User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Klotho allele lowers APOE4-associated risk of Alzheimer’s
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
FROM JAMA NEUROLOGY
Doing things right vs. doing the right things
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.) 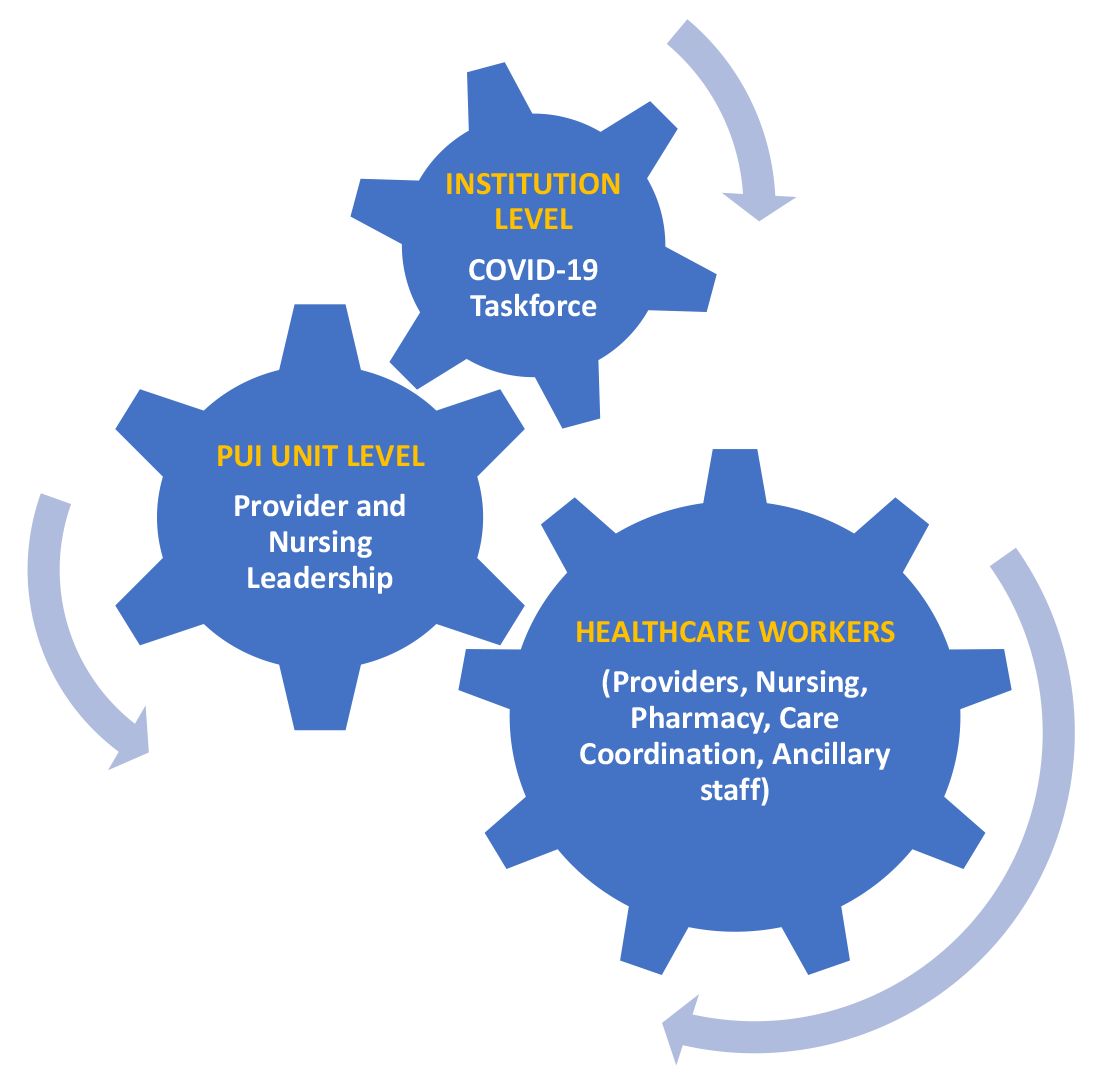
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara (psunkara@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert (wlippert@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris (chrmorri@wakehealth.edu) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang (chuang@wakehealth.edu) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A framework for a COVID-19 Person Under Investigation unit
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.) 
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara (psunkara@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert (wlippert@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris (chrmorri@wakehealth.edu) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang (chuang@wakehealth.edu) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.) 
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara (psunkara@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert (wlippert@wakehealth.edu) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris (chrmorri@wakehealth.edu) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang (chuang@wakehealth.edu) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
Coronavirus tests are being fast-tracked by the FDA, but it’s unclear how accurate they are
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Pandemic necessitates new strategies to treat migraine
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
FROM HEADACHE
‘We’re in great distress here,’ infusion center CMO says
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Infusion center directors shuffle treatment services in the era of COVID-19
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
CDC issues new return-to-work guidelines
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
The 7 strategies of highly effective people facing the COVID-19 pandemic
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
American Lung Association announces $25 million initiative to end COVID-19
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.