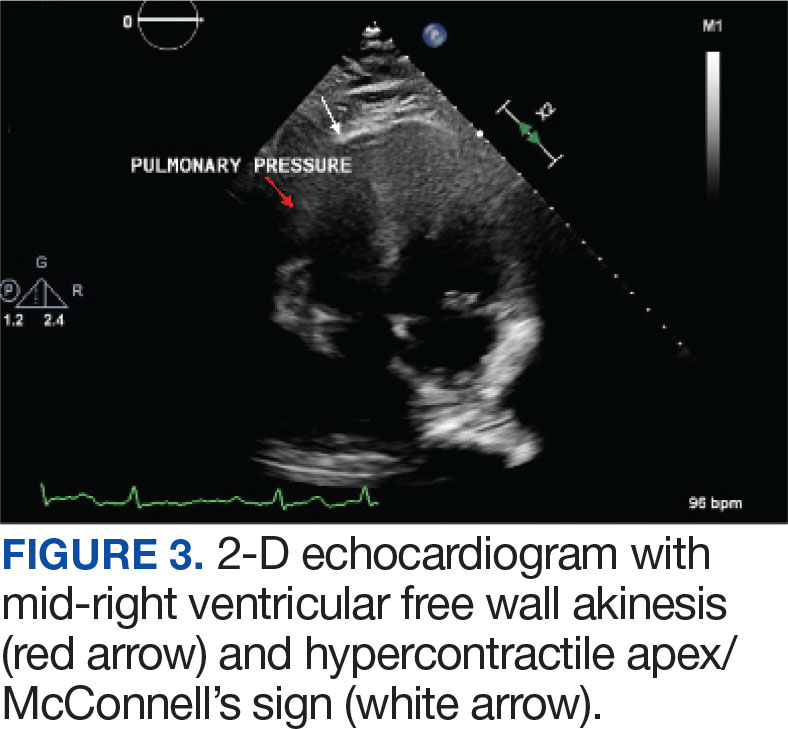
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.
Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
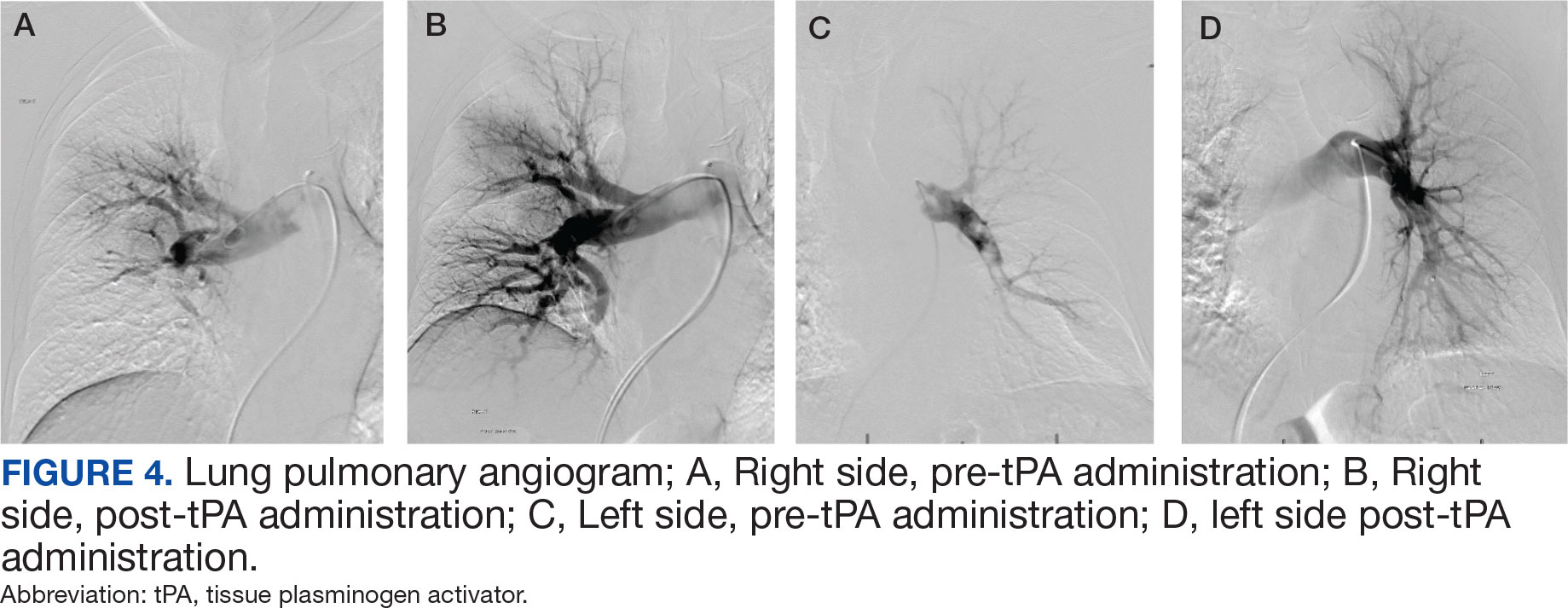
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.
DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.

Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.

DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
Pulmonary embolism (PE) is a common cause of morbidity and mortality in the general population.1 The incidence of PE has been reported to range from 39 to 115 per 100,000 persons per year and has remained stable.2 Although mortality rates have declined, they remain high.3 The clinical presentation is nonspecific, making diagnosis and management challenging. A crucial and difficult aspect in the management of patients with PE is weighing the risks vs benefits of treatment, including thrombolytic therapy and other invasive procedures, which carry inherent risks. These factors have led to the development of PE response teams (PERTs) in some hospitals to implement effective multidisciplinary protocols that facilitate prompt diagnosis, management, and follow-up.4
CASE PRESENTATIONS
Case 1
New onset seizures and cardiac arrest in the treatment of saddle PE. A 54-year-old male who worked as a draftsman and truck driver with a history of hypertension and nephrolithiasis presented to the emergency department (ED) with progressive shortness of breath for 2 weeks. On the morning of ED presentation the patient experienced an episode of severe shortness of breath, lightheadedness, and chest pressure. He reported no other symptoms such as palpitations, nausea, vomiting, abdominal discomfort, or extremity pain or swelling. He reported no recent travel, immunization, falls, or surgery. Upon evaluation, the patient was found to be in no acute distress, with stable vital signs and laboratory results except for 2 elevated results: > 20 μg/mL D-dimer (reference range, < 0.5 μg/mL) and N-terminal prohormone brain natriuretic peptide (proBNP) level, 3455 pg/mL (reference range, < 125 pg/mL for patients aged < 75 years). Electrocardiogram showed T-wave inversions in leads V2 to V4. Imaging revealed a saddle PE and left popliteal deep venous thrombosis (Figure 1). The patient received an anticoagulation loading dose and was started on heparin drip upon admission to the medical intensive care unit (MICU) for further management and monitoring. The Interventional Radiology Service recommended full anticoagulation with consideration of reperfusion therapies if deterioration developed.

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).

indicated by arrows in the pulmonary trunk extending to the left pulmonary artery (A),
and obliterating right pulmonary artery and branches of left pulmonary artery (B).
While in the MICU, point-of-care ultrasound findings were confirmed with official echocardiogram by the cardiology service, which demonstrated a preserved ejection fraction of 60% to 65%, a D-shaped left ventricle with septal wall hypokinesis secondary to right heart strain (Figure 2), a markedly elevated right ventricular systolic pressure (RVSP) of 73 mm Hg, and a mean pulmonary artery pressure (mPAP) of 38 mm Hg. The patient’s blood pressure progressively decreased, heart rate increased, and he required increased oxygen supplementation. The case was discussed with the Pharmacy Service, and since the patient had no contraindications to thrombolytic therapy, the appropriate dosage was calculated and 100 mg intravenous (IV) tissue plasminogen activator (tPA) was administered over 2 hours.

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).

flattening and deviation to left in direction (A) and septal deviation to left with
formation of D-sign (B).
About 40 minutes into tPA infusion, the patient suddenly experienced marked shortness of breath, diaphoresis, and anxiety with seizure-like involuntary movements; as a result, the infusion was stopped. He also had episodes of posturing, mental status decline, and briefly going in and out of consciousness, which lasted about 3 minutes before he lost consciousness and pulse. High-quality advanced cardiac life support was initiated, followed by endotracheal intubation. Despite a secured airway and return of spontaneous circulation, the patient remained hypotensive and continued to have seizure-like activity.
The patient was administered a total of 8 mg of lorazepam, sedated with propofol, initiated at 5 μg/kg/min, titrated to stop seizure activity at 15μg/kg/min, and later maintained at 10 μg/kg/min, for a RASS of -1, and started on norepinephrine 0.1 μg/kg/min for acute stabilization. Head computed tomography without contrast showed no acute intracranial pathology as etiology of seizures. Seizure etiology differential at this time was broad; however, hypoxemia due to PE and medication adverse effects were strongly suspected.
The patient’s condition improved, and vasopressor therapy was tapered off the next day. Four days later, the patient was weaned from mechanical ventilation and transferred to the step-down unit. Echocardiogram obtained 48 hours after tPA infusion showed essentially normal left ventricular function (60%-65%), a RVSP of 17 mm Hg and mPAP of 13 mm Hg. The patient’s ProBNP levels markedly decreased to 137 pg/mL. Postextubation, the neurologic examination was at baseline. The Neurology Service recommended temporary treatment with levetiracetam, 1000 mg every 12 hours, and the Hematology Service recommended transitioning to direct oral anticoagulation with follow-up. The patient presented significant clinical and respiratory improvement and was referred for home-based physical rehabilitation as recommended by the physical medicine and rehabilitation service before being discharged.
Case 2
Localized tPA infusion for bilateral PEs via infusion catheters. A 91-year-old male with no history of smoking and a medical history of hypertension, diabetes mellitus, prostate cancer (> 20 years postradiotherapy) and severe osteoarthritis was receiving treatment in the medical ward for medication-induced liver injury secondary to an antibiotic for a urinary tract infection. During the night the patient developed hypotension (86/46 mm Hg), shortness of breath, tachypnea, desaturation, nonradiating retrosternal chest pain, and tachycardia. The hypotension resolved after a 500-mL 0.9 normal saline bolus, and hypoxemia improved with supplemental oxygen via Venturi mask. Chest computed tomography angiography was performed immediately and revealed extensive bilateral acute PE, located most proximally in the right main pulmonary artery (PA) and on the left in the proximal lobar branches, with associated right heart strain. The patient was started on IV heparin with a bolus of 5000 units (80 u/kg) followed by a drip with a partial thromboplastin time goal of 62-103 seconds and transferred to MICU.
Laboratory findings were notable for proBNP that increased from 115 pg/mL to 4470 pg/mL (reference range, < 450 pg/mL for patients aged 75 years) and elevated troponin levels at 218 ng/L to 295 ng/L (reference range, < 22 ng/L), exhibiting chemical evidence of right heart strain. Initial echocardiogram showed mid-right ventricular free wall akinesis with a hypercontractile apex, suggestive of PE (McConnell’s sign) (Figure 3). Interventional Radiology Service was consulted and recommended tPA infusion given that the patient had bilateral PEs and stable blood pressure.

Pulmonary angiogram showed elevated pressures in the right PA of 64/21 mm Hg and the left PA pressures of 63/20 mm Hg. Mechanical disruption of the larger right lower PA thrombus was achieved via a pigtail catheter followed by bilateral catheter bolus infusions of 2 mg tPA (alteplase) and a continuous tPA infusion 0.5 mg/h for 24 hours, in conjunction with a heparin infusion.
After 24 hours of tPA infusion, the catheters were removed, with posttreatment pulmonary angiography demonstrating right and left PA pressures of 42/15 mm Hg and 40/16 mm Hg, respectively. Pre- and postlocalized tPA infusion treatment images are provided for visual comparison (Figure 4). An echocardiogram performed after tPA infusion showed no signs of pulmonary hypertension. The Hematology Service provided recommendations regarding anticoagulation, and after completion of tPA infusion, the patient was transitioned to an unfractioned heparin infusion and subsequently to direct oral anticoagulation prior to transfer back to the medical ward, hemodynamically stable and asymptomatic.

DISCUSSION
PE management can be a straightforward decision when the patient meets criteria for hemodynamic instability, or with small PE burden. In contrast, management can be more challenging in intermediate-risk (submassive) PE when patients remain hemodynamically stable but show signs of cardiopulmonary stress, such as right heart strain, elevated troponins, or increased proBNP levels.2 In these situations, case-by- case evaluation is warranted. A PERT can assess the most beneficial treatment approach by considering factors such as right ventricular dysfunction, hemodynamic status, clot burden, and clinical deterioration despite appropriate anticoagulation. The evidence supporting the benefits these organized teams can provide is growing. These case reports emphasize the need for a multidisciplinary and systematic approach in these complex cases, especially in the management of intermediate-risk PE patients.
Currently, the Veterans Affairs Caribbean Healthcare System does not have an organized PERT, although a multidisciplinary approach was applied in the management of these patients. A systematic, structured team could have decreased time to interventions and alleviated the burden of physician decision-making. Having such a team would streamline the diagnostic pathway for patients presenting from a ward or emergency department with suspected PE.
We present 2 cases of patients found to have a high clot burden from PEs. The patients were initially hemodynamically stable (intermediate-risk PE), but later required systemic or localized thrombolysis due to hemodynamic deterioration despite adequate anticoagulation. Despite similar diagnoses and etiologies, these patients were successfully managed using different approaches, yielding positive outcomes. This reflects the complexity and variability in diagnosing and managing intermediate-risk PE in patients with different comorbidities and clot burden effects. In Case 1, our multidisciplinary approach was obtained via consults to selected services such as interventional radiology, cardiology, and direct involvement of pharmacy. An organized PERT conceivably would have allowed quicker discussions among these services, including hematology, to provide recommendations and collaborative support upon the patient’s arrival to the ED. Additionally, with a PERT team, a systematic approach to these patients could have allowed for an earlier official echocardiogram report for evaluation of right heart strain and develop an adequate therapeutic plan in a timely manner.
In Case 2, consultation with the Interventional Radiology Service yielded a better therapeutic plan, utilizing localized tPA infusion for this older adult patient with increased risk of bleeding with systemic tPA infusion. Having a PERT presents an opportunity to optimize PE management through early recognition, diagnosis, and treatment by institutional consensus from an interdisciplinary team.5,6 These response teams may improve outcomes and prognosis for patients with PE, especially where diagnosis and management is not clear.
The definite etiology of seizure activity in the first case pre- and postcardiac arrest, in the context of no acute intracranial process, remains unknown. Reports have emerged about postreperfusion seizures in acute ischemic stroke, as well as cases of seizures masquerading as PE as the primary presentation. 7,8 However, there were no reports of patients developing seizures post tPA infusion for the treatment of PE. This report may shed light into possible complications secondary to tPA infusion, raising awareness among physicians and encouraging further investigation into its possible etiologies.
CONCLUSIONS
Management of PE can be challenging in patients that meet criteria for intermediate risk. PERTs are a tool that allow for a multidisciplinary, standardized and systematic approach with a diagnostic and treatment algorithm that conceivably would result in a better consensus and therapeutic approach.
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
- Thompson BT, Kabrhel C. Epidemiology and pathogenesis of acute pulmonary embolism in adults. UpToDate. Wolters Kluwer. Updated December 4, 2023. Accessed February 26, 2025. https://www.uptodate.cn/contents/epidemiology-and-pathogenesis-of-acute-pulmonary-embolism-in-adults
- Kulka HC, Zeller A, Fornaro J, Wuillemin WA, Konstantinides S, Christ M. Acute pulmonary embolism– its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch Arztebl Int. 2021;118(37):618-628. doi:10.3238/arztebl.m2021.0226
- Zghouzi M, Mwansa H, Shore S, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577. doi:10.1513/AnnalsATS.202302-091OC
- Channick RN. The pulmonary embolism response team: why and how? Semin Respir Crit Care Med. 2021;42(2):212-217. doi:10.1055/s-0041-1722963
- Rosovsky R, Zhao K, Sista A, Rivera-Lebron B, Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315-330. doi:10.1002/rth2.12216
- Glazier JJ, Patiño-Velasquez S, Oviedo C. The pulmonary embolism response team: rationale, operation, and outcomes. Int J Angiol. 2022;31(3):198-202. doi:10.1055/s-0042-1750328
- Lekoubou A, Fox J, Ssentongo P. Incidence and association of reperfusion therapies with poststroke seizures: a systematic review and meta-analysis. Stroke. 2020;51(9):2715-2723.doi:10.1161/STROKEAHA.119. 028899
- Alemany M, Nuñez A, Falip M, et al. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. A prospective long-term follow-up study. Seizure. 2021;89:5-9. doi:10.1016/j.seizure.2021.04.011
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment
The Need for a Multidisciplinary Approach for Successful High-Risk Pulmonary Embolism Treatment