User login
Iododerma Simulating Cryptococcal Infection
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
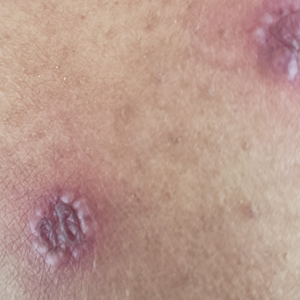
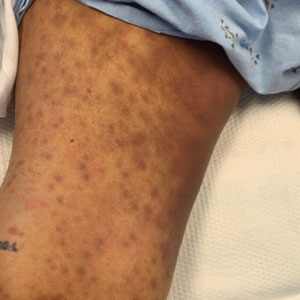
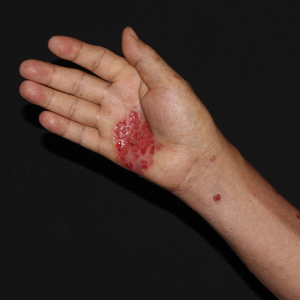
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
Practice Points
- Halogenodermas are rare cutaneous reactions to excess exposure to or ingestion of halogen-containing drugs or substances such as bromine, iodine (iododerma), fluorine, and rarely lithium.
- The clinical presentation of a halogenoderma varies; the most characteristic manifestation is a vegetative or exudative plaque with a peripheral rim of pustules.
- Histologically, lesions of a halogenoderma are characterized by pseudoepitheliomatous hyperplasia associated with numerous intraepidermal microabscesses overlying a dense mixed inflammatory infiltrate of neutrophils, plasma cells, eosinophils, histiocytes, and scattered multinucleated giant cells.
- Rarely, the dermal infiltrate of a halogenoderma contains abundant acellular bodies surrounded by capsulelike vacuolated spaces mimicking Cryptococcus neoformans.
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

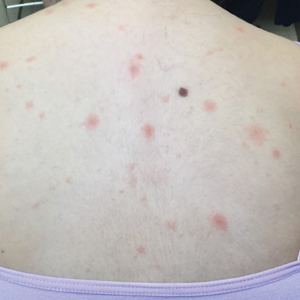
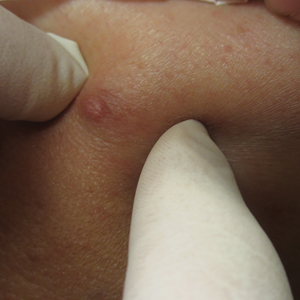
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
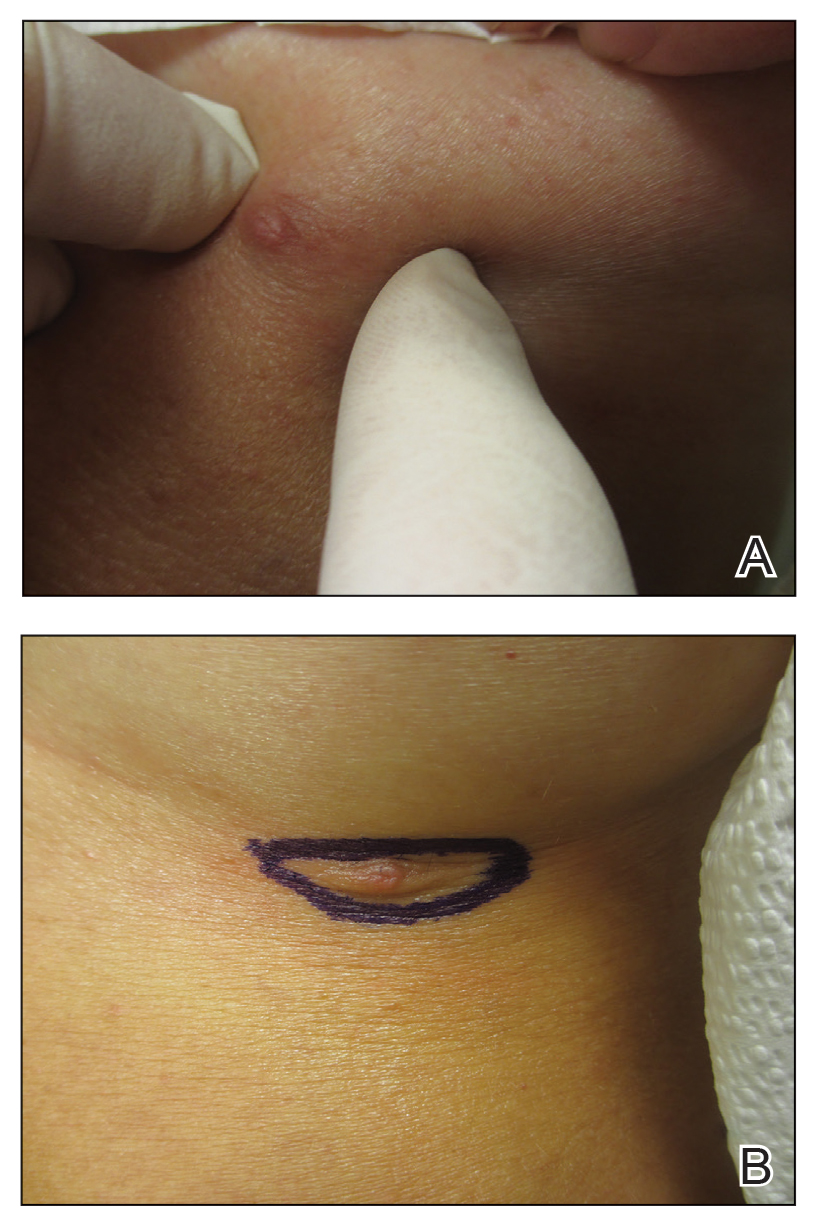
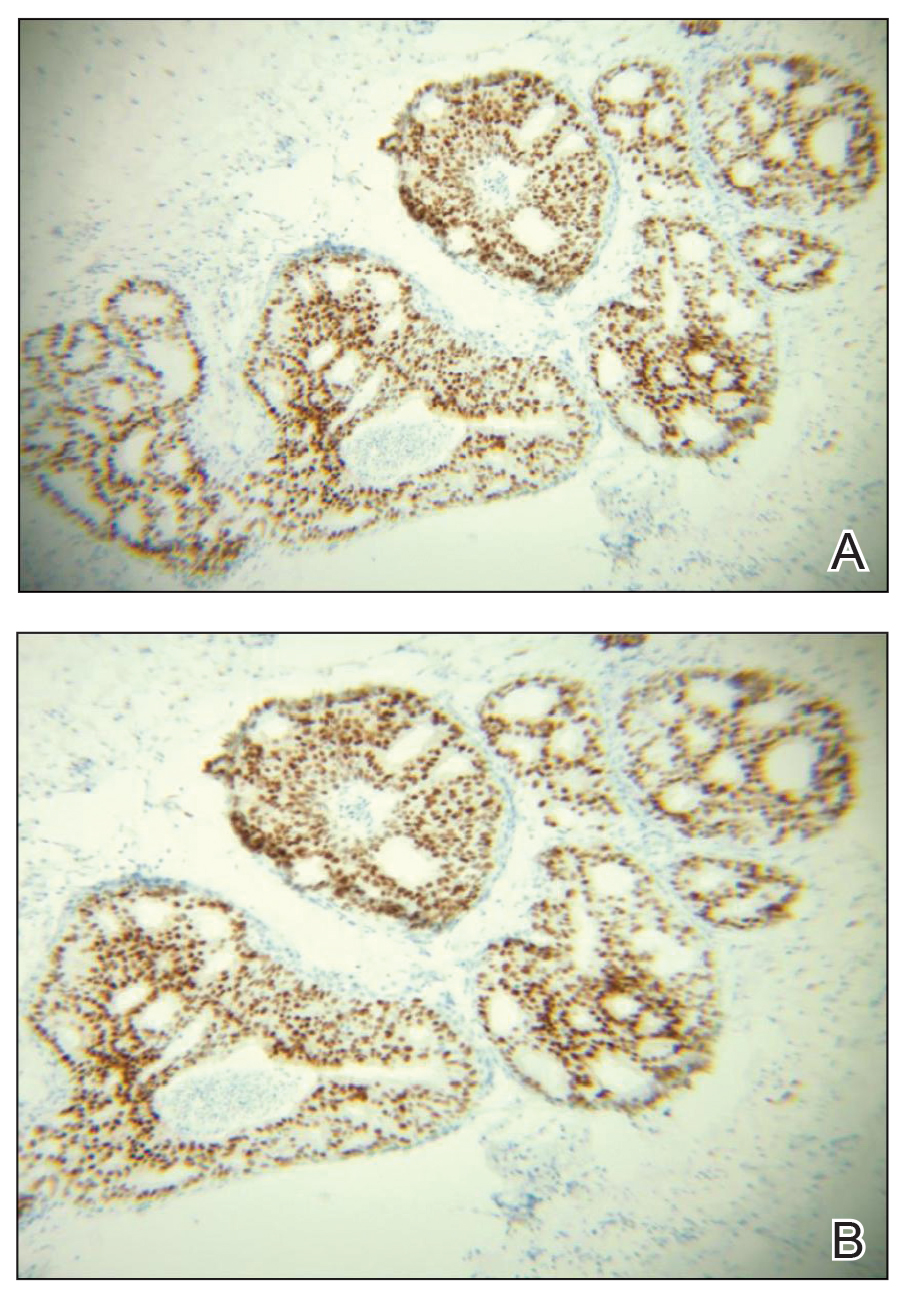
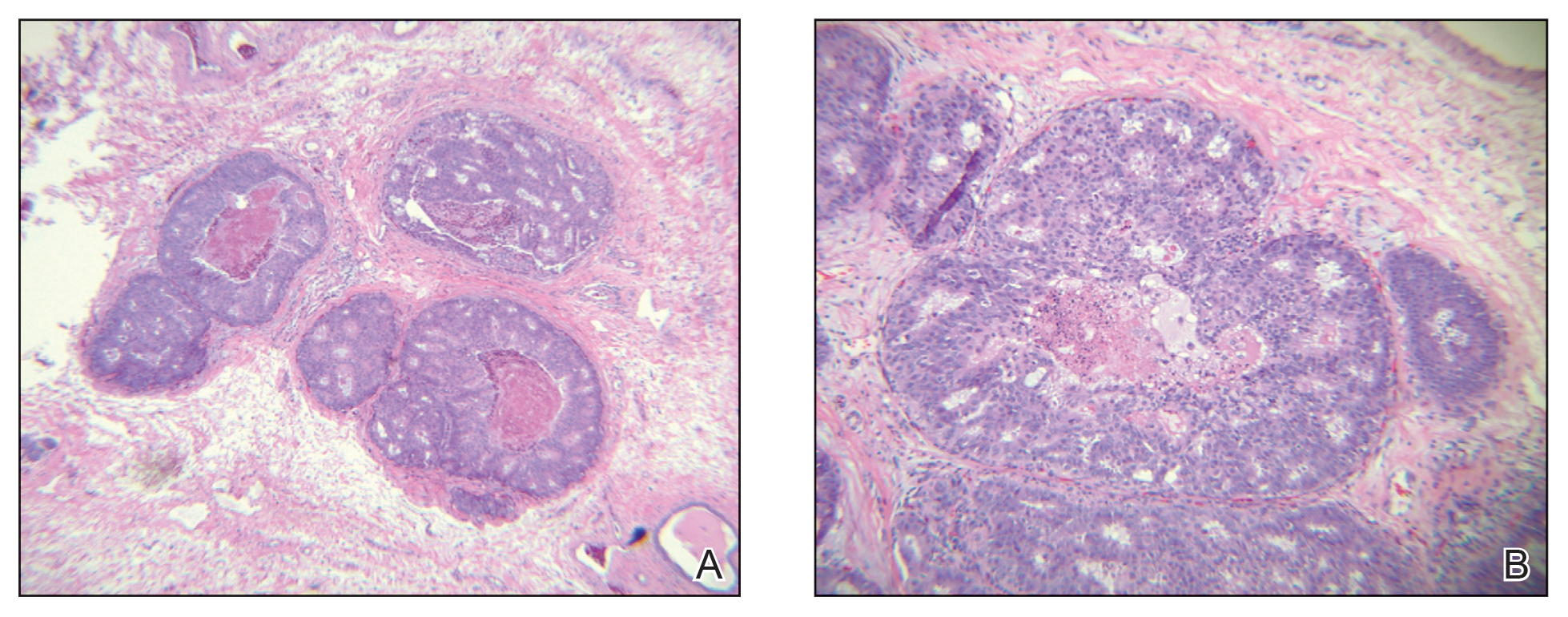
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
Palifermin-Associated Cutaneous Papular Rash of the Head and Neck
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.
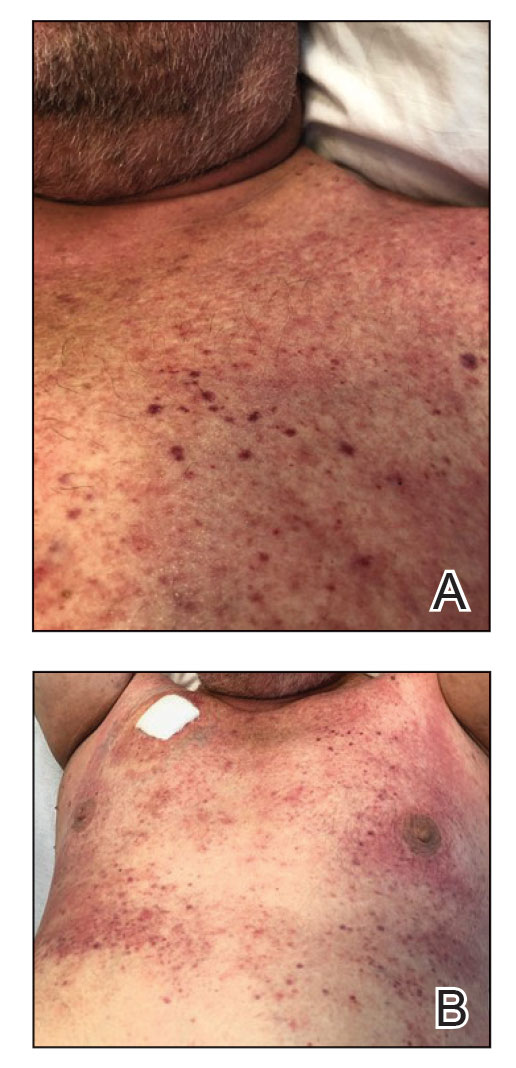
Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein.Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining.These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein.Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining.These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein.Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining.These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
Practice Points
- Palifermin is a recombinant keratinocyte growth factor that is US Food and Drug Administration approved to prevent oral mucositis in patients undergoing chemotherapy or radiation therapy.
- Histologically, the rash can resemble verrucae with evidence of hypergranulosis, hyperorthokeratosis, and papillomatosis.
- Cutaneous reactions have been reported with use of palifermin and generally are benign and self-limited with removal of the offending agent.
Adjuvant Scalp Rolling for Patients With Refractory Alopecia Areata
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
Practice Points
- Alopecia areata (AA) is an autoimmune hair loss disorder with few effective treatments and no cure.
- Scalp rolling is a promising new treatment option that may stimulate hair regrowth by both direct collagen induction and indirect synergy with the use of topical medications.
- Dermatologists should be aware of scalp rolling as a safe, affordable, and potentially effective adjuvant to conventional therapy for AA.
Cryptococcus neoformans Panniculitis Unmasked: A Paradoxical Reaction to Therapy
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.
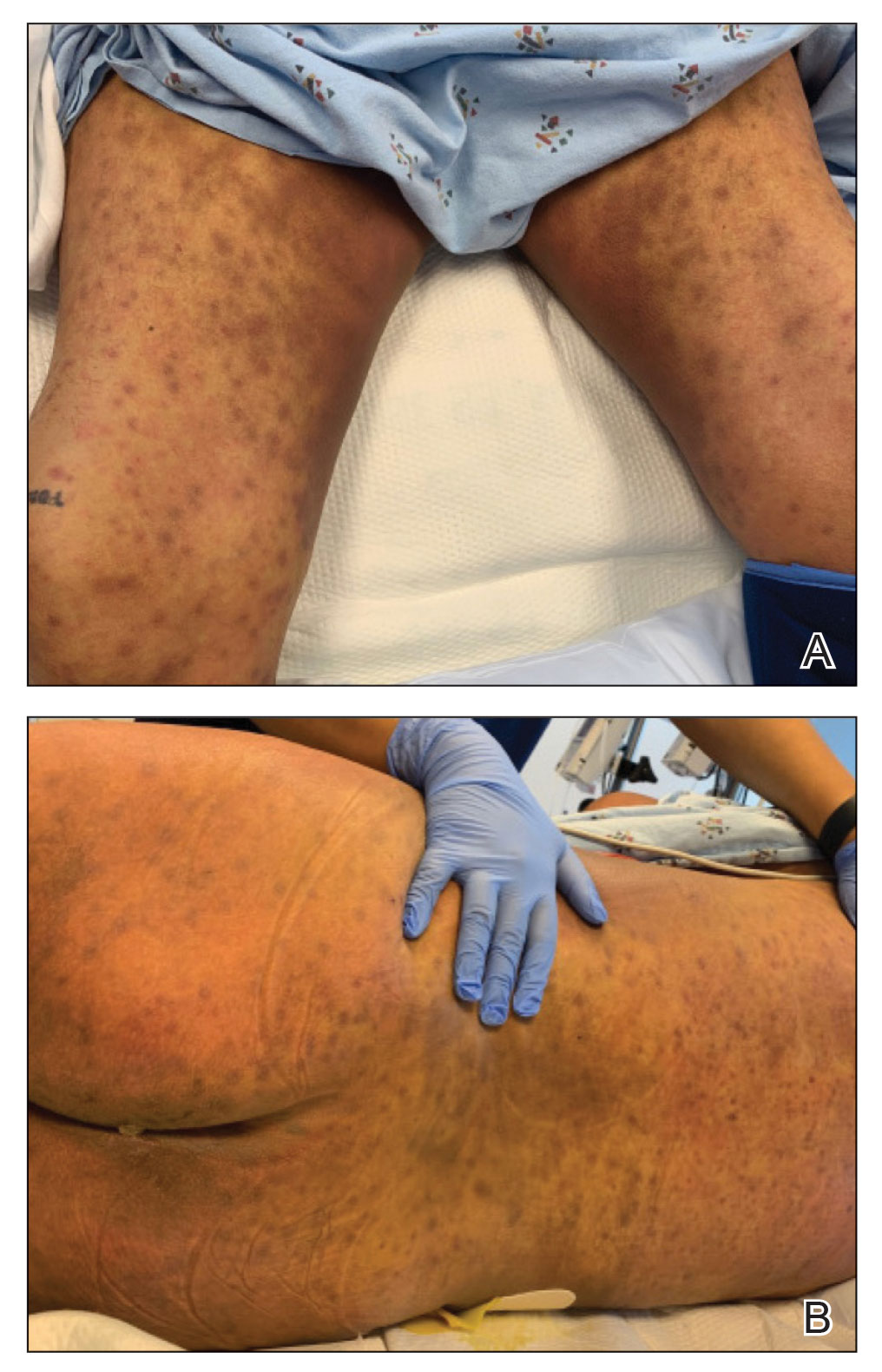
The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.
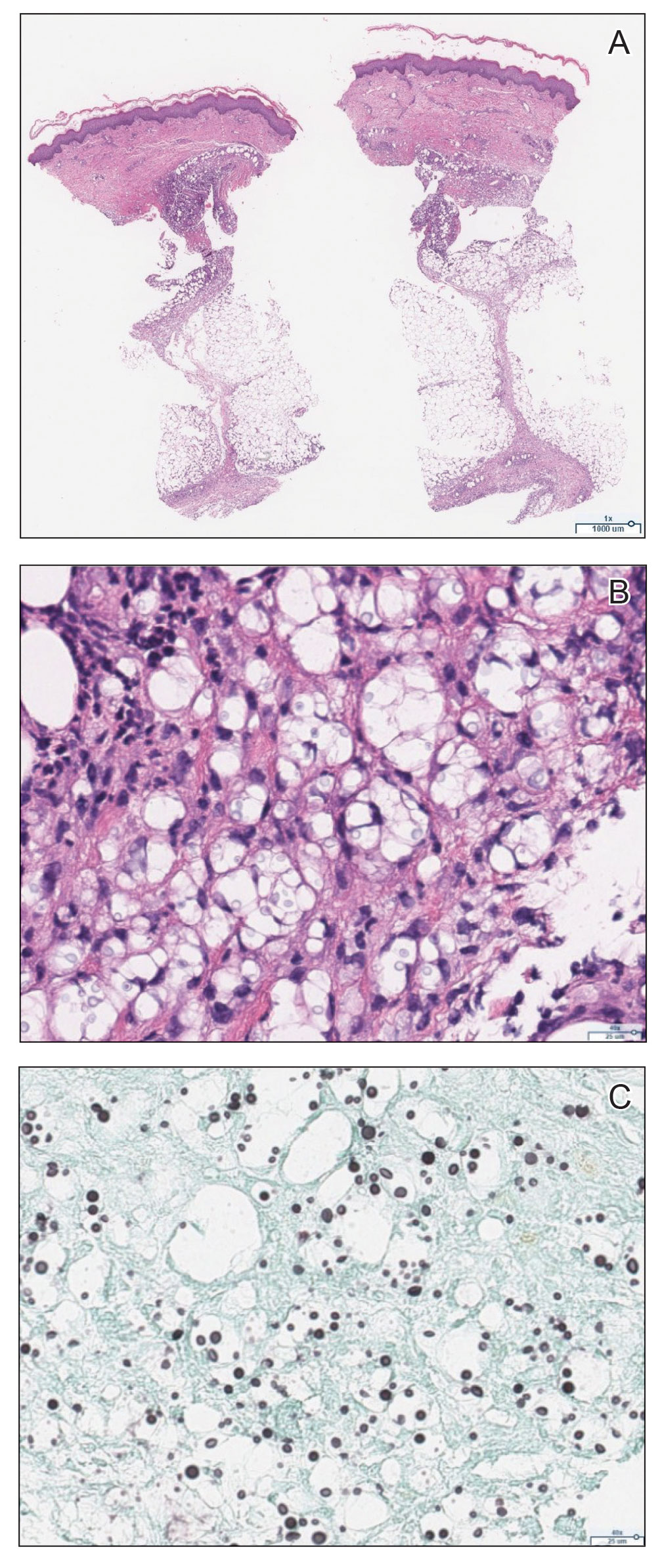
The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
Practice Points
- Panniculitis caused by Cryptococcus neoformans is a rare complication in solid organ transplant recipients.
- Subclinical panniculitis from C neoformans may be unmasked during paradoxical inflammatory reactions as early as days following immunosuppressant withdrawal and treatment initiation.
Methemoglobinemia Induced by Application of an Anesthetic Cream
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.
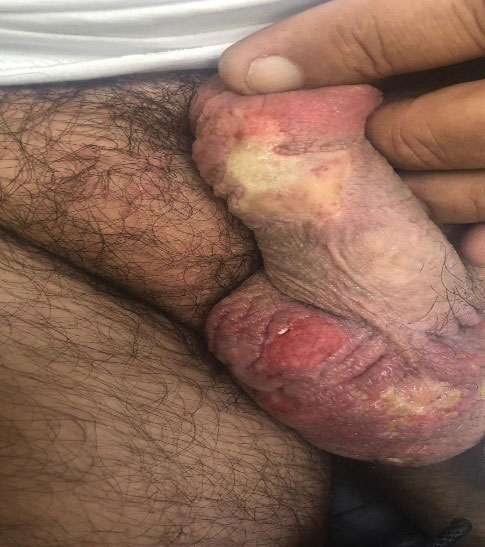
After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
Practice Points
- Consideration should be given to patients applying anesthetic creams to areas with high absorption capacity.
- Dermatologists should be aware of methemoglobinemia as a serious adverse effect of local anesthetics and guide patients accordingly, as patients do not tend to consider these products to be drugs.
Penile Herpes Vegetans in a Patient With Well-controlled HIV
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.
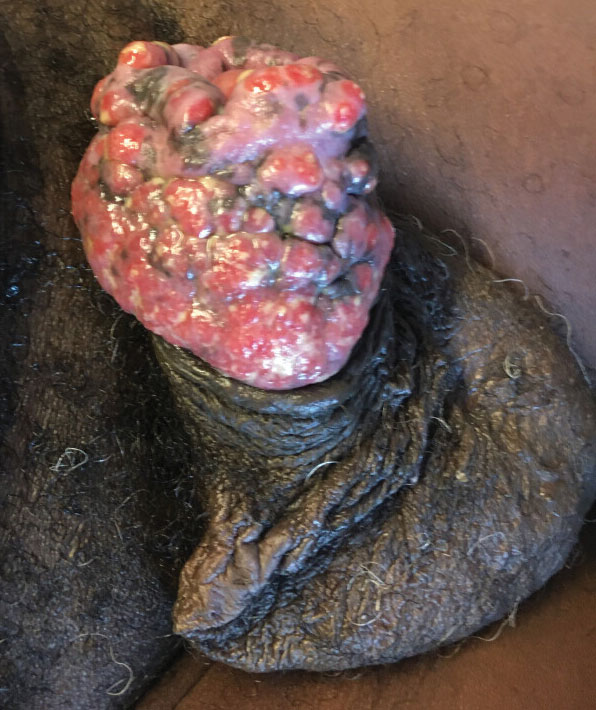
A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.
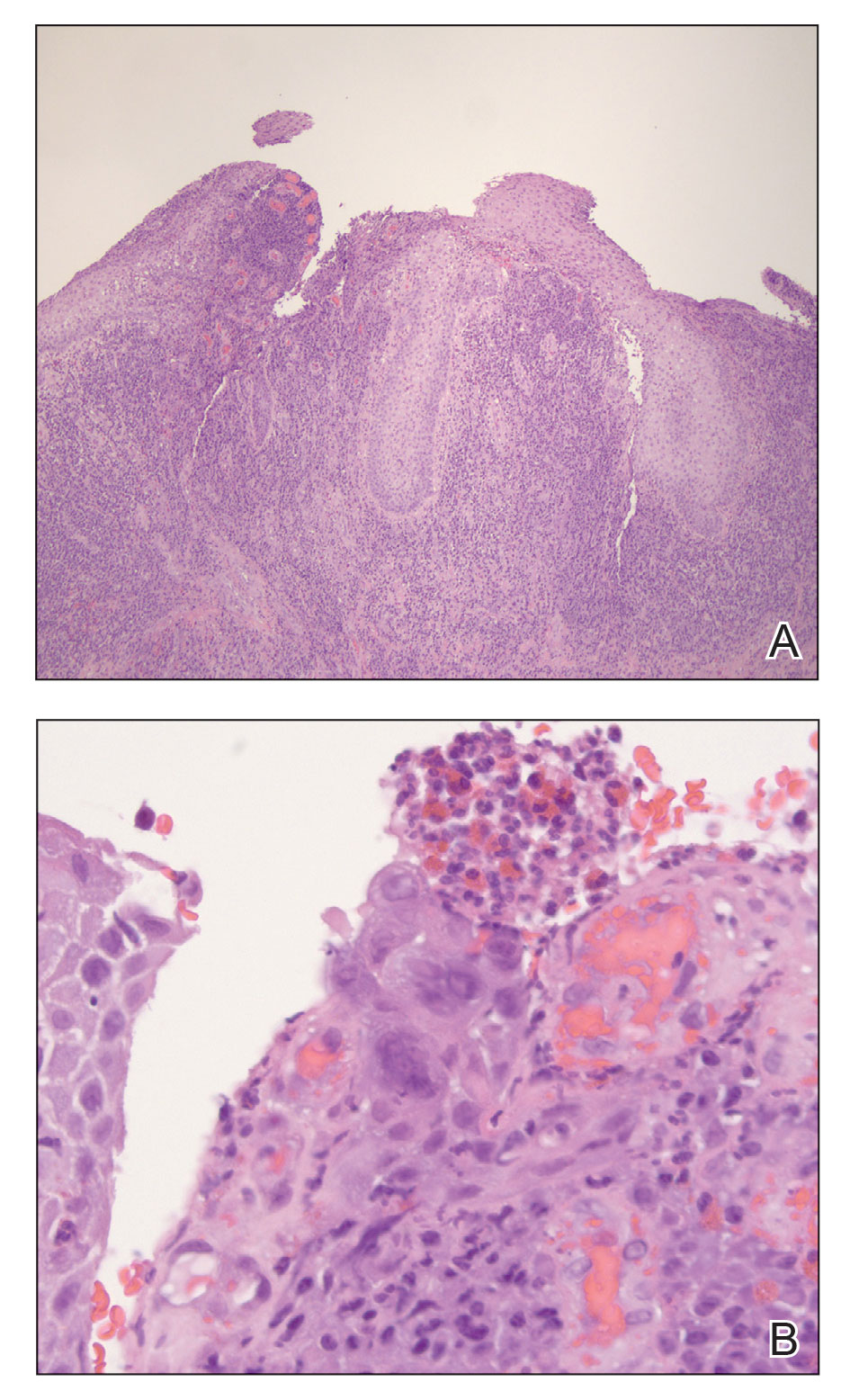
Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.
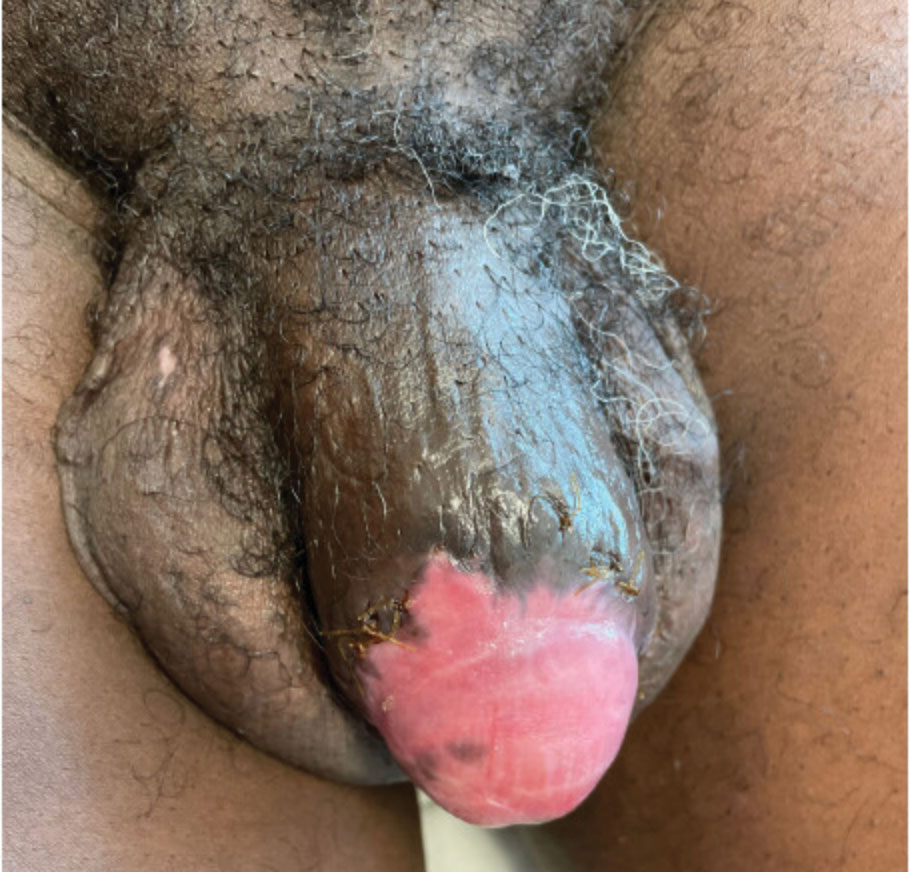
Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
Practice Points
- Maintain a high clinical suspicion for herpes vegetans (HV) in a patient who has a history of immunosuppression and presents with exophytic genital lesions.
- A history of resistance to acyclovir requires a multimodal approach to treatment of HV lesions, including medical and surgical therapies.
Treatment of an Unresectable Cutaneous Squamous Cell Carcinoma With ED&C and 5-FU
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.
Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.
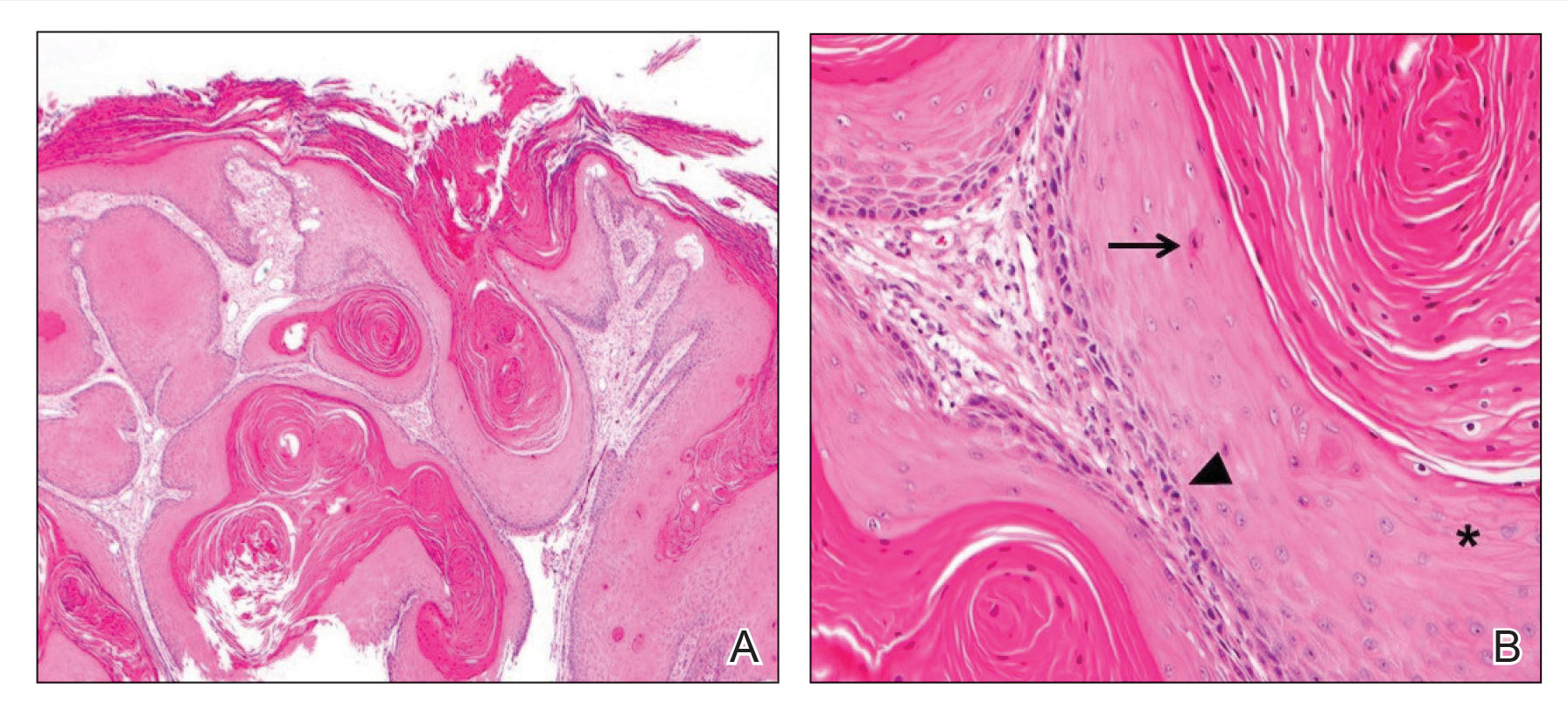
Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
Practice Points
- In a subset of cases of cutaneous squamous cell carcinoma (cSCC), the tumor is not amenable to surgical resection or other standard treatment modalities.
- Electrodesiccation and curettage followed by topical 5-fluorouracil may be an effective option in eliminating unresectable primary cSCCs that do not respond to intralesional treatment.
Porocarcinoma Development in a Prior Trauma Site
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.
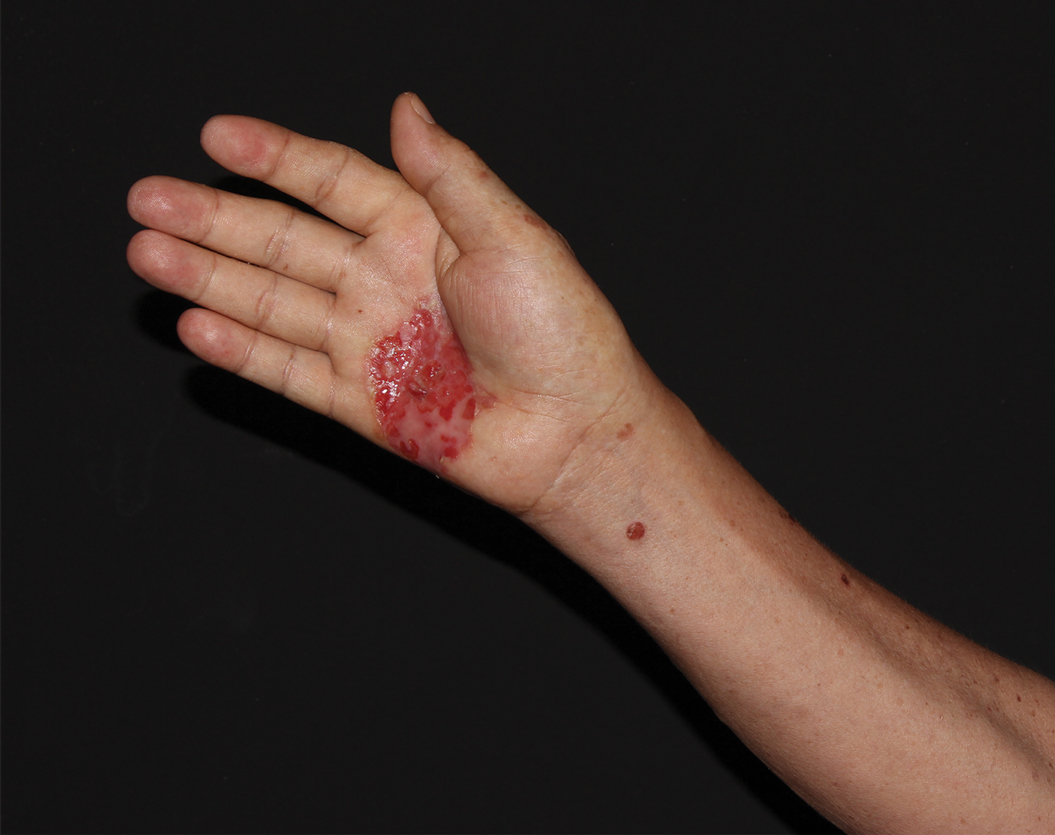
Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.
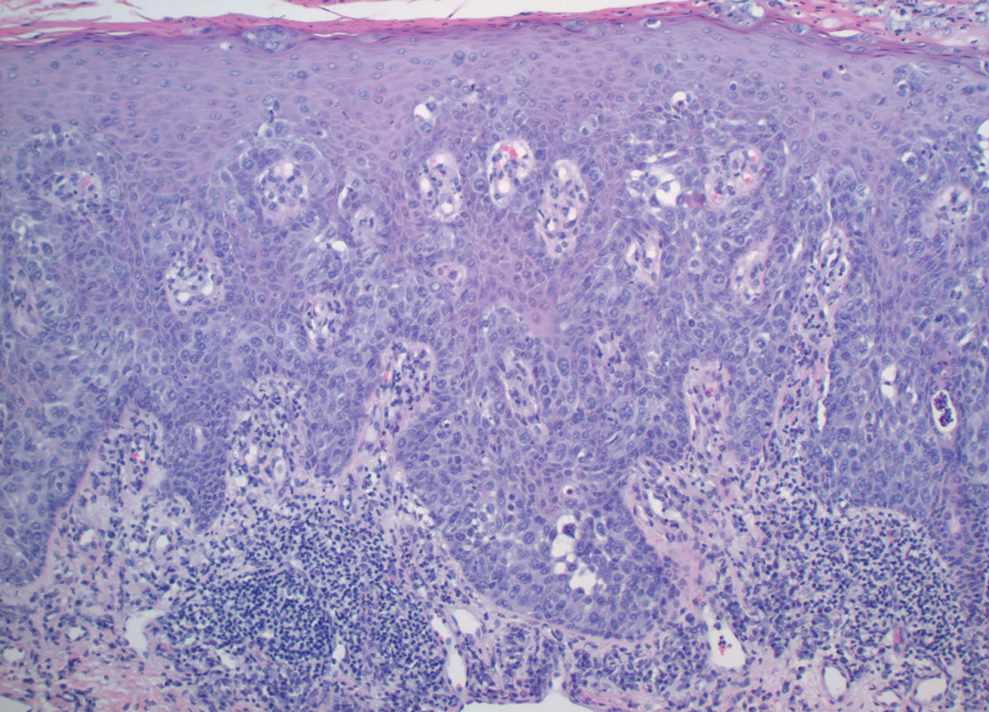
The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.
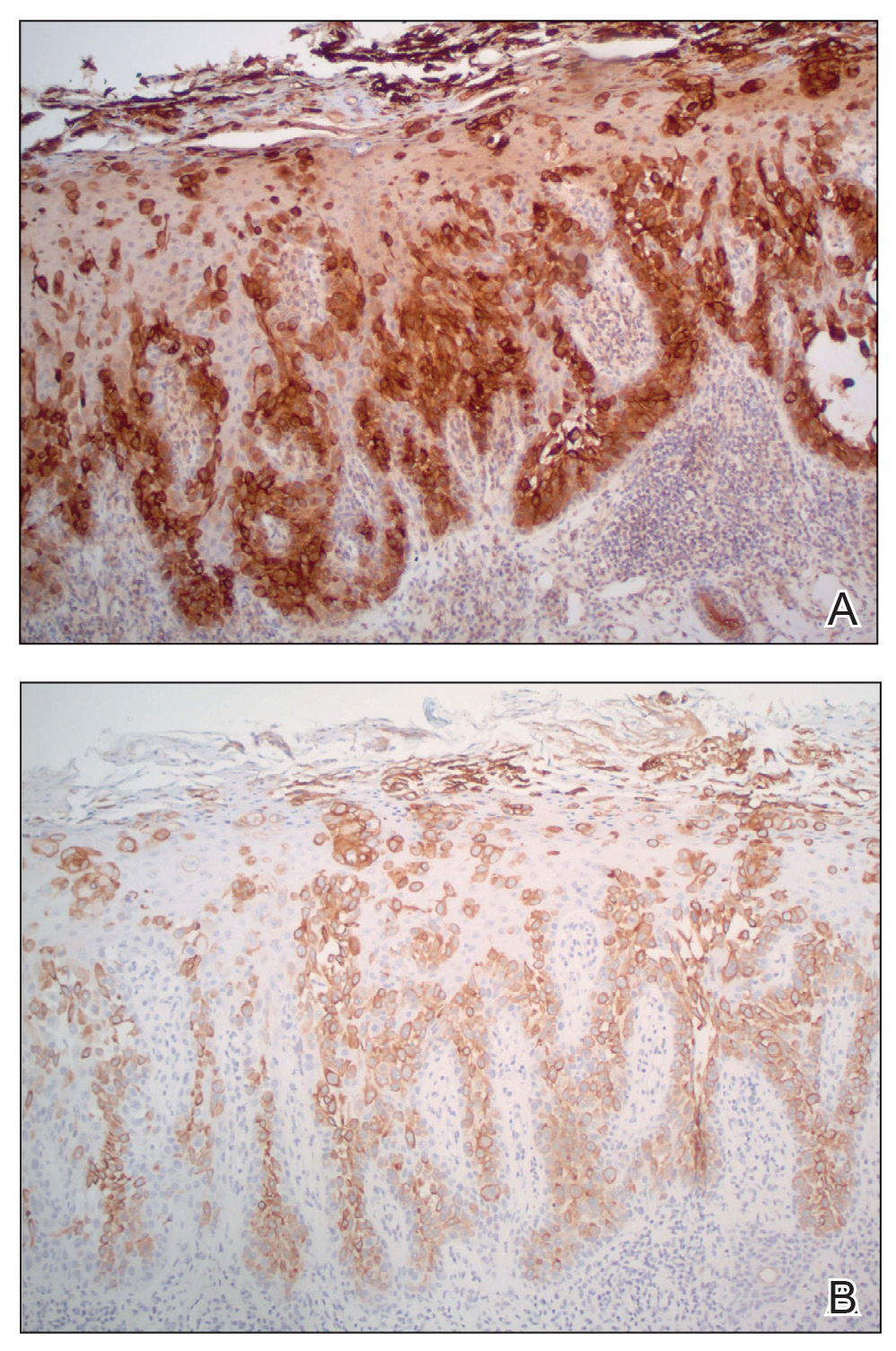
Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
Practice Points
- Porocarcinoma is a rare, potentially aggressive, glandular malignancy that should be a clinical consideration in patients presenting with a cutaneous neoplasm.
- Although wide local excision historically has been the treatment of choice for porocarcinoma, Mohs micrographic surgery has demonstrated excellent cure rates.
- Patients with unresectable or metastatic porocarcinomas have a poor prognosis but may respond to combination chemotherapy regimens.