User login
Eruptive Keratoacanthomas After Nivolumab Treatment of Stage III Melanoma
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
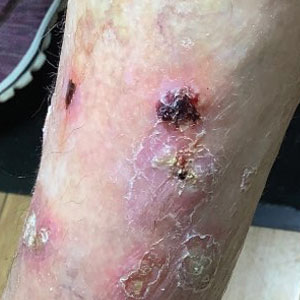
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.
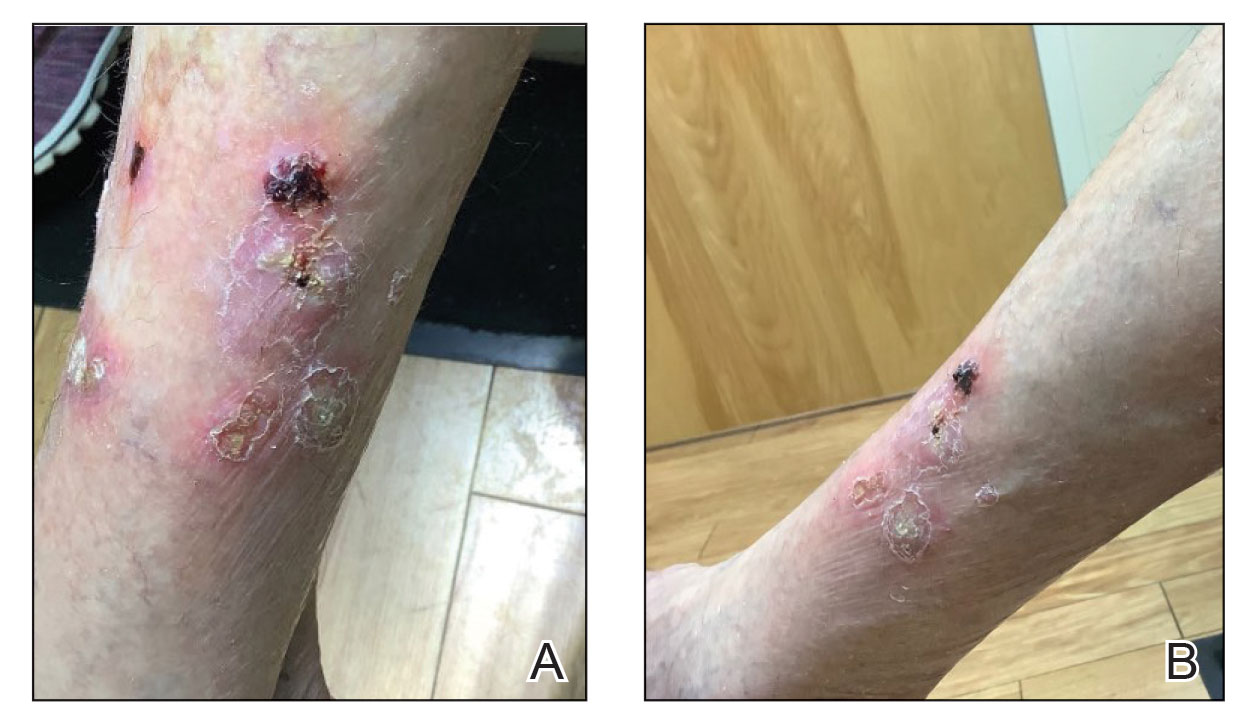
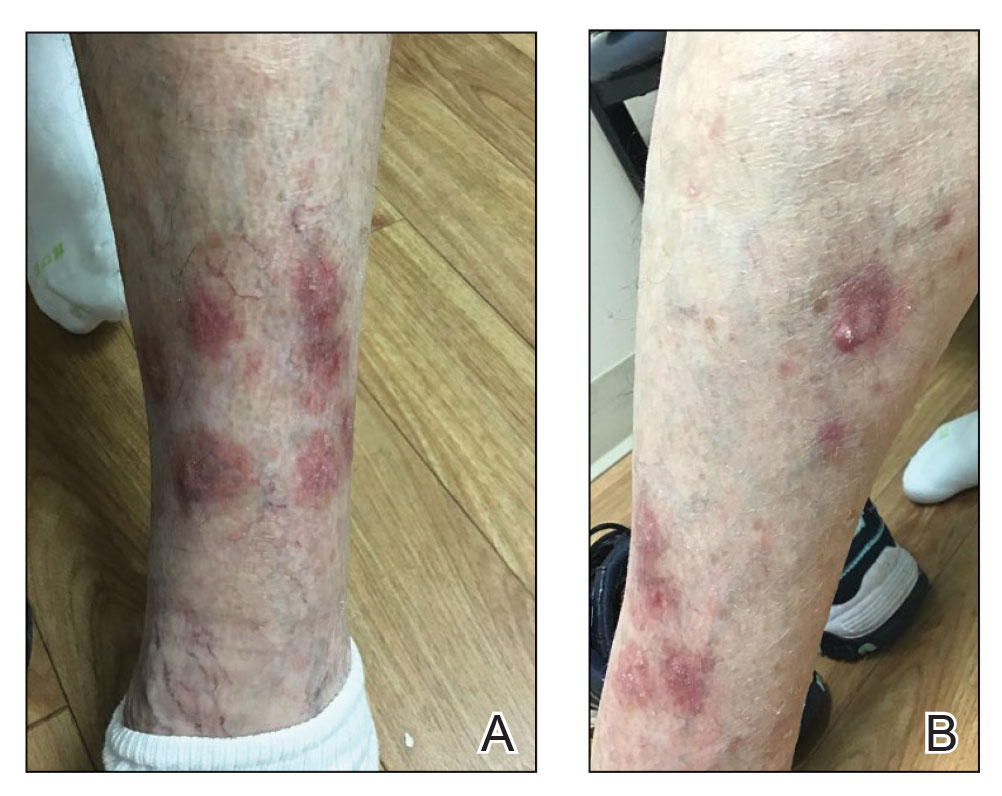
Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.
We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.

Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.

We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
To the Editor:
Programmed cell death protein 1 (PD-1) inhibitors have been widely used in the treatment of various cancers. Programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 located on cancer cells will bind to PD-1 receptors on T cells and suppress them, which will prevent cancer cell destruction. Programmed cell death protein 1 inhibitors block the binding of PD-L1 to cancer cells, which then prevents T-cell immunosuppression.1 However, cutaneous adverse effects have been associated with PD-1 inhibitors. Dermatitis associated with PD-1 inhibitor therapy occurs more frequently in patients with cutaneous tumors such as melanoma compared to those with head and neck cancers.2 Curry et al1 reported that treatment with an immune checkpoint blockade can lead to immune-related adverse effects, most commonly affecting the gastrointestinal tract, liver, and skin. The same report cited dermatologic toxicity as an adverse effect in approximately 39% of patients treated with anti–PD-1 and approximately 17% of anti–PD-L1.1 The 4 main categories of dermatologic toxicities to immunotherapies in general include inflammatory disorders, immunobullous disorders, alterations of keratinocytes, and alteration of melanocytes. The most common adverse effects from the use of the PD-1 inhibitor nivolumab were skin rashes, not otherwise specified (14%–20%), pruritus (13%–18%), and vitiligo (~8%).1 Of the cutaneous dermatitic reactions to PD-1 and PD-L1 inhibitors that were biopsied, the 2 most common were lichenoid dermatitis and spongiotic dermatitis.2 Seldomly, there have been reports of keratoacanthomas (KAs) in association with anti–PD-1 therapy.3
A KA is a common skin tumor that appears most frequently as a solitary lesion and is thought to arise from the hair follicle.4 It resembles squamous cell carcinoma and commonly regresses within months without intervention. Exposure to UV light is a known risk factor for the development of KAs.
Eruptive KAs have been found in association with 10 cases of various cancers treated with the PD-1 inhibitors pembrolizumab and nivolumab.3 Multiple lesions on photodistributed areas of the body were reported in all 10 cases. Various treatments were used in these 10 cases—doxycycline and niacinamide, electrodesiccation and curettage, clobetasol ointment and/or intralesional triamcinolone, cryotherapy, imiquimod, or no treatment—as well as the cessation of PD-1 inhibitor therapy, with 4 cases continuing therapy and 6 cases discontinuing therapy. Nine cases regressed by 6 months; electrodesiccation and curettage of the lesions was used in the tenth case.3 We report a case of eruptive KA after 1 cycle of nivolumab therapy for metastatic melanoma.
A 79-year-old woman with stage III melanoma presented to her dermatologist after developing generalized pruritic lichenoid eruptions involving the torso, arms, and legs, as well as erosions on the lips, buccal mucosa, and palate 1 month after starting nivolumab therapy. The patient initially presented to dermatology with an irregularly shaped lesion on the left upper back 3 months prior. Biopsy results at that time revealed a diagnosis of malignant melanoma, lentigo maligna type. The lesion was 1.5-mm thick and classified as Clark level IV with a mitotic count of 6 per mm2. Molecular genetic studies showed expression of PD-L1 and no expression of c-KIT. The patient underwent wide local excision, and a sentinel lymph node biopsy was positive. Positron emission tomography did not show any hypermetabolic lesions, and magnetic resonance imaging did not indicate brain metastasis. The patient underwent an axillary dissection, which did not show any residual melanoma. She was started on adjuvant immunotherapy with intravenous nivolumab 480 mg monthly and developed pruritic crusted lesions on the arms, legs, and torso 1 month later, which prompted follow-up to dermatology.
At the current presentation 4 months after the onset of lesions, physical examination revealed lichenoid patches with serous crusting that were concentrated on the torso but also affected the arms and legs. She developed erosions on the upper and lower lips, buccal mucosa, and hard and soft palates, as well as painful, erythematous, dome-shaped papules and nodules on the legs (Figure 1). Her oncologist previously had initiated treatment at the onset of the lesions with clobetasol cream and valacyclovir for the lesions, but the patient showed no improvement.

Four months after the onset of the lesions, the patient was re-referred to her dermatologist, and a biopsy was performed on the left lower leg that showed squamous cell carcinoma, KA type. Additionally, flat erythematous patches were seen on the legs that were consistent with a lichenoid drug eruption. Two weeks later, she was started on halobetasol propionate ointment 0.05% for treatment of the KAs. At 2-week follow-up, 5 months after the onset of the lesions, the patient showed no signs of improvement. An oral prednisone taper of 60 mg for 3 days, 40 mg for 3 days, and then 20 mg daily for a total of 4 weeks was started to treat the lichenoid dermatitis and eruptive KAs. At the next follow-up 6.5 months following the first eruptive KAs, she was no longer using topical or oral steroids, she did not have any new eruptive KAs, and old lesions showed regression (Figure 2). The patient still experienced postinflammatory erythema and hyperpigmentation at the location of the KAs but showed improvement of the lichenoid drug eruption.

We describe a case of eruptive KAs after use of a PD-1 inhibitor for treatment of melanoma. Our patient developed eruptive KAs after only 1 nivolumab treatment. Another report described onset of eruptive KAs after 1 month of nivolumab infusions.3 The KAs experienced by our patient took 6.5 months to regress, which is unusual compared to other case reports in which the KAs self-resolved within a few months, though one other case described lesions that persisted for 6 months.3
Our patient was treated with topical steroids and an oral steroid taper for the concomitant lichenoid drug eruption. It is unknown if the steroids affected the course of the KAs or if they spontaneously regressed on their own. Freites-Martinez et al5 described that regression of KAs may be related to an immune response, but corticosteroids are inherently immunosuppressive. They hypothesized that corticosteroids help to temper the heightened immune response of eruptive KAs.5
Our patient had oral ulcers, which may have been indicative of an oral lichenoid drug eruption, as well as skin lesions representative of a cutaneous lichenoid drug eruption. This is a favorable reaction, as lichenoid dermatitis is thought to represent successful PD-1 inhibition and therefore a better response to oncologic therapies.2 Comorbid lichenoid drug eruption lesions and eruptive KAs may be suggestive of increased T-cell activity,2,6,7 though some prior case studies have reported eruptive KAs in isolation.3
Discontinuation of immunotherapy due to development of eruptive KAs presents a challenge in the treatment of underlying malignancies such as melanoma. Immunotherapy was discontinued in 7 of 11 cases due to these cutaneous reactions.3 Similarly, our patient underwent only 1 cycle of immunotherapy before developing eruptive KAs and discontinuing PD-1 inhibitor therapy. If we are better able to treat eruptive KAs, then patients can remain on immunotherapy to treat underlying malignancies. Crow et al8 showed improvement in lesions when 3 patients with eruptive KAs were treated with hydroxychloroquine; the Goeckerman regimen consisting of steroids, UVB phototherapy, and crude coal tar; and Unna boots with zinc oxide and compression stockings. The above may be added to a list of possible treatments to consider for hastening the regression of eruptive KAs.
Our patient’s clinical course was similar to reports on the regressive nature of eruptive KAs within 6 months after initial eruption. Although it is likely that KAs will regress on their own, treatment modalities that speed up recovery are a future source for research.
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176.
- Min Lee CK, Li S, Tran DC, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047-1052. doi:10.1016/j.jaad.2018.05.035
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Bednarek R, Marks K, Lin G. Eruptive keratoacanthomas secondary to nivolumab immunotherapy. Int J Dermatol. 2018;57:E28-E29.
- Feldstein SI, Patel F, Kim E, et al. Eruptive keratoacanthomas arising in the setting of lichenoid toxicity after programmed cell death 1 inhibition with nivolumab. J Eur Acad Dermatol Venereol. 2018;32:E58-E59.
- Crow LD, Perkins I, Twigg AR, et al. Treatment of PD-1/PD-L1 inhibitor-induced dermatitis resolves concomitant eruptive keratoacanthomas. JAMA Dermatol. 2020;156:598-600. doi:10.1001/jamadermatol.2020.0176
Practice Points
- Eruptive keratoacanthomas (KAs) are a rare buttransient adverse effect of programmed cell death protein 1 (PD-1) inhibitor therapy.
- Nivolumab, a human monoclonal IgG4 antibody, is used as an antitumor treatment for melanoma by blocking PD-1.
- Possible new treatments may hasten the regression of eruptive KAs, which could allow patients to continue PD-1 inhibitor therapy.