User login
Primary Effusion Lymphoma: An Infiltrative Plaque in a Patient With HIV
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.
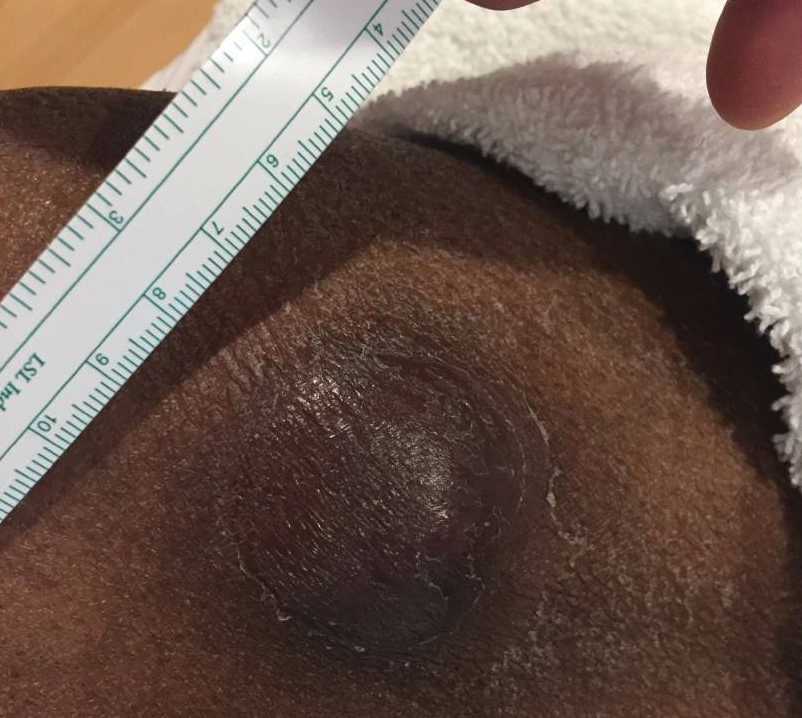
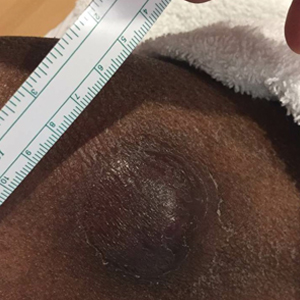
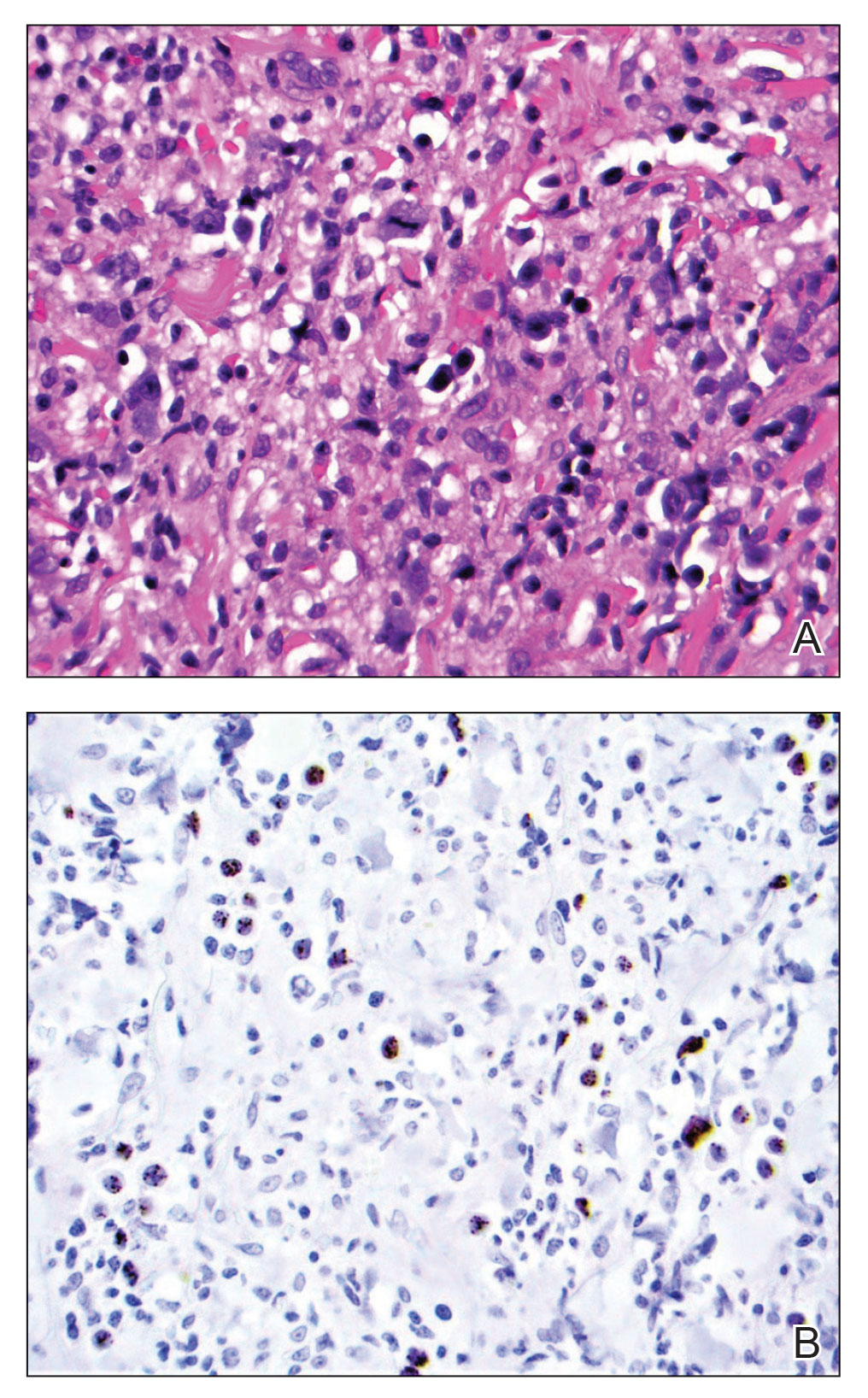
At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.
Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.
Drug-induced Linear IgA Bullous Dermatosis in a Patient With a Vancomycin-impregnated Cement Spacer
Case Report
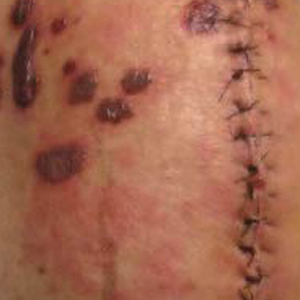
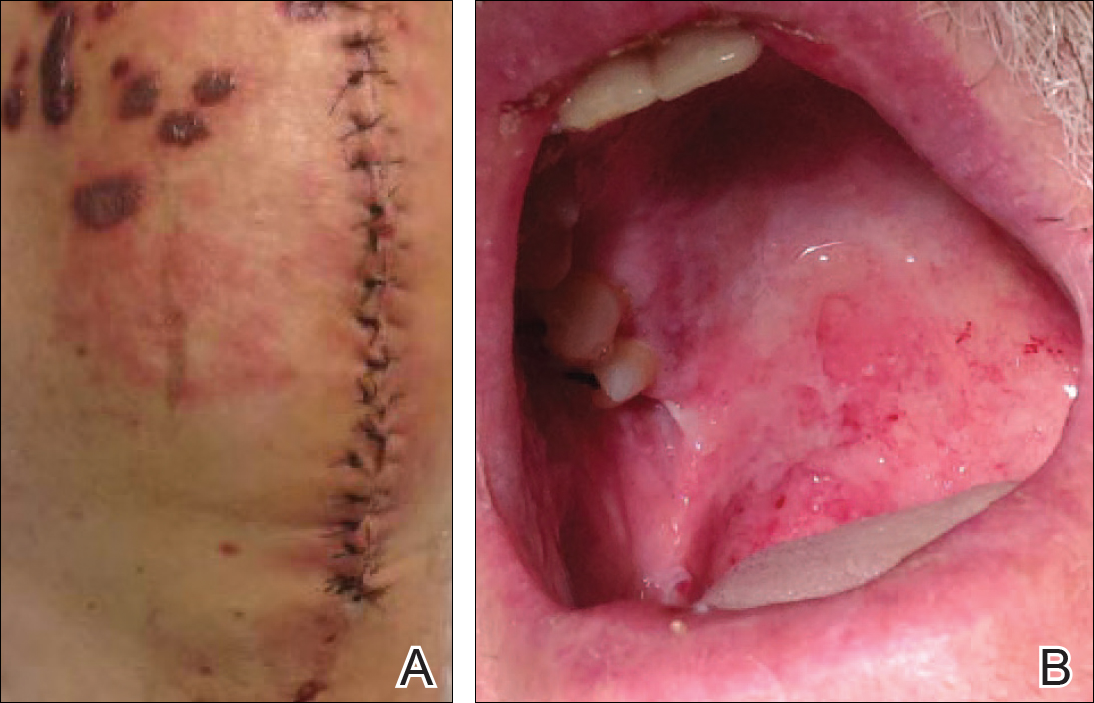
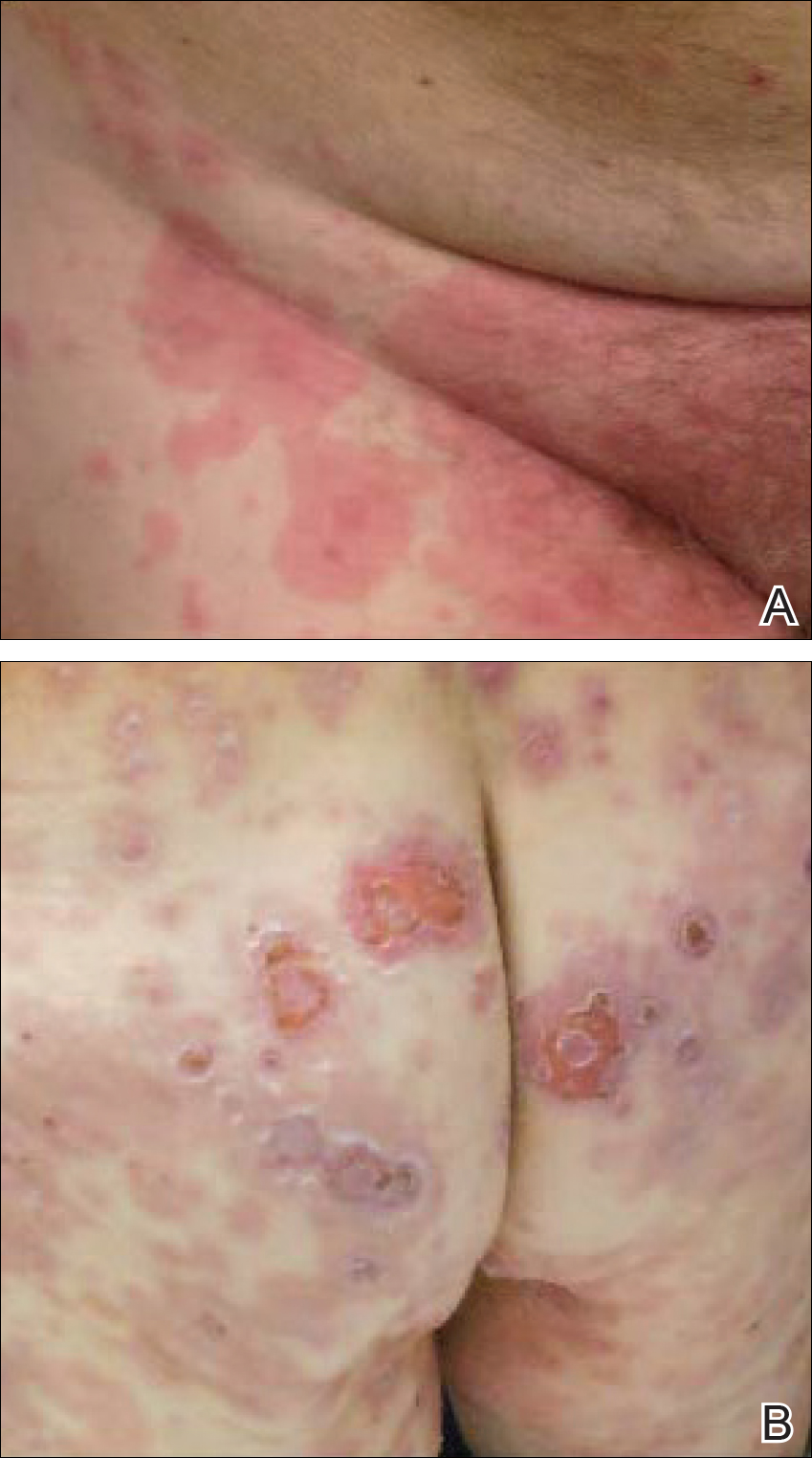
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.
Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.
Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
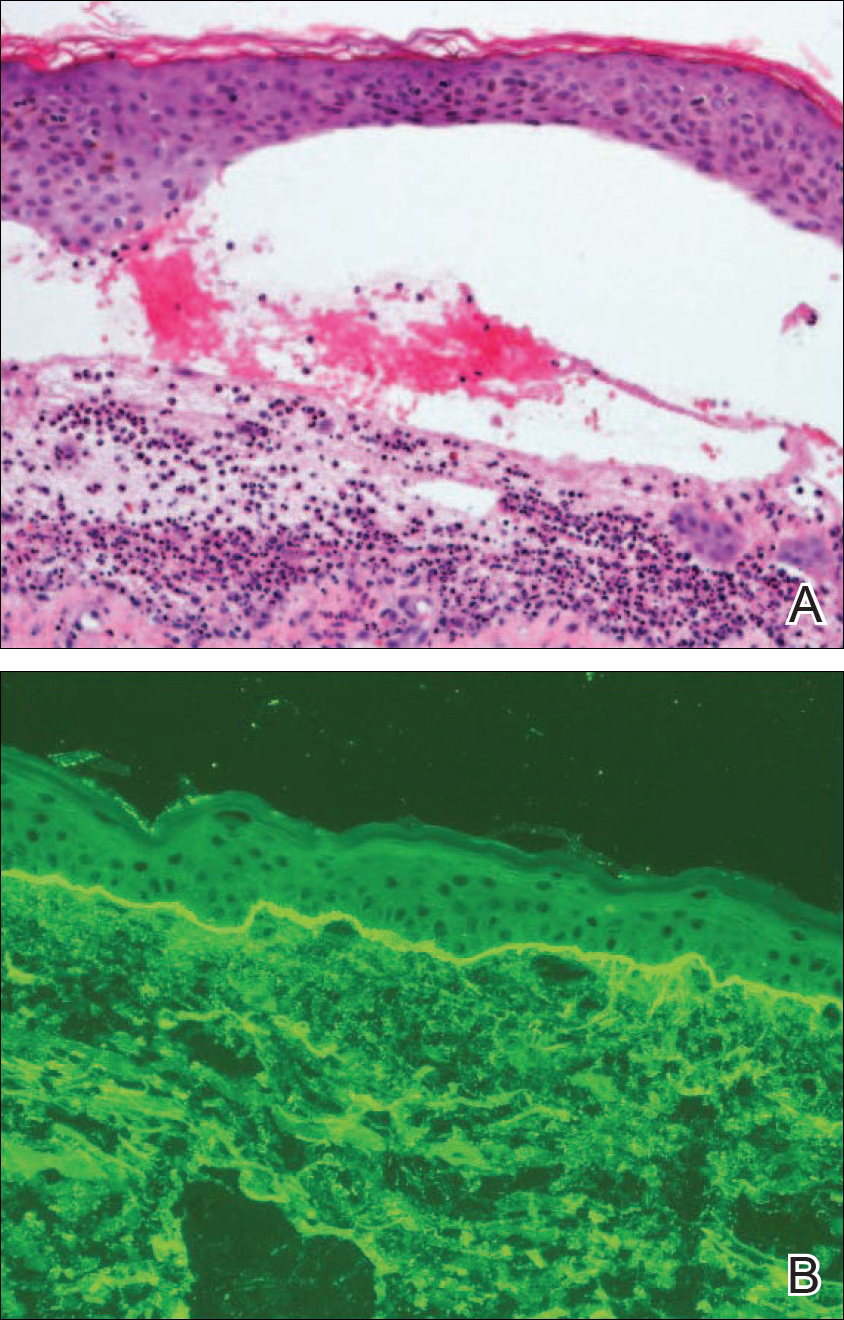
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Practice Points
- Linear IgA bullous dermatosis (LABD) is an autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction.
- A substantial number of cases of LABD are drug related, with vancomycin most commonly implicated.
- While antibiotic-impregnated cement spacers deliver high concentrations of local medications, systemic reactions are still possible.
- Dapsone is the first-line treatment for LABD.