User login
Biotin interference a concern in hormonal assays
Biotin supplementation showed signs of interference with biotinylated assays in a crossover trial.
The proposed benefits of biotin (vitamin B7), including stimulating hair growth and assisting in the treatment of various forms of diabetes, have made it a popular supplement. Supplementation generally leads to artificially high levels of biotin, which was shown to cause inaccurate results in biotinylated assays, according to Danni Li, PhD, of the advanced research and diagnostic laboratory at the University of Minnesota, Minneapolis, and her colleagues.
They analyzed the results of both biotinylated and nonbiotinylated assays of six patients – two women and four men – who ingested 10 mg/day of biotin supplement for 7 days. Two blood samples were obtained in the course of the study: one prior to starting biotin supplementation as a baseline and one a week ofter biotin supplementation had ended. The assays tested the presence of nine hormones and two nonhormones using multiple diagnostic systems to run the assays. In total, 37 immunoassays were conducted on each sample (JAMA. 2017;318[12]:1150-60. doi: 10.1001/jama.2017.13705).
Two immunoassay testing techniques were used in the diagnostic assays. The sandwich technique was used in testing TSH, parathyroid hormone (PTH), prolactin, N-terminal pro-brain natriuretic peptide (NT-proBNP), PSA, and ferritin and competitive technique was used in testing total T4, total T3, free T4, free T3, and 25-OHD. In assays utilizing competitive techniques, false highs were reported in three Roche cobas e602 machines and one Siemens Vista Dimension 1500 machine.
Assays utilizing the sandwich technique experienced false decreases in TSH, PTH, and NT-proBNP when compared with baseline measurements in Roche cobas e602 and OCD Vitros 5600 machines. A predominance of the falsely low results were present in the assays conducted by the OCD Vitros machine, with significant changes from baseline measurements. TSH experienced a 94% decrease of 1.67 mIU/L, PTH experienced a 61% decrease of 25.8 pg/mL, and NT-proBNP falsely decreased by more than 13.9 pg/mL. In Roche cobas e602 assays, TSH levels were falsely low and measurements decreased by 0.72 mIU/L (37%) when compared with baseline measurements.
Biotin did not interfere in all biotinylated assays and was only observed in 9 of the 23 (39%) of the assays conducted. Specifically, 4 of 15 (27%) sandwich immunoassays were falsely decreased while five of eight (63%) competitive binding assays were falsely increased.
“Among the 23 biotinylated assays studied, biotin interference was of greatest clinical significance in the OCD Vitros TSH assay, where falsely decreased TSH concentrations (to less than 0.15 mU/L) could have resulted in misdiagnosis of thyrotoxicosis in otherwise euthyroid individuals,” according to Dr. Li and her associates, “Likewise, falsely decreased OCD Vitros NT-proBNP, to lower than assay detection limits, could possibly result in failure to identify congestive heart failure.”
One investigator received funding and nonfinanical support from Siemens Healthcare Diagnostics, one reported receiving financial support from Abbott Laboratories, and another reported receiving personal fees from Roche and Abbott Laboratories. Dr. Li and the other researchers had no relevant financial disclosures.
Biotin supplementation showed signs of interference with biotinylated assays in a crossover trial.
The proposed benefits of biotin (vitamin B7), including stimulating hair growth and assisting in the treatment of various forms of diabetes, have made it a popular supplement. Supplementation generally leads to artificially high levels of biotin, which was shown to cause inaccurate results in biotinylated assays, according to Danni Li, PhD, of the advanced research and diagnostic laboratory at the University of Minnesota, Minneapolis, and her colleagues.
They analyzed the results of both biotinylated and nonbiotinylated assays of six patients – two women and four men – who ingested 10 mg/day of biotin supplement for 7 days. Two blood samples were obtained in the course of the study: one prior to starting biotin supplementation as a baseline and one a week ofter biotin supplementation had ended. The assays tested the presence of nine hormones and two nonhormones using multiple diagnostic systems to run the assays. In total, 37 immunoassays were conducted on each sample (JAMA. 2017;318[12]:1150-60. doi: 10.1001/jama.2017.13705).
Two immunoassay testing techniques were used in the diagnostic assays. The sandwich technique was used in testing TSH, parathyroid hormone (PTH), prolactin, N-terminal pro-brain natriuretic peptide (NT-proBNP), PSA, and ferritin and competitive technique was used in testing total T4, total T3, free T4, free T3, and 25-OHD. In assays utilizing competitive techniques, false highs were reported in three Roche cobas e602 machines and one Siemens Vista Dimension 1500 machine.
Assays utilizing the sandwich technique experienced false decreases in TSH, PTH, and NT-proBNP when compared with baseline measurements in Roche cobas e602 and OCD Vitros 5600 machines. A predominance of the falsely low results were present in the assays conducted by the OCD Vitros machine, with significant changes from baseline measurements. TSH experienced a 94% decrease of 1.67 mIU/L, PTH experienced a 61% decrease of 25.8 pg/mL, and NT-proBNP falsely decreased by more than 13.9 pg/mL. In Roche cobas e602 assays, TSH levels were falsely low and measurements decreased by 0.72 mIU/L (37%) when compared with baseline measurements.
Biotin did not interfere in all biotinylated assays and was only observed in 9 of the 23 (39%) of the assays conducted. Specifically, 4 of 15 (27%) sandwich immunoassays were falsely decreased while five of eight (63%) competitive binding assays were falsely increased.
“Among the 23 biotinylated assays studied, biotin interference was of greatest clinical significance in the OCD Vitros TSH assay, where falsely decreased TSH concentrations (to less than 0.15 mU/L) could have resulted in misdiagnosis of thyrotoxicosis in otherwise euthyroid individuals,” according to Dr. Li and her associates, “Likewise, falsely decreased OCD Vitros NT-proBNP, to lower than assay detection limits, could possibly result in failure to identify congestive heart failure.”
One investigator received funding and nonfinanical support from Siemens Healthcare Diagnostics, one reported receiving financial support from Abbott Laboratories, and another reported receiving personal fees from Roche and Abbott Laboratories. Dr. Li and the other researchers had no relevant financial disclosures.
Biotin supplementation showed signs of interference with biotinylated assays in a crossover trial.
The proposed benefits of biotin (vitamin B7), including stimulating hair growth and assisting in the treatment of various forms of diabetes, have made it a popular supplement. Supplementation generally leads to artificially high levels of biotin, which was shown to cause inaccurate results in biotinylated assays, according to Danni Li, PhD, of the advanced research and diagnostic laboratory at the University of Minnesota, Minneapolis, and her colleagues.
They analyzed the results of both biotinylated and nonbiotinylated assays of six patients – two women and four men – who ingested 10 mg/day of biotin supplement for 7 days. Two blood samples were obtained in the course of the study: one prior to starting biotin supplementation as a baseline and one a week ofter biotin supplementation had ended. The assays tested the presence of nine hormones and two nonhormones using multiple diagnostic systems to run the assays. In total, 37 immunoassays were conducted on each sample (JAMA. 2017;318[12]:1150-60. doi: 10.1001/jama.2017.13705).
Two immunoassay testing techniques were used in the diagnostic assays. The sandwich technique was used in testing TSH, parathyroid hormone (PTH), prolactin, N-terminal pro-brain natriuretic peptide (NT-proBNP), PSA, and ferritin and competitive technique was used in testing total T4, total T3, free T4, free T3, and 25-OHD. In assays utilizing competitive techniques, false highs were reported in three Roche cobas e602 machines and one Siemens Vista Dimension 1500 machine.
Assays utilizing the sandwich technique experienced false decreases in TSH, PTH, and NT-proBNP when compared with baseline measurements in Roche cobas e602 and OCD Vitros 5600 machines. A predominance of the falsely low results were present in the assays conducted by the OCD Vitros machine, with significant changes from baseline measurements. TSH experienced a 94% decrease of 1.67 mIU/L, PTH experienced a 61% decrease of 25.8 pg/mL, and NT-proBNP falsely decreased by more than 13.9 pg/mL. In Roche cobas e602 assays, TSH levels were falsely low and measurements decreased by 0.72 mIU/L (37%) when compared with baseline measurements.
Biotin did not interfere in all biotinylated assays and was only observed in 9 of the 23 (39%) of the assays conducted. Specifically, 4 of 15 (27%) sandwich immunoassays were falsely decreased while five of eight (63%) competitive binding assays were falsely increased.
“Among the 23 biotinylated assays studied, biotin interference was of greatest clinical significance in the OCD Vitros TSH assay, where falsely decreased TSH concentrations (to less than 0.15 mU/L) could have resulted in misdiagnosis of thyrotoxicosis in otherwise euthyroid individuals,” according to Dr. Li and her associates, “Likewise, falsely decreased OCD Vitros NT-proBNP, to lower than assay detection limits, could possibly result in failure to identify congestive heart failure.”
One investigator received funding and nonfinanical support from Siemens Healthcare Diagnostics, one reported receiving financial support from Abbott Laboratories, and another reported receiving personal fees from Roche and Abbott Laboratories. Dr. Li and the other researchers had no relevant financial disclosures.
FROM JAMA
Key clinical point:
Major finding: 9 of 23 biotinylated (39%) showed signs of interference from biotin ingestion.
Data source: Nonrandomized crossover trial of six participants at an academic medical center.
Disclosures: One investigator received funding and nonfinanical support from Siemens Healthcare Diagnostics, one reported receiving financial support from Abbott Laboratories, and another reported receiving personal fees from Roche and Abbott Laboratories. Dr. Li and the other researchers had no relevant financial disclosures.
It’s a beautiful day to discuss inflammatory bowel disease
Uma Mahadevan, MD, AGAF, and I moderated this session on IBD, and we were fortunate enough to secure four of the best IBD educators in the AGA.
David Rubin, MD, AGAF, opened with “Selecting the correct therapy for your outpatients with IBD: From mesalamine to biologics.” Treatment goals have evolved from symptom control to remission based on measures of inflammation (e.g., serum C-reactive protein, fecal calprotectin, or endoscopy). For ulcerative colitis (UC), high-risk markers include extensive disease, deep ulcers, younger age at diagnosis, elevated biomarkers, and early need for steroids or hospitalization. For Crohn’s disease (CD), these include younger age, extensive involvement, and fistulizing disease. The 5-aminosalicylate drugs remain a backbone in mild to moderate UC. Judicious use of corticosteroids is reasonable, but we need an exit strategy. The thiopurines are decent drugs, but studies have called into question their efficacy as monotherapy, and safety issues persist. Methotrexate is underutilized. The anti–tumor necrosis factor (TNF) biologics are excellent therapies but controversies persist as to whether these drugs require combination therapy or if they can be managed as “optimized monotherapy” with therapeutic drug monitoring (TDM). There are now two infliximab biosimilars available in the U.S.. Vedolizumab is an efficacious gut-selective anti-integrin (for both CD and UC). Ustekinumab, an anti-IL-12/23 antibody, is now available for moderate to severe CD, and has a favorable safety profile.
Fernando Velayos, MD, AGAF, discussed “Surveillance for dysplasia: What is the standard of care in 2017?” General principles for surveillance colonoscopy in IBD include having quiescent disease, since inflammation can reduce ability to detect lesions, and good colonic preparation. The three U.S. society guidelines recommend starting surveillance after 8 years of disease. Patients with concomitant primary schlerosing cholangitis should begin surveillance immediately. Frequency of surveillance ranges every 1-3 years depending on histology. A meta-analysis showed a higher incremental dysplasia yield with chromoendoscopy compared to standard white-light colonoscopy. If visible dysplasia can be endoscopically resected, then continued surveillance rather than colectomy is recommended.
Sunanda Kane, MD, AGAF, discussed “Managing special populations: the transitioning adolescent, the gravid, and the elderly.” The transition from pediatric to adult IBD care is a high-risk time because the patient may be lost to follow-up or not adhere to the medical regimen, resulting in increased risk of flare. Successful transition requires developmental maturity of the patient, a certain style of parental involvement, and care coordination of the medical team. For women with IBD considering pregnancy, active IBD at the time of conception significantly increases the risk of flare. Women with CD who have no history of perianal disease don’t have an increased risk of perianal disease with vaginal delivery. A meta-analysis of the risk of congenital malformations with thiopurines found no significant association. Infliximab levels were likely to rise in the mother during the second and third trimesters (versus no increase with adalimumab), so one could consider TDM to guide dosing. In the PIANO study, anti-TNF therapy in the third trimester was neither associated with adverse pregnancy outcomes nor with infections up to 1 year for children. Patients who develop IBD later in life are more likely to have colonic inflammation. Elderly UC patients are more likely to require surgery, and postop mortality is higher for both CD and UC.
This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017. Dr. Loftus is a professor of medicine, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn.
Uma Mahadevan, MD, AGAF, and I moderated this session on IBD, and we were fortunate enough to secure four of the best IBD educators in the AGA.
David Rubin, MD, AGAF, opened with “Selecting the correct therapy for your outpatients with IBD: From mesalamine to biologics.” Treatment goals have evolved from symptom control to remission based on measures of inflammation (e.g., serum C-reactive protein, fecal calprotectin, or endoscopy). For ulcerative colitis (UC), high-risk markers include extensive disease, deep ulcers, younger age at diagnosis, elevated biomarkers, and early need for steroids or hospitalization. For Crohn’s disease (CD), these include younger age, extensive involvement, and fistulizing disease. The 5-aminosalicylate drugs remain a backbone in mild to moderate UC. Judicious use of corticosteroids is reasonable, but we need an exit strategy. The thiopurines are decent drugs, but studies have called into question their efficacy as monotherapy, and safety issues persist. Methotrexate is underutilized. The anti–tumor necrosis factor (TNF) biologics are excellent therapies but controversies persist as to whether these drugs require combination therapy or if they can be managed as “optimized monotherapy” with therapeutic drug monitoring (TDM). There are now two infliximab biosimilars available in the U.S.. Vedolizumab is an efficacious gut-selective anti-integrin (for both CD and UC). Ustekinumab, an anti-IL-12/23 antibody, is now available for moderate to severe CD, and has a favorable safety profile.
Fernando Velayos, MD, AGAF, discussed “Surveillance for dysplasia: What is the standard of care in 2017?” General principles for surveillance colonoscopy in IBD include having quiescent disease, since inflammation can reduce ability to detect lesions, and good colonic preparation. The three U.S. society guidelines recommend starting surveillance after 8 years of disease. Patients with concomitant primary schlerosing cholangitis should begin surveillance immediately. Frequency of surveillance ranges every 1-3 years depending on histology. A meta-analysis showed a higher incremental dysplasia yield with chromoendoscopy compared to standard white-light colonoscopy. If visible dysplasia can be endoscopically resected, then continued surveillance rather than colectomy is recommended.
Sunanda Kane, MD, AGAF, discussed “Managing special populations: the transitioning adolescent, the gravid, and the elderly.” The transition from pediatric to adult IBD care is a high-risk time because the patient may be lost to follow-up or not adhere to the medical regimen, resulting in increased risk of flare. Successful transition requires developmental maturity of the patient, a certain style of parental involvement, and care coordination of the medical team. For women with IBD considering pregnancy, active IBD at the time of conception significantly increases the risk of flare. Women with CD who have no history of perianal disease don’t have an increased risk of perianal disease with vaginal delivery. A meta-analysis of the risk of congenital malformations with thiopurines found no significant association. Infliximab levels were likely to rise in the mother during the second and third trimesters (versus no increase with adalimumab), so one could consider TDM to guide dosing. In the PIANO study, anti-TNF therapy in the third trimester was neither associated with adverse pregnancy outcomes nor with infections up to 1 year for children. Patients who develop IBD later in life are more likely to have colonic inflammation. Elderly UC patients are more likely to require surgery, and postop mortality is higher for both CD and UC.
This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017. Dr. Loftus is a professor of medicine, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn.
Uma Mahadevan, MD, AGAF, and I moderated this session on IBD, and we were fortunate enough to secure four of the best IBD educators in the AGA.
David Rubin, MD, AGAF, opened with “Selecting the correct therapy for your outpatients with IBD: From mesalamine to biologics.” Treatment goals have evolved from symptom control to remission based on measures of inflammation (e.g., serum C-reactive protein, fecal calprotectin, or endoscopy). For ulcerative colitis (UC), high-risk markers include extensive disease, deep ulcers, younger age at diagnosis, elevated biomarkers, and early need for steroids or hospitalization. For Crohn’s disease (CD), these include younger age, extensive involvement, and fistulizing disease. The 5-aminosalicylate drugs remain a backbone in mild to moderate UC. Judicious use of corticosteroids is reasonable, but we need an exit strategy. The thiopurines are decent drugs, but studies have called into question their efficacy as monotherapy, and safety issues persist. Methotrexate is underutilized. The anti–tumor necrosis factor (TNF) biologics are excellent therapies but controversies persist as to whether these drugs require combination therapy or if they can be managed as “optimized monotherapy” with therapeutic drug monitoring (TDM). There are now two infliximab biosimilars available in the U.S.. Vedolizumab is an efficacious gut-selective anti-integrin (for both CD and UC). Ustekinumab, an anti-IL-12/23 antibody, is now available for moderate to severe CD, and has a favorable safety profile.
Fernando Velayos, MD, AGAF, discussed “Surveillance for dysplasia: What is the standard of care in 2017?” General principles for surveillance colonoscopy in IBD include having quiescent disease, since inflammation can reduce ability to detect lesions, and good colonic preparation. The three U.S. society guidelines recommend starting surveillance after 8 years of disease. Patients with concomitant primary schlerosing cholangitis should begin surveillance immediately. Frequency of surveillance ranges every 1-3 years depending on histology. A meta-analysis showed a higher incremental dysplasia yield with chromoendoscopy compared to standard white-light colonoscopy. If visible dysplasia can be endoscopically resected, then continued surveillance rather than colectomy is recommended.
Sunanda Kane, MD, AGAF, discussed “Managing special populations: the transitioning adolescent, the gravid, and the elderly.” The transition from pediatric to adult IBD care is a high-risk time because the patient may be lost to follow-up or not adhere to the medical regimen, resulting in increased risk of flare. Successful transition requires developmental maturity of the patient, a certain style of parental involvement, and care coordination of the medical team. For women with IBD considering pregnancy, active IBD at the time of conception significantly increases the risk of flare. Women with CD who have no history of perianal disease don’t have an increased risk of perianal disease with vaginal delivery. A meta-analysis of the risk of congenital malformations with thiopurines found no significant association. Infliximab levels were likely to rise in the mother during the second and third trimesters (versus no increase with adalimumab), so one could consider TDM to guide dosing. In the PIANO study, anti-TNF therapy in the third trimester was neither associated with adverse pregnancy outcomes nor with infections up to 1 year for children. Patients who develop IBD later in life are more likely to have colonic inflammation. Elderly UC patients are more likely to require surgery, and postop mortality is higher for both CD and UC.
This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017. Dr. Loftus is a professor of medicine, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn.
Cultivating competencies for value-based care
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.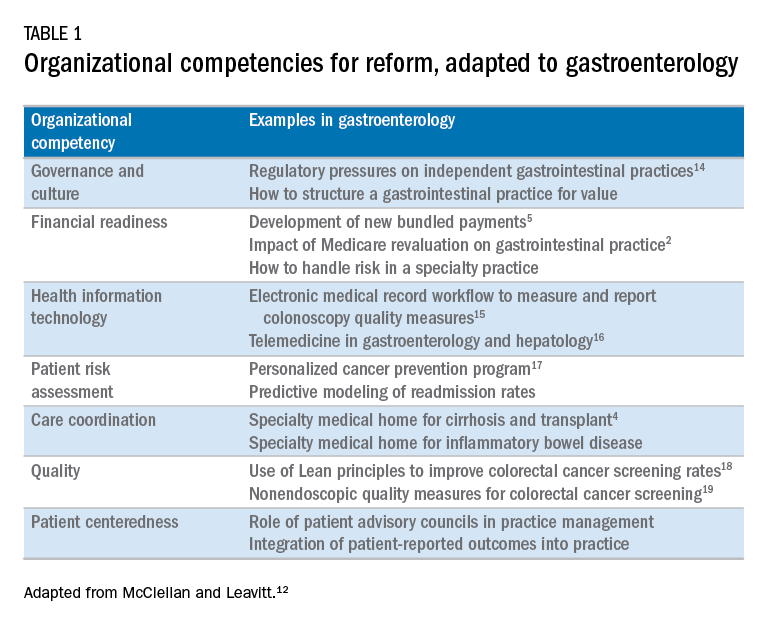
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
It is my privilege this month to assume responsibility for the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology. I am honored to join an impressive board of editors led by Dr Fasiha Kanwal, and anchored by global leaders in the field of gastroenterology and hepatology. This board of editors promises to continue the high level of excellence that has propelled the journal to its preeminent position among clinical journals. I am confident that the practice management section will uphold that tradition and continue to meet the expectation of our readers. I would like to mark this transition by acknowledging the history of the practice management section of Clinical Gastroenterology and Hepatology and outlining a vision for the future.
The section was introduced in 2010 under the leadership of Dr. Joel V. Brill. The section, titled “Practice Management: Opportunities and Challenges,” aimed to help practices navigate the disparate issues facing the field. Some of these issues included use of capnography in endoscopy, the importance of registries for quality reporting, and the burdens of meaningful use on physician practices. Dr Brill introduced this section in a video in May 2010 (https://www.youtube.com/watch?v=8FMsc2Wl5E8). Dr. Brill’s reference to these “interesting and challenging times” in gastroenterology resonates even more loudly today.
Over the next 5 years, the Road Ahead section will continue and strengthen its focus on the current and emerging issues facing gastroenterology and hepatology practices. I believe that high-value care will continue to be a high priority for patients and payers alike. Early results with payment reform around value have been mixed, in large part because of challenges in health systems and practices developing the competencies required for such reform.12 These competencies include governance and culture, financial readiness, health information technology, patient risk assessment, care coordination, quality, and patient centeredness. I will use this conceptual framework of organizational competencies, and their application in gastroenterology and hepatology, to help curate the Road Ahead section (Table 1). Key themes will include the following:
- • Governance and culture: The structure of health delivery systems, as conceptualized by Donabedian,13 is a key determinant of quality. Structural attributes include regulatory requirements on gastrointestinal practices, such as the rules governing use of anesthesia providers in ambulatory surgical settings; role of allied health professionals in clinical settings; and the impact of financial incentives in driving provider behavior.
- • Financial readiness: Value-based reimbursement, accountable care, medical homes, reference pricing, and physician tiering are some of the new terms in this era of value-based medicine. It is important for practices to assess patient costs longitudinally and manage financial risks. The Road Ahead section will continue to include papers that describe the impact of these reforms on gastroenterology and hepatology practices while providing guidance on implementation of these new models of care. Some examples include papers on the effect of payment policy on specialty practices, the development of a medical home in inflammatory bowel disease, and the physician experience with episode-based payments for colonoscopy.
- • Health information technology: All of the organizational competencies required for reform rely on a robust information technology platform that collects meaningful data and harnesses that data for analytic purposes. These platforms can be enterprise systems deployed by large health delivery systems or smaller, more nimble platforms, created by innovative start-up companies. The Road Ahead will include papers that share best practices in the use of these platforms to provide high-quality and cost-efficient care. In addition, the Road Ahead will continue to explore the use of health information technology to expand the reach of clinicians beyond brick and mortar clinics.
- • Patient risk assessment: Tailoring interventions to high-risk patients is necessary to deploy limited resources in a cost-effective manner. Risk assessment is also needed to more accurately and effectively personalize care for patients with chronic conditions. The Road Ahead will include papers that evaluate risk assessment tools and/or describe real-life implementation of these tools in different contexts.
- • Care coordination: The ability to provide team-based longitudinal care across the continuum of care will be integral to providing high value health care. The Road Ahead will serve as a means to disseminate best practices and innovative methods to care for increasingly complex patients, especially those with chronic diseases, such as cirrhosis and inflammatory bowel disease. For example, papers will explore the implementation of specialty medical homes, patient navigators, community-based care services, and involvement of patients in their own care.
- • Quality improvement: Providing high-value care by definition will require clinicians to accurately measure the quality of care provided to patients and use data to guide process improvement. The Road Ahead will continue to serve as an educational resource for clinicians with papers that discuss challenges and opportunities in quality measurement and improvement. Similarly, this section will present data on novel or impactful quality-improvement initiatives.
- • Patient centeredness: Patient experience measures and patient-reported outcomes are becoming increasingly important as meaningful indicators of quality. These measures are designed to ensure that patient perspectives are incorporated into the governance, design, and delivery of health care. The Road Ahead will serve as a dissemination mechanism for sharing best practices in developing, validating, implementing, and tracking patient-reported outcomes.
I consider Dr. Brill and Dr. Allen as mentors who have taught me tremendously about the business of medicine and the importance of physician leadership. I had the opportunity to coauthor several papers and book chapters with them. More recently, I have had the privilege to work closely with them in my role as the Chair of the American Gastroenterological Association Quality Measures Committee. It is an honor to now join their league as the editor for the Road Ahead section of Clinical Gastroenterology and Hepatology. These are indeed big shoes to fill. The section will retain the “Road Ahead” title in an acknowledgement of the continued importance of the issues outlined by Dr Allen. We will build on this theme to focus on not just the destination, but also the bumps in the road, the unexpected curves, the rest areas, beautiful vistas, and the indulgent road food. Hopefully no accidents along the way!
References
1. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692-6.
2. Dorn, S.D., Vesy, C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2017;14:924-8.
3. Dorn, S.D. The road ahead 3.0: changing payments, changing practice. Clin Gastroenterol Hepatol. 2016;14:785-9.
4. Meier, S.K., Shah, N.D., Talwalkar, J.A. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
5. Mehta, S.J. Bundled payment for gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2016;14:1681-4.
6. Weizman, A.V., Mosko, J., Bollegala, N., et al. Quality improvement primer series: launching a quality improvement initiative. Clin Gastroenterol Hepatol. 2017;14:1067-71.
7. Bernstein, M., Hou, J.K., Weizman, A.V., et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2017;14:1371-5.
8. Bollegala, N., Patel, K., Mosko, J.D., et al. Quality improvement primer series: the plan-do-study-act cycle and data display. Clin Gastroenterol Hepatol. 2016;14:1230-3.
9. Adams, M.A. Covert recording by patients of encounters with gastroenterology providers: path to empowerment or breach of trust?. Clin Gastroenterol Hepatol. 2017;15:13-6.
10. Oza, V.M., El-Dika, S., and Adams, M.A. Reaching safe harbor: legal implications of clinical practice guidelines. Clin Gastroenterol Hepatol. 2016;14:172-4.
11. Lin, M., Pappas, S.C., Sellin, J., et al. Curbside consultations: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2016;14:2-4.
12. McClellan, M.B., Leavitt, M.O. Competencies and tools to shift payments from volume to value. JAMA. 2016; 316: 1655–1656
13. Donabedian, A. Evaluating the quality of medical care. Milbank Q. 1966;44:166-203.
14. Rosenberg, F.B., Kim, L.S., Ketover, S.R. Challenges facing independent integrated gastroenterology. Clin Gastroenterol Hepatol. 2017;15:335-8.
15. Leiman, D.A., Metz, D.C., Ginsberg, G.G., et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14:333-7.
16. Cross, R.K., Kane, S. Integration of telemedicine into clinical gastroenterology and hepatology Practice. Clin Gastroenterol Hepatol. 2017;15:175-81.
17. Llor, X. Building a cancer genetics and prevention program. Clin Gastroenterol Hepatol. 2016;14:1516-20.
18. Patel, K.K., Cummings, S., Sellin, J., et al. Applying Lean design principles to a gastrointestinal endoscopy program for uninsured patients improves health care utilization. Clin Gastroenterol Hepatol. 2015;13:1556-9.
19. Saini, S.D., Adams, M.A., Brill, J.V., et al. Colorectal cancer screening quality measures: beyond colonoscopy. Clin Gastroenterol Hepatol. 2016;14:644-7.
Dr. Gellad is an associate professor of medicine in the division of gastroenterology at Durham VA Medical Center, Durham, N.C.; and Duke Clinical Research Institute, Durham, N.C. He reports a consulting relationship with Merck & Co. and he is also a cofounder and equity holder in Higgs Boson, LLC. He is funded by Veterans Affairs Health Services Research and Development Career Development Award (CDA 14-158 ).
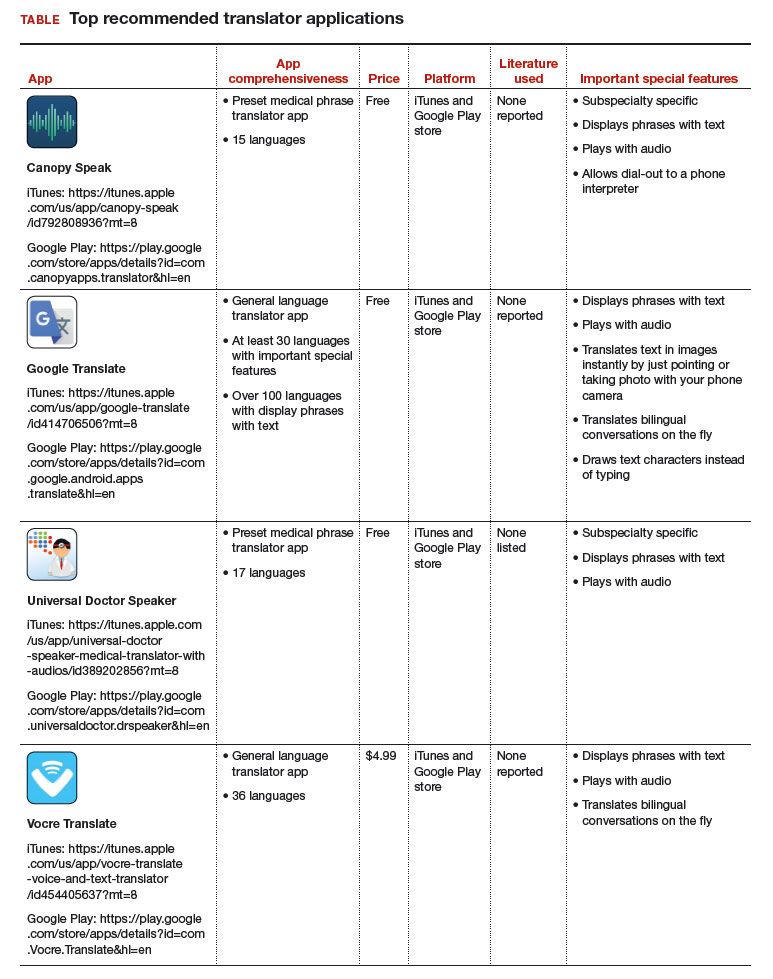
Top translator apps can help you communicate with patients who have limited English proficiency
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
No benefit seen for routine low-dose oxygen after stroke
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
koakes@frontlinemedcom.com
On Twitter @karioakes
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
koakes@frontlinemedcom.com
On Twitter @karioakes
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
koakes@frontlinemedcom.com
On Twitter @karioakes
FROM JAMA
Key clinical point:
Major finding: The poststroke death and disability odds ratio was 0.97 for those receiving continuous low-dose oxygen, compared with controls.
Data source: Single-blind, multisite, randomized, controlled trial of 8,003 patients admitted with acute stroke.
Disclosures: Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
Sen. Collins deals likely fatal blow to GOP’s health bill
Sen. Susan Collins (R-Maine) became the third GOP senator to confirm a no vote on the latest Republican attempt to repeal and replace the Affordable Care Act (ACA), a move that likely seals the bill’s fate.
The bill has the support of President Trump and many GOP leaders but has been roundly criticized by medical groups for insuring fewer Americans, failing to provide adequate protections for people with preexisting conditions, and barring Planned Parenthood from Medicaid participation.
“Sweeping reforms to our health care system and to Medicaid can’t be done well in a compressed time frame, especially when the actual bill is a moving target,” Sen. Collins said in a Sept. 25 statement announcing her opposition to a bill sponsored by Sen. Lindsey Graham (R-S.C.), Sen. Bill Cassidy (R-La.), Sen. Dean Heller (R-Nev.), and Sen. Ron Johnson (R-Wis.).
Since its introduction in mid September, the bill has undergone at least four published revisions, including providing additional funding to Maine and Alaska in an effort to flip Sen. Collins and Sen. Lisa Murkowski (R-Alaska) to the yes column. Both were no votes that helped kill a previous ACA repeal and replace effort.
“The fact is, Maine still loses money under whichever version of the Graham-Cassidy bill we consider because the bill uses what could be described as a ‘give with one hand, take with the other’ distribution model” to maintain the budget neutrality of the Medicaid block grants sent to states, Sen. Collins said. “Huge Medicaid cuts down the road more than offset any short-term influx of money.”
Sen. Collins’ opposition came on the heels of a damaging analysis from the Congressional Budget Office (CBO). The preliminary analysis looked at an earlier version of the bill and found that the number of people with comprehensive health insurance would be reduced by millions and the impact would be particularly large starting in 2020, when the bill would make changes to Medicaid funding and the nongroup insurance market.
Since Senate Republicans are using the budget reconciliation process to pass this legislation, they only need 50 votes to pass the legislation, with Vice President Mike Pence providing the tie-breaking vote. But with a slim 52-seat majority, there was little margin for error. Sen. John McCain (R-Ariz.) had already announced his opposition to the Graham-Cassidy bill on Sept. 22, primarily on process grounds.
“We should not be content to pass health care legislation on a party-line basis, as Democrats did when they rammed Obamacare through Congress in 2009. If we do, our success could be as short-lived as theirs, when the political winds shift, as they regularly do,” Sen. McCain said in a statement. “The issue is too important, and too many lives are at risk, for us to leave the American people guessing from one election to the next whether and how they will acquire health insurance. A bill of this impact requires a bipartisan approach.”
He praised the work of Senate Health, Education, Labor & Pensions Committee Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.), who have been working together on a small, focused package aimed at stabilizing the individual insurance market.
“Senators Alexander and Murray have been negotiating in good faith to fix some of the problems with Obamacare,” Sen. McCain said. “But I fear that the prospect of one last attempt at a strictly Republican bill has left the impression that their efforts cannot succeed. I hope they will resume their work should this last attempt at a partisan solution fail.”
Sen. Rand Paul (R-Ky.) also came out publicly against the Graham-Cassidy bill, though his opposition stems from it not going far enough in repealing elements of the ACA.
During a Sept. 25 Senate Finance Committee hearing on the Graham-Cassidy bill, Teresa Miller, acting secretary of the Pennsylvania Department of Human Services, testified that the real problem – the cost of health care – is getting pushed aside as everyone focuses on the coverage issue.
“This whole debate, for the last several years, has been about coverage and we haven’t been talking about the cost of health care,” Ms. Miller told the committee. “At the end of the day, insurance is a reflection of the cost of health care. So if we don’t have a debate in this county and discussion about how we get at the underlying cost of care, we have a major problem. That’s really the debate we should be having.”
Senate Majority Leader Mitch McConnell (R-Ky.) has not signaled whether he would still bring Graham-Cassidy to a vote, given that it appears not to have the votes needed for passage. If he chooses to move forward with the bill, the vote would need to happen before the end of the day on Sept. 30 to use the budget reconciliation process and gain passage with a simple majority.
Sen. Susan Collins (R-Maine) became the third GOP senator to confirm a no vote on the latest Republican attempt to repeal and replace the Affordable Care Act (ACA), a move that likely seals the bill’s fate.
The bill has the support of President Trump and many GOP leaders but has been roundly criticized by medical groups for insuring fewer Americans, failing to provide adequate protections for people with preexisting conditions, and barring Planned Parenthood from Medicaid participation.
“Sweeping reforms to our health care system and to Medicaid can’t be done well in a compressed time frame, especially when the actual bill is a moving target,” Sen. Collins said in a Sept. 25 statement announcing her opposition to a bill sponsored by Sen. Lindsey Graham (R-S.C.), Sen. Bill Cassidy (R-La.), Sen. Dean Heller (R-Nev.), and Sen. Ron Johnson (R-Wis.).
Since its introduction in mid September, the bill has undergone at least four published revisions, including providing additional funding to Maine and Alaska in an effort to flip Sen. Collins and Sen. Lisa Murkowski (R-Alaska) to the yes column. Both were no votes that helped kill a previous ACA repeal and replace effort.
“The fact is, Maine still loses money under whichever version of the Graham-Cassidy bill we consider because the bill uses what could be described as a ‘give with one hand, take with the other’ distribution model” to maintain the budget neutrality of the Medicaid block grants sent to states, Sen. Collins said. “Huge Medicaid cuts down the road more than offset any short-term influx of money.”
Sen. Collins’ opposition came on the heels of a damaging analysis from the Congressional Budget Office (CBO). The preliminary analysis looked at an earlier version of the bill and found that the number of people with comprehensive health insurance would be reduced by millions and the impact would be particularly large starting in 2020, when the bill would make changes to Medicaid funding and the nongroup insurance market.
Since Senate Republicans are using the budget reconciliation process to pass this legislation, they only need 50 votes to pass the legislation, with Vice President Mike Pence providing the tie-breaking vote. But with a slim 52-seat majority, there was little margin for error. Sen. John McCain (R-Ariz.) had already announced his opposition to the Graham-Cassidy bill on Sept. 22, primarily on process grounds.
“We should not be content to pass health care legislation on a party-line basis, as Democrats did when they rammed Obamacare through Congress in 2009. If we do, our success could be as short-lived as theirs, when the political winds shift, as they regularly do,” Sen. McCain said in a statement. “The issue is too important, and too many lives are at risk, for us to leave the American people guessing from one election to the next whether and how they will acquire health insurance. A bill of this impact requires a bipartisan approach.”
He praised the work of Senate Health, Education, Labor & Pensions Committee Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.), who have been working together on a small, focused package aimed at stabilizing the individual insurance market.
“Senators Alexander and Murray have been negotiating in good faith to fix some of the problems with Obamacare,” Sen. McCain said. “But I fear that the prospect of one last attempt at a strictly Republican bill has left the impression that their efforts cannot succeed. I hope they will resume their work should this last attempt at a partisan solution fail.”
Sen. Rand Paul (R-Ky.) also came out publicly against the Graham-Cassidy bill, though his opposition stems from it not going far enough in repealing elements of the ACA.
During a Sept. 25 Senate Finance Committee hearing on the Graham-Cassidy bill, Teresa Miller, acting secretary of the Pennsylvania Department of Human Services, testified that the real problem – the cost of health care – is getting pushed aside as everyone focuses on the coverage issue.
“This whole debate, for the last several years, has been about coverage and we haven’t been talking about the cost of health care,” Ms. Miller told the committee. “At the end of the day, insurance is a reflection of the cost of health care. So if we don’t have a debate in this county and discussion about how we get at the underlying cost of care, we have a major problem. That’s really the debate we should be having.”
Senate Majority Leader Mitch McConnell (R-Ky.) has not signaled whether he would still bring Graham-Cassidy to a vote, given that it appears not to have the votes needed for passage. If he chooses to move forward with the bill, the vote would need to happen before the end of the day on Sept. 30 to use the budget reconciliation process and gain passage with a simple majority.
Sen. Susan Collins (R-Maine) became the third GOP senator to confirm a no vote on the latest Republican attempt to repeal and replace the Affordable Care Act (ACA), a move that likely seals the bill’s fate.
The bill has the support of President Trump and many GOP leaders but has been roundly criticized by medical groups for insuring fewer Americans, failing to provide adequate protections for people with preexisting conditions, and barring Planned Parenthood from Medicaid participation.
“Sweeping reforms to our health care system and to Medicaid can’t be done well in a compressed time frame, especially when the actual bill is a moving target,” Sen. Collins said in a Sept. 25 statement announcing her opposition to a bill sponsored by Sen. Lindsey Graham (R-S.C.), Sen. Bill Cassidy (R-La.), Sen. Dean Heller (R-Nev.), and Sen. Ron Johnson (R-Wis.).
Since its introduction in mid September, the bill has undergone at least four published revisions, including providing additional funding to Maine and Alaska in an effort to flip Sen. Collins and Sen. Lisa Murkowski (R-Alaska) to the yes column. Both were no votes that helped kill a previous ACA repeal and replace effort.
“The fact is, Maine still loses money under whichever version of the Graham-Cassidy bill we consider because the bill uses what could be described as a ‘give with one hand, take with the other’ distribution model” to maintain the budget neutrality of the Medicaid block grants sent to states, Sen. Collins said. “Huge Medicaid cuts down the road more than offset any short-term influx of money.”
Sen. Collins’ opposition came on the heels of a damaging analysis from the Congressional Budget Office (CBO). The preliminary analysis looked at an earlier version of the bill and found that the number of people with comprehensive health insurance would be reduced by millions and the impact would be particularly large starting in 2020, when the bill would make changes to Medicaid funding and the nongroup insurance market.
Since Senate Republicans are using the budget reconciliation process to pass this legislation, they only need 50 votes to pass the legislation, with Vice President Mike Pence providing the tie-breaking vote. But with a slim 52-seat majority, there was little margin for error. Sen. John McCain (R-Ariz.) had already announced his opposition to the Graham-Cassidy bill on Sept. 22, primarily on process grounds.
“We should not be content to pass health care legislation on a party-line basis, as Democrats did when they rammed Obamacare through Congress in 2009. If we do, our success could be as short-lived as theirs, when the political winds shift, as they regularly do,” Sen. McCain said in a statement. “The issue is too important, and too many lives are at risk, for us to leave the American people guessing from one election to the next whether and how they will acquire health insurance. A bill of this impact requires a bipartisan approach.”
He praised the work of Senate Health, Education, Labor & Pensions Committee Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.), who have been working together on a small, focused package aimed at stabilizing the individual insurance market.
“Senators Alexander and Murray have been negotiating in good faith to fix some of the problems with Obamacare,” Sen. McCain said. “But I fear that the prospect of one last attempt at a strictly Republican bill has left the impression that their efforts cannot succeed. I hope they will resume their work should this last attempt at a partisan solution fail.”
Sen. Rand Paul (R-Ky.) also came out publicly against the Graham-Cassidy bill, though his opposition stems from it not going far enough in repealing elements of the ACA.
During a Sept. 25 Senate Finance Committee hearing on the Graham-Cassidy bill, Teresa Miller, acting secretary of the Pennsylvania Department of Human Services, testified that the real problem – the cost of health care – is getting pushed aside as everyone focuses on the coverage issue.
“This whole debate, for the last several years, has been about coverage and we haven’t been talking about the cost of health care,” Ms. Miller told the committee. “At the end of the day, insurance is a reflection of the cost of health care. So if we don’t have a debate in this county and discussion about how we get at the underlying cost of care, we have a major problem. That’s really the debate we should be having.”
Senate Majority Leader Mitch McConnell (R-Ky.) has not signaled whether he would still bring Graham-Cassidy to a vote, given that it appears not to have the votes needed for passage. If he chooses to move forward with the bill, the vote would need to happen before the end of the day on Sept. 30 to use the budget reconciliation process and gain passage with a simple majority.
Breast density and optimal screening for breast cancer
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.
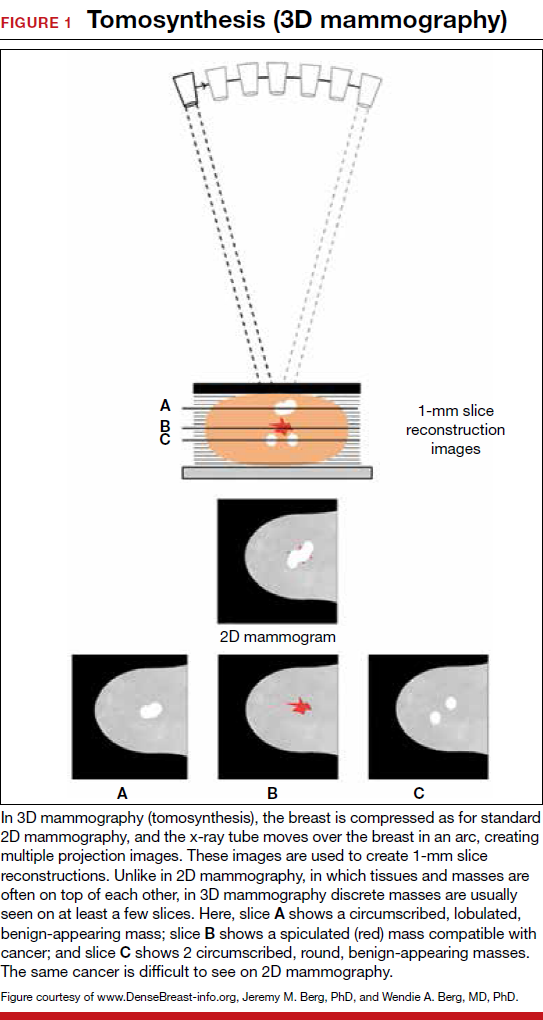
Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).
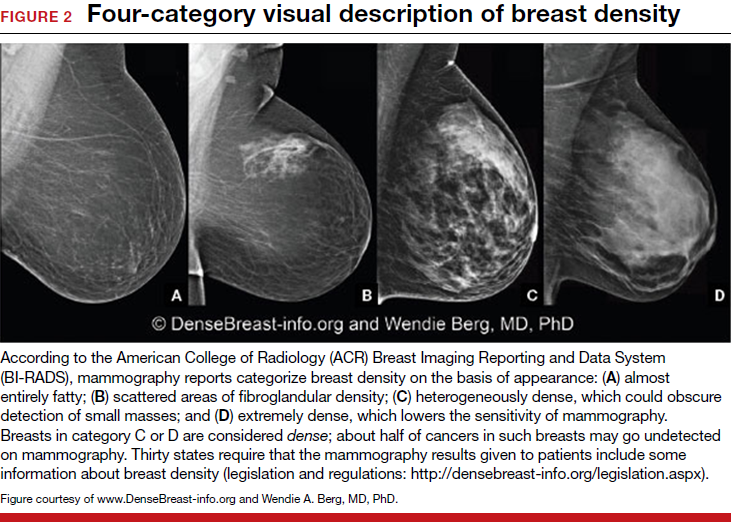
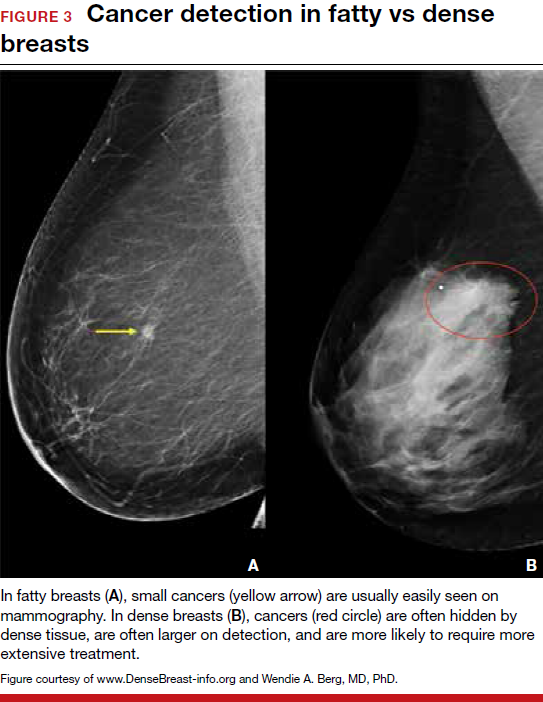
3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts
MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
Investigational Alzheimer’s Disease Drug Is Not Effective in Phase III Trials
LONDON—An investigational drug did not effectively treat symptoms of mild to moderate Alzheimer’s disease in phase III trials, according to results presented at the 2017 Alzheimer’s Association International Conference.
Investigators evaluated the efficacy and safety of idalopirdine, a selective 5-HT6 receptor antagonist, as adjunctive therapy in patients taking acetylcholinesterase inhibitors. Researchers conducted three 24-week, double-blind, placebo-controlled trials that included more than 2,500 patients. Patients received 10, 30, or 60 mg/day of idalopirdine or placebo.
The drug had a good safety and tolerability profile, but did not effectively alleviate clinical symptoms, said Alireza Atri, MD, PhD, Ray Dolby Endowed Chair in Brain Health Research at California Pacific Medical Center in San Francisco and Lecturer in Neurology at Harvard Medical School in Boston.
Serotonin 5-HT6 receptor antagonists nevertheless may prove effective in other trials with different compounds and doses, he said. “We have to wait for the other clinical trials to see whether they are positive,” Dr. Atri said.
Proof-of-Concept Findings
In a phase II study in patients with moderate Alzheimer’s disease (defined as a Mini-Mental State Examination [MMSE] score from 12 to 19), idalopirdine (90 mg/day [30 mg tid]) added to stable donepezil treatment significantly improved cognitive performance, compared with donepezil monotherapy.
The phase III trials included patients age 50 or older with mild to moderate Alzheimer’s disease (defined as MMSE score from 12 to 22). The main primary end point was change from baseline to Week 24 in Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-cog) total score. Other co-primary end points were change from baseline to Week 24 in Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL23) and Clinical Global Impression of Change (ADCS-CGIC) scores. Significant benefit over placebo for the main primary end point and at least one co-primary end point were needed to establish efficacy.
The phase III trials had similar designs. STARSHINE enrolled patients taking donepezil who received placebo, idalopirdine (30 mg/day), or idalopirdine (60 mg/day). STARBEAM enrolled patients taking donepezil who received placebo, idalopirdine (10 mg/day), or idalopirdine (30 mg/day). STARBRIGHT enrolled patients taking any acetylcholinesterase inhibitor who received placebo or idalopirdine (60 mg/day).
About 4,000 patients were screened, and 2,525 patients were randomized across the three studies. Patients had a mean age of 74, baseline ADAS-cog score of 26, and baseline MMSE score of 17. Patients on average had been diagnosed with Alzheimer’s disease for 2.2 years and had been on cholinesterase therapy for 1.7 years. About 55% to 60% of patients were APOE positive, and 62% to 65% were female.
None of the doses of idalopirdine met the prespecified efficacy criteria. An analysis suggested some indication of potential efficacy for the 60-mg/day dose in patients with moderate Alzheimer’s disease (ie, an MMSE score between 12 and 18), but there was no consistent replication across all end points, Dr. Atri said.
High Rate of Study Completion
Serious adverse events occurred in about 5% of patients who received placebo and idalopirdine (10 mg/day), and in about 6% to 7% of patients who received idalopirdine (30 mg/day) or idalopirdine (60 mg/day).
The percentage of patients with treatment-emergent adverse events was similar between the idalopirdine and placebo groups. Transient, dose-dependent increases in liver enzymes and vomiting were infrequently observed (< 5%).
The high rate of study completion and enrollment in the extension study (about 90%) suggests unmet need for therapies to treat patients with mild to moderate Alzheimer’s disease, Dr. Atri said.
One limitation of the trials was that patients did not need to have evidence of Alzheimer’s disease biomarkers to participate. In addition, investigators excluded patients who were taking memantine.
Researchers plan to further analyze the data from the phase III trials, Dr. Atri said. Lundbeck, based in Valby, Denmark, developed idalopirdine. The company announced in February that the phase III results did not support seeking regulatory approval for the drug.
—Jake Remaly
Suggested Reading
Ferrero H, Solas M, Francis PT, Ramirez MJ. Serotonin 5-HT6 receptor antagonists in Alzheimer’s disease: Therapeutic rationale and current development status. CNS Drugs. 2017;31(1):19-32.
Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13(11):1092-1099.
LONDON—An investigational drug did not effectively treat symptoms of mild to moderate Alzheimer’s disease in phase III trials, according to results presented at the 2017 Alzheimer’s Association International Conference.
Investigators evaluated the efficacy and safety of idalopirdine, a selective 5-HT6 receptor antagonist, as adjunctive therapy in patients taking acetylcholinesterase inhibitors. Researchers conducted three 24-week, double-blind, placebo-controlled trials that included more than 2,500 patients. Patients received 10, 30, or 60 mg/day of idalopirdine or placebo.
The drug had a good safety and tolerability profile, but did not effectively alleviate clinical symptoms, said Alireza Atri, MD, PhD, Ray Dolby Endowed Chair in Brain Health Research at California Pacific Medical Center in San Francisco and Lecturer in Neurology at Harvard Medical School in Boston.
Serotonin 5-HT6 receptor antagonists nevertheless may prove effective in other trials with different compounds and doses, he said. “We have to wait for the other clinical trials to see whether they are positive,” Dr. Atri said.
Proof-of-Concept Findings
In a phase II study in patients with moderate Alzheimer’s disease (defined as a Mini-Mental State Examination [MMSE] score from 12 to 19), idalopirdine (90 mg/day [30 mg tid]) added to stable donepezil treatment significantly improved cognitive performance, compared with donepezil monotherapy.
The phase III trials included patients age 50 or older with mild to moderate Alzheimer’s disease (defined as MMSE score from 12 to 22). The main primary end point was change from baseline to Week 24 in Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-cog) total score. Other co-primary end points were change from baseline to Week 24 in Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL23) and Clinical Global Impression of Change (ADCS-CGIC) scores. Significant benefit over placebo for the main primary end point and at least one co-primary end point were needed to establish efficacy.
The phase III trials had similar designs. STARSHINE enrolled patients taking donepezil who received placebo, idalopirdine (30 mg/day), or idalopirdine (60 mg/day). STARBEAM enrolled patients taking donepezil who received placebo, idalopirdine (10 mg/day), or idalopirdine (30 mg/day). STARBRIGHT enrolled patients taking any acetylcholinesterase inhibitor who received placebo or idalopirdine (60 mg/day).
About 4,000 patients were screened, and 2,525 patients were randomized across the three studies. Patients had a mean age of 74, baseline ADAS-cog score of 26, and baseline MMSE score of 17. Patients on average had been diagnosed with Alzheimer’s disease for 2.2 years and had been on cholinesterase therapy for 1.7 years. About 55% to 60% of patients were APOE positive, and 62% to 65% were female.
None of the doses of idalopirdine met the prespecified efficacy criteria. An analysis suggested some indication of potential efficacy for the 60-mg/day dose in patients with moderate Alzheimer’s disease (ie, an MMSE score between 12 and 18), but there was no consistent replication across all end points, Dr. Atri said.
High Rate of Study Completion
Serious adverse events occurred in about 5% of patients who received placebo and idalopirdine (10 mg/day), and in about 6% to 7% of patients who received idalopirdine (30 mg/day) or idalopirdine (60 mg/day).
The percentage of patients with treatment-emergent adverse events was similar between the idalopirdine and placebo groups. Transient, dose-dependent increases in liver enzymes and vomiting were infrequently observed (< 5%).
The high rate of study completion and enrollment in the extension study (about 90%) suggests unmet need for therapies to treat patients with mild to moderate Alzheimer’s disease, Dr. Atri said.
One limitation of the trials was that patients did not need to have evidence of Alzheimer’s disease biomarkers to participate. In addition, investigators excluded patients who were taking memantine.
Researchers plan to further analyze the data from the phase III trials, Dr. Atri said. Lundbeck, based in Valby, Denmark, developed idalopirdine. The company announced in February that the phase III results did not support seeking regulatory approval for the drug.
—Jake Remaly
Suggested Reading
Ferrero H, Solas M, Francis PT, Ramirez MJ. Serotonin 5-HT6 receptor antagonists in Alzheimer’s disease: Therapeutic rationale and current development status. CNS Drugs. 2017;31(1):19-32.
Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13(11):1092-1099.
LONDON—An investigational drug did not effectively treat symptoms of mild to moderate Alzheimer’s disease in phase III trials, according to results presented at the 2017 Alzheimer’s Association International Conference.
Investigators evaluated the efficacy and safety of idalopirdine, a selective 5-HT6 receptor antagonist, as adjunctive therapy in patients taking acetylcholinesterase inhibitors. Researchers conducted three 24-week, double-blind, placebo-controlled trials that included more than 2,500 patients. Patients received 10, 30, or 60 mg/day of idalopirdine or placebo.
The drug had a good safety and tolerability profile, but did not effectively alleviate clinical symptoms, said Alireza Atri, MD, PhD, Ray Dolby Endowed Chair in Brain Health Research at California Pacific Medical Center in San Francisco and Lecturer in Neurology at Harvard Medical School in Boston.
Serotonin 5-HT6 receptor antagonists nevertheless may prove effective in other trials with different compounds and doses, he said. “We have to wait for the other clinical trials to see whether they are positive,” Dr. Atri said.
Proof-of-Concept Findings
In a phase II study in patients with moderate Alzheimer’s disease (defined as a Mini-Mental State Examination [MMSE] score from 12 to 19), idalopirdine (90 mg/day [30 mg tid]) added to stable donepezil treatment significantly improved cognitive performance, compared with donepezil monotherapy.
The phase III trials included patients age 50 or older with mild to moderate Alzheimer’s disease (defined as MMSE score from 12 to 22). The main primary end point was change from baseline to Week 24 in Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-cog) total score. Other co-primary end points were change from baseline to Week 24 in Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL23) and Clinical Global Impression of Change (ADCS-CGIC) scores. Significant benefit over placebo for the main primary end point and at least one co-primary end point were needed to establish efficacy.
The phase III trials had similar designs. STARSHINE enrolled patients taking donepezil who received placebo, idalopirdine (30 mg/day), or idalopirdine (60 mg/day). STARBEAM enrolled patients taking donepezil who received placebo, idalopirdine (10 mg/day), or idalopirdine (30 mg/day). STARBRIGHT enrolled patients taking any acetylcholinesterase inhibitor who received placebo or idalopirdine (60 mg/day).
About 4,000 patients were screened, and 2,525 patients were randomized across the three studies. Patients had a mean age of 74, baseline ADAS-cog score of 26, and baseline MMSE score of 17. Patients on average had been diagnosed with Alzheimer’s disease for 2.2 years and had been on cholinesterase therapy for 1.7 years. About 55% to 60% of patients were APOE positive, and 62% to 65% were female.
None of the doses of idalopirdine met the prespecified efficacy criteria. An analysis suggested some indication of potential efficacy for the 60-mg/day dose in patients with moderate Alzheimer’s disease (ie, an MMSE score between 12 and 18), but there was no consistent replication across all end points, Dr. Atri said.
High Rate of Study Completion
Serious adverse events occurred in about 5% of patients who received placebo and idalopirdine (10 mg/day), and in about 6% to 7% of patients who received idalopirdine (30 mg/day) or idalopirdine (60 mg/day).
The percentage of patients with treatment-emergent adverse events was similar between the idalopirdine and placebo groups. Transient, dose-dependent increases in liver enzymes and vomiting were infrequently observed (< 5%).
The high rate of study completion and enrollment in the extension study (about 90%) suggests unmet need for therapies to treat patients with mild to moderate Alzheimer’s disease, Dr. Atri said.
One limitation of the trials was that patients did not need to have evidence of Alzheimer’s disease biomarkers to participate. In addition, investigators excluded patients who were taking memantine.
Researchers plan to further analyze the data from the phase III trials, Dr. Atri said. Lundbeck, based in Valby, Denmark, developed idalopirdine. The company announced in February that the phase III results did not support seeking regulatory approval for the drug.
—Jake Remaly
Suggested Reading
Ferrero H, Solas M, Francis PT, Ramirez MJ. Serotonin 5-HT6 receptor antagonists in Alzheimer’s disease: Therapeutic rationale and current development status. CNS Drugs. 2017;31(1):19-32.
Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13(11):1092-1099.
Multifaceted Intervention Improves Anticoagulant Use in Patients With Atrial Fibrillation
A customized educational intervention significantly increases the use of oral anticoagulants among patients with atrial fibrillation at risk for stroke, according to data published online ahead of print August 28 in Lancet. The intervention also appears to reduce the risk of stroke.
“If this intervention could be broadly applied, which we believe is possible, the public health implications would be substantial,” said Christopher B. Granger, MD, Professor of Medicine at Duke University School of Medicine in Durham, North Carolina. “More than 33 million people worldwide have atrial fibrillation, which is a leading cause of stoke. Improving adherence to anticoagulation therapy would be a lifesaver.”
Intervention Included Education and Monitoring
Dr. Granger and colleagues conducted IMPACT-AF—a prospective, cluster-randomized, controlled trial—to evaluate the effect of a multifaceted educational intervention on the use of oral anticoagulation in patients with atrial fibrillation, compared with usual care. The study was conducted in Argentina, Brazil, China, India, and Romania. Eligible patients were 18 or older, had atrial fibrillation not resulting from reversible causes, and had an indication for oral anticoagulation. Patients with an absolute contraindication to oral anticoagulation, those with a mechanical prosthetic valve, and those who were not able to have one year of follow-up were excluded.
Clusters (ie, sites) in each country were paired and randomized 1:1 to receive an educational intervention or usual care. The intervention’s two components were education and regular monitoring. Education was provided through various media (eg, web-based materials, videos, and guideline recommendations) to patients, their families, and healthcare providers. It was customized for each country and described the benefits, risks, and costs of anticoagulant therapies. The monitoring component, which included feedback, was designed to promote anticoagulant initiation among appropriate candidates who were not being treated, prevent discontinuation among treated patients, and improve adherence. The investigators collected data at baseline, six months, and 12 months at all sites. The intervention sites had additional telephone calls or patient visits at one month, three months, and nine months.
The primary end point was the change in the proportion of patients treated with oral anticoagulants from baseline to one year. Key secondary end points included the proportion of patients who were on oral anticoagulation at baseline, six months, and 12 months; and the proportion of patients who were not on oral anticoagulation at baseline, but were on this therapy at six months and 12 months. Other secondary clinical outcomes included all-cause death, stroke, transient ischemic attack, and major bleeding.
Anticoagulant Use Increased
In all, researchers enrolled 2,281 participants at 48 clusters. Mean age was approximately 70, and about 47% of participants were women. Five patients (three in the intervention group) were lost to follow-up after baseline. The median follow-up duration was 12 months. Age, sex, educational level, and socioeconomic factors were well balanced between the two groups. The intervention group, however, had a higher proportion of patients with permanent atrial fibrillation, history of major bleeding, systemic embolism, and uncontrolled hypertension, and a lower proportion of patients with rheumatic valvular heart disease, heart failure or left ventricular dysfunction, vascular disease, and previous myocardial infarction, than the control group.
The proportion of patients on oral anticoagulation increased from 68% at baseline to 80% at one year in the intervention group and from 64% to 67% in the control group. The absolute difference between groups in the change of oral anticoagulation use was 9.1%. This result yielded an odds ratio of 3.28, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group. The effect size in favor of the intervention was consistent across prespecified subgroups. The primary outcome result was consistent in all five countries.
Furthermore, 95% of patients who were on oral anticoagulants at baseline in the intervention group and 94% of patients in the control group continued taking oral anticoagulants at one year. For patients who were not on oral anticoagulants at baseline, 48% in the intervention group and 18% in the control group were on oral anticoagulants at one year. This result yielded an odds ratio of 4.60, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group.
“Our study also found a reduction in strokes in the intervention group, compared with the control group,” said Renato D. Lopes, MD, PhD, Professor of Medicine at Duke University School of Medicine and principal investigator for Brazil. The hazard ratio of stroke was 0.48 for the intervention group, compared with the control group. “While this was a secondary outcome, it highlights the potential benefit of improved anticoagulation care,” he added.
The number needed to treat was 100 patients exposed to intervention to prevent one stroke event over one year. The rates of all-cause death and the composite of stroke, systemic embolism, or major bleeding did not differ between the intervention and control groups.
Intervention Might Help Patients Worldwide
The majority of participants were using vitamin K antagonists at baseline, and data suggest that non-vitamin K antagonists have advantages (eg, reduced intracerebral bleeds) over this class of oral anticoagulants. Approximately 8% of participants in the intervention group had switched from vitamin K antagonists to non-vitamin K antagonists at one year, while participants in the control group did not change their medication. “A similar educational approach could be used for all anticoagulant classes with even greater benefit to patients,” said Michael D. Ezekowitz, MD, a cardiologist at Lankenau Medical Center in Wynnewood, Pennsylvania, and Anthony P. Kent, MD, a resident at Bridgeport Hospital in Bridgeport, Connecticut, in an accompanying editorial.
The study was limited to five countries and relied on a technological intervention, which might hinder the generalization of the results to broader clinical practice, said Drs. Ezekowitz and Kent. Nevertheless, “we are confident that the impact of IMPACT-AF will benefit patients with atrial fibrillation worldwide,” they concluded.
—Erik Greb
Suggested Reading
Ezekowitz MD, Kent AP. The impact of IMPACT-AF. Lancet. 2017 Aug 28 [Epub ahead of print].
Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017 Aug 28 [Epub ahead of print].
A customized educational intervention significantly increases the use of oral anticoagulants among patients with atrial fibrillation at risk for stroke, according to data published online ahead of print August 28 in Lancet. The intervention also appears to reduce the risk of stroke.
“If this intervention could be broadly applied, which we believe is possible, the public health implications would be substantial,” said Christopher B. Granger, MD, Professor of Medicine at Duke University School of Medicine in Durham, North Carolina. “More than 33 million people worldwide have atrial fibrillation, which is a leading cause of stoke. Improving adherence to anticoagulation therapy would be a lifesaver.”
Intervention Included Education and Monitoring
Dr. Granger and colleagues conducted IMPACT-AF—a prospective, cluster-randomized, controlled trial—to evaluate the effect of a multifaceted educational intervention on the use of oral anticoagulation in patients with atrial fibrillation, compared with usual care. The study was conducted in Argentina, Brazil, China, India, and Romania. Eligible patients were 18 or older, had atrial fibrillation not resulting from reversible causes, and had an indication for oral anticoagulation. Patients with an absolute contraindication to oral anticoagulation, those with a mechanical prosthetic valve, and those who were not able to have one year of follow-up were excluded.
Clusters (ie, sites) in each country were paired and randomized 1:1 to receive an educational intervention or usual care. The intervention’s two components were education and regular monitoring. Education was provided through various media (eg, web-based materials, videos, and guideline recommendations) to patients, their families, and healthcare providers. It was customized for each country and described the benefits, risks, and costs of anticoagulant therapies. The monitoring component, which included feedback, was designed to promote anticoagulant initiation among appropriate candidates who were not being treated, prevent discontinuation among treated patients, and improve adherence. The investigators collected data at baseline, six months, and 12 months at all sites. The intervention sites had additional telephone calls or patient visits at one month, three months, and nine months.
The primary end point was the change in the proportion of patients treated with oral anticoagulants from baseline to one year. Key secondary end points included the proportion of patients who were on oral anticoagulation at baseline, six months, and 12 months; and the proportion of patients who were not on oral anticoagulation at baseline, but were on this therapy at six months and 12 months. Other secondary clinical outcomes included all-cause death, stroke, transient ischemic attack, and major bleeding.
Anticoagulant Use Increased
In all, researchers enrolled 2,281 participants at 48 clusters. Mean age was approximately 70, and about 47% of participants were women. Five patients (three in the intervention group) were lost to follow-up after baseline. The median follow-up duration was 12 months. Age, sex, educational level, and socioeconomic factors were well balanced between the two groups. The intervention group, however, had a higher proportion of patients with permanent atrial fibrillation, history of major bleeding, systemic embolism, and uncontrolled hypertension, and a lower proportion of patients with rheumatic valvular heart disease, heart failure or left ventricular dysfunction, vascular disease, and previous myocardial infarction, than the control group.
The proportion of patients on oral anticoagulation increased from 68% at baseline to 80% at one year in the intervention group and from 64% to 67% in the control group. The absolute difference between groups in the change of oral anticoagulation use was 9.1%. This result yielded an odds ratio of 3.28, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group. The effect size in favor of the intervention was consistent across prespecified subgroups. The primary outcome result was consistent in all five countries.
Furthermore, 95% of patients who were on oral anticoagulants at baseline in the intervention group and 94% of patients in the control group continued taking oral anticoagulants at one year. For patients who were not on oral anticoagulants at baseline, 48% in the intervention group and 18% in the control group were on oral anticoagulants at one year. This result yielded an odds ratio of 4.60, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group.
“Our study also found a reduction in strokes in the intervention group, compared with the control group,” said Renato D. Lopes, MD, PhD, Professor of Medicine at Duke University School of Medicine and principal investigator for Brazil. The hazard ratio of stroke was 0.48 for the intervention group, compared with the control group. “While this was a secondary outcome, it highlights the potential benefit of improved anticoagulation care,” he added.
The number needed to treat was 100 patients exposed to intervention to prevent one stroke event over one year. The rates of all-cause death and the composite of stroke, systemic embolism, or major bleeding did not differ between the intervention and control groups.
Intervention Might Help Patients Worldwide
The majority of participants were using vitamin K antagonists at baseline, and data suggest that non-vitamin K antagonists have advantages (eg, reduced intracerebral bleeds) over this class of oral anticoagulants. Approximately 8% of participants in the intervention group had switched from vitamin K antagonists to non-vitamin K antagonists at one year, while participants in the control group did not change their medication. “A similar educational approach could be used for all anticoagulant classes with even greater benefit to patients,” said Michael D. Ezekowitz, MD, a cardiologist at Lankenau Medical Center in Wynnewood, Pennsylvania, and Anthony P. Kent, MD, a resident at Bridgeport Hospital in Bridgeport, Connecticut, in an accompanying editorial.
The study was limited to five countries and relied on a technological intervention, which might hinder the generalization of the results to broader clinical practice, said Drs. Ezekowitz and Kent. Nevertheless, “we are confident that the impact of IMPACT-AF will benefit patients with atrial fibrillation worldwide,” they concluded.
—Erik Greb
Suggested Reading
Ezekowitz MD, Kent AP. The impact of IMPACT-AF. Lancet. 2017 Aug 28 [Epub ahead of print].
Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017 Aug 28 [Epub ahead of print].
A customized educational intervention significantly increases the use of oral anticoagulants among patients with atrial fibrillation at risk for stroke, according to data published online ahead of print August 28 in Lancet. The intervention also appears to reduce the risk of stroke.
“If this intervention could be broadly applied, which we believe is possible, the public health implications would be substantial,” said Christopher B. Granger, MD, Professor of Medicine at Duke University School of Medicine in Durham, North Carolina. “More than 33 million people worldwide have atrial fibrillation, which is a leading cause of stoke. Improving adherence to anticoagulation therapy would be a lifesaver.”
Intervention Included Education and Monitoring
Dr. Granger and colleagues conducted IMPACT-AF—a prospective, cluster-randomized, controlled trial—to evaluate the effect of a multifaceted educational intervention on the use of oral anticoagulation in patients with atrial fibrillation, compared with usual care. The study was conducted in Argentina, Brazil, China, India, and Romania. Eligible patients were 18 or older, had atrial fibrillation not resulting from reversible causes, and had an indication for oral anticoagulation. Patients with an absolute contraindication to oral anticoagulation, those with a mechanical prosthetic valve, and those who were not able to have one year of follow-up were excluded.
Clusters (ie, sites) in each country were paired and randomized 1:1 to receive an educational intervention or usual care. The intervention’s two components were education and regular monitoring. Education was provided through various media (eg, web-based materials, videos, and guideline recommendations) to patients, their families, and healthcare providers. It was customized for each country and described the benefits, risks, and costs of anticoagulant therapies. The monitoring component, which included feedback, was designed to promote anticoagulant initiation among appropriate candidates who were not being treated, prevent discontinuation among treated patients, and improve adherence. The investigators collected data at baseline, six months, and 12 months at all sites. The intervention sites had additional telephone calls or patient visits at one month, three months, and nine months.
The primary end point was the change in the proportion of patients treated with oral anticoagulants from baseline to one year. Key secondary end points included the proportion of patients who were on oral anticoagulation at baseline, six months, and 12 months; and the proportion of patients who were not on oral anticoagulation at baseline, but were on this therapy at six months and 12 months. Other secondary clinical outcomes included all-cause death, stroke, transient ischemic attack, and major bleeding.
Anticoagulant Use Increased
In all, researchers enrolled 2,281 participants at 48 clusters. Mean age was approximately 70, and about 47% of participants were women. Five patients (three in the intervention group) were lost to follow-up after baseline. The median follow-up duration was 12 months. Age, sex, educational level, and socioeconomic factors were well balanced between the two groups. The intervention group, however, had a higher proportion of patients with permanent atrial fibrillation, history of major bleeding, systemic embolism, and uncontrolled hypertension, and a lower proportion of patients with rheumatic valvular heart disease, heart failure or left ventricular dysfunction, vascular disease, and previous myocardial infarction, than the control group.
The proportion of patients on oral anticoagulation increased from 68% at baseline to 80% at one year in the intervention group and from 64% to 67% in the control group. The absolute difference between groups in the change of oral anticoagulation use was 9.1%. This result yielded an odds ratio of 3.28, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group. The effect size in favor of the intervention was consistent across prespecified subgroups. The primary outcome result was consistent in all five countries.
Furthermore, 95% of patients who were on oral anticoagulants at baseline in the intervention group and 94% of patients in the control group continued taking oral anticoagulants at one year. For patients who were not on oral anticoagulants at baseline, 48% in the intervention group and 18% in the control group were on oral anticoagulants at one year. This result yielded an odds ratio of 4.60, representing the proportional increase in anticoagulation use from baseline to one year in the intervention group, compared with the control group.
“Our study also found a reduction in strokes in the intervention group, compared with the control group,” said Renato D. Lopes, MD, PhD, Professor of Medicine at Duke University School of Medicine and principal investigator for Brazil. The hazard ratio of stroke was 0.48 for the intervention group, compared with the control group. “While this was a secondary outcome, it highlights the potential benefit of improved anticoagulation care,” he added.
The number needed to treat was 100 patients exposed to intervention to prevent one stroke event over one year. The rates of all-cause death and the composite of stroke, systemic embolism, or major bleeding did not differ between the intervention and control groups.
Intervention Might Help Patients Worldwide
The majority of participants were using vitamin K antagonists at baseline, and data suggest that non-vitamin K antagonists have advantages (eg, reduced intracerebral bleeds) over this class of oral anticoagulants. Approximately 8% of participants in the intervention group had switched from vitamin K antagonists to non-vitamin K antagonists at one year, while participants in the control group did not change their medication. “A similar educational approach could be used for all anticoagulant classes with even greater benefit to patients,” said Michael D. Ezekowitz, MD, a cardiologist at Lankenau Medical Center in Wynnewood, Pennsylvania, and Anthony P. Kent, MD, a resident at Bridgeport Hospital in Bridgeport, Connecticut, in an accompanying editorial.
The study was limited to five countries and relied on a technological intervention, which might hinder the generalization of the results to broader clinical practice, said Drs. Ezekowitz and Kent. Nevertheless, “we are confident that the impact of IMPACT-AF will benefit patients with atrial fibrillation worldwide,” they concluded.
—Erik Greb
Suggested Reading
Ezekowitz MD, Kent AP. The impact of IMPACT-AF. Lancet. 2017 Aug 28 [Epub ahead of print].
Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017 Aug 28 [Epub ahead of print].
Reengineering your office to be perfect for your patients
Independent of the Affordable Care Act or any upcoming changes in health care, the focus of an ObGyn practice remains paramount: the patient comes first.
The “recipe” for creating patient satisfaction and service excellence is predicated upon the mission of your practice and creating a shared vision with your employees. An action plan that is created and “visited/revisited”on a regular basis will serve to keep all abreast of the latest information to enhance the quality of patient care. It goes without saying, the ObGyn must first “lead by example” and always strive for satisfied patients who will tell their friends about your practice.
Start with the right tools
To organize a practice well, you need the right tools, which ideally include mission and vision statements and an action plan with goals and objectives.
Mission statement
A mission statement can be developed by the ObGyn(s) in your office or in concert with your staff. It should include:
- the “here and now” focus on the current approach to patient care
- why the practice exists (Develop a brief description of your practice, including the desired patient population.)
- the products and services offered and why and how those services are provided.
Here is an example of a mission statement for an ObGyn practice: “Our mission is to provide excellent, exceptional, personalized care for women of all ages in a warm and friendly environment. We incorporate leading-edge technology in our practice and continue to be a leader in obstetrics and gynecology.”
Vision statement
A vision statement should be developed in concert with your staff. It should include:
- the “then and there” focus on the historic perspective of your practice
- the ObGyn(s) and staff vision of the future
- what the ObGyn(s) and staff want to create.
The vision statement should energize and excite your personnel, create a shared and meaningful purpose, inspire passion and interest, and convey the values you want to share in your practice.
Here is an example of a vision statement for an ObGyn practice: “We aim to become the premier obstetrics and gynecology pro-vider to residents of (location) community.”
Action plan: Setting goals
To succeed, an ObGyn practice needs to:
- develop targets and challenges reflecting periodic (quarterly) meetings with staff and new entity development in the practice
- establish benchmarks and measurable parameters (How do you compare with other local practices? Set criteria/metrics to assess your progress.)
- ensure that the objectives support the goals (Develop goals and objectives over a defined period of time.)
- revisit the goals (Have they have been met? Do they need revision?)
Goals and objectives are essential for the continued health of your practice. This is all predicated upon developing a competitive advantage and then maintaining it.
Read about how to make a positive first impression on a new patient.
Is the environment welcoming?
When we examine a practice from the patient’s point of view, a good starting place is with the front desk. Have you looked at your front desk “from the outside in?” In one sense, this is the showcase of your practice.
Related article:
Four pillars of a successful practice: 2. Attract new patients
The first impression: Appointment scheduling
The first impression a patient receives about your practice occurs when she attempts to set up an appointment. Perhaps you might ask someone to call in to schedule an appointment. Is the caller immediately put on hold? Are your personnel courteous on the phone? Can she be seen quickly if she has a problem? How long is the wait for an annual exam? A test run can be very revealing.
Walk in the front door
When a patient walks in the door, does the physical office space radiate a friendly, relaxed atmosphere? Walk through the waiting room, then consultation and exam rooms as if you are a patient seeing it for the first time. Have you created an environment in which patients sense a well-organized office and the esprit de corps of the personnel? Does it look and smell fresh and clean? This all sends a loud and clear positive message about your practice.1–3
Here are some suggestions for making a waiting room more inviting:
- Provide a seating arrangement that is “patient centered.” For example, semi- circular arrangements allow easy viewing of any monitors in the waiting room.
- WiFi is a great addition. Post several signs with the user name and password.
- Offer computers for patients to use to complete registration
- Set up a fish tank. If well-maintained, it can be soothing to many people.
- Display medical information pamphlets, even if they are rarely taken.
- Provide a big screen television that offers information about your practice, including personnel and procedures.
Streaming ads for physician offices are available. One platform, Outcome Health (https://www.outcomehealth.com), provides flat-screen TVs and tablets that show patient education videos.4 Another vendor, Patient Point (http://patientpoint.com), offers waiting room networks, editorials, and other communications designed to support “the goals of improving healthcare.”5 Other available media include channel news and music programming to relax patients.6
Wait times. A patient’s perceived wait time and the actual wait time are often quite different. How long she waits to see the ObGyn is “numero uno” with regard to patient satisfaction and can be a key source of annoyance, irritability, stress, and anger.
Does someone inform waiting patients that the ObGyn is running late? Does staff at the front desk or perhaps your medical assistant inquire, “Can I get you anything? The doctor is running late,” or “Dr. Jones has just finished delivering a baby. He’ll be here in 10 minutes. He’ll see you first.”
Consultation and exam rooms
Suggestions to develop a relaxing environment in your consultation and exam rooms are7:
- decorate the walls with soft, pastel colors
- use “spa aesthetics” to create a colorful atmosphere with appropriate lighting, artwork, and modern furnishings
- present a few magazines neatly and update them periodically
- stock and appropriately maintain the patients rooms with medical supplies
- remember, “Subjects perceive people more positively in beautiful rooms than in ugly rooms.”5
Read about how to keep your patients satisfied and your business stable.
Set the lead example
The need for open and supportive communication between you and your office staff cannot be overly emphasized. An ideal office staff member understands and shares in the vision, is aware of stated goals and objectives, is responsive to patient needs, and wants to create a win-win environment.
Frequently discuss your expectations with your staff. Expect them to be responsive, courteous, competent, have good communication skills, and be influenced by the appearance of the physical environ-ment. Provide support and educational tools to help them successfully perform their work.
Related article:
Four pillars of a successful practice: 1. Keep your current patients happy
Discover your patients’ vision of customer service
Formal measurement of patient satisfaction began with Professor Irwin Press at the University of Notre Dame. Rod Ganey, a sociologist and statistician, then developed the Press Ganey Patient Satisfaction Survey. These points earlier conveyed by Maslow and Mintz8 addressed the “effects of esthetic surroundings.” Color and art proved to be preferences in an esthetically pleasing environment. Additional historical information has been provided by Siegrist, who addressed “the patient experience.”9 He cites the myth that patients do not fill out satisfaction surveys. Indeed they do. Patient satisfaction is not a personality contest but rather a reflection of the health care provider’s investment of time and effort to offer patient-centered care. Siegrist also notes that the patient’s family plays a key role in how a patient perceives her experience with her health care professional.9
The federal government has been actively involved in assessing patient satisfaction in the hospital setting since 2002. This is reflected in the Centers for Medicare and Medicaid Services, the Agency for Healthcare Research and Quality, and Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys. The HCAHPS is a 27-question survey randomly administered to adult inpatients after discharge.10–12
The following metrics are often included in patient satisfaction surveys9,10:
- rating of hospital care from 0 (lowest) to 10 (highest)
- percentage of patients who would recommend a practice to family and friends
- number of patients who say their health care providers always communicate well
- the number of patients who report that the office is always clean and friendly.
Use of search engines focused on health care patient surveys can provide a number of options for clinicians to use in their practice.
Tips on patient satisfaction
Several interesting tips from the busi-ness world can be applied to an ObGyn’s practice14:
- You will only hear from 4% of unhappy customers.
- One dissatisfied customer tells 9.
- 95% of customers with resolved issues will do business with you again.
- If a problem is not addressed, that patient will tell 10 others.
- Resolve the problem and 5 people will know about it.
- It costs 5 times as much effort to gain 1 new customer.
- Loyal customers in 1 area of service are good prospects for other (new) services.
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
Tell stories about good, satisfied patients
Sharing the stories of satisfied patients motivates others to consider coming to your practice. To develop these stories, offer a “suggestion box” where patients can leave compliments or comments about their experiences. Ask patients to record their positive reviews (be sure to obtain written consent before recording and publishing). Show the videos on the big-screen TVs in your waiting room and include patient reviews (written, audio, and video) on your website.15
Related article:
Four pillars of a successful practice: 4. Motivate your staff
Reevaluate periodically
Encouraging team spirit makes good business sense. Offer staff members bonuses for coming up with improved processes. Provide educational programs for staff on patient care, technology, etc. If a difficult experience occurs, discuss it openly with staff members without accusing, asking them for suggestions to improve the situation.16
To assess the monetary value of your practice, you need to know what contributes to your profit margin and overhead. What investments are the most profitable? Then monitor each segment of the office practice.
Should you proceed with a purchase? Should you take on a new hire? Let's look at one excellent model from the Boston Consulting Group (FIGURE) that provides insight into "low and high performance" aspects of business or practice.1
In the matrix, Stars use large amounts of cash and are leaders in cash generation. Stars lead to development of a Cash Cow, which are entities that generate profits and cash with low investment prerequisites. Dogs are segments of product and service line(s) that should be carefully reevaluated. A decision must be made to liquidate if the problem cannot be corrected. Question Marks have the worst cash characteristics of all and are associated with high demands and low profit margin(s).1
SWOT analysis
A SWOT analysis is most helpful when assessing a practice in real time. The basic tenets are2:
Strengths:
- prestigious reputation
- technological expertise
Weaknesses:
- antiquated computer system
- lack of experience in specific areas
Opportunities:
- growing market demand for a specific product or procedure
- provision of unique services
Threats:
- changing demographics
- competitive practices
- changes in health care third-party payers.
The American College of Obstetricians and Gynecologists (ACOG) has developed an "ACOG Medical Home Toolkit" to allow ObGyns to assess how significant the changes regarding payers will be to their practice. Sections include the patient/practice partnership support; clinical care information; community resources; care delivery management; performance measurement and improvement; and payment and finance.3 The toolkit is available for download from the ACOG website.
References
- Morrison A, Wensley R. Boxing up or boxed in? A short history of the Boston Consulting Group Share/Growth Matrix. J Market Manag. 1993;7(2):105-129. http://www.tandfonline.com/doi/abs/10.1080/0267257X.1991.9964145.
- Klasko SK, Toub DB. It's not a plan without a business plan. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:36-37.
- American Congress of Obstetricians and Gynecologists. ACOG Medical Home Toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Practice-Management-and-Managed-Care/ACOG-Medical-Home-Toolkit. Accessed August 14, 2017.
Bottom line
Ensuring that your patients have an outstanding experience is a smart business strategy. A unified approach that includes team members’ involvement to create a patient-centered environment will provide a quality experience and encourage patients to recommend your ObGyn practice to others.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Ulrich RS. Evidence-based environmental design for improving medical outcomes: Paper Delivered at a Conference Entitled Healing by Design: Building for Health Care in the 21st Century. Montreal: McGill University Health Centre; 2000. http://www.brikbase.org/sites/default/files/Evidence%20Based%20Environmental%20Design%20for%20Improving%20Medical.pdf. Accessed August 15, 2017.
- Becker F, Douglass S. The ecology of the patient visit: physical attractiveness, waiting times and perceived quality of care. J Ambul Care Manag. 2008;31(2):128–141.
- Becker F, Sweeney B, Parsons K. Ambulatory facility design and patients’ perceptions of healthcare quality. HERD. 2008;1(4):35–54.
- Outcome Health Website. https://www.outcomehealth.com/. Accessed August 14, 2017.
- Mazer SE. The waiting room: Where suffering begins. Healing Healthcare Systems website. http://www.healinghealth.com/waiting-room-suffering-begins/. Published November 7, 2014. Accessed August 14, 2017.
- Patient Point Programs Website. http://patientpoint.com/. Accessed August 14, 2017.
- Almquist J, Kelly C, Bromberg J, Bryant S, Christianson T, Montori V. Consultation room design and the clinical encounter: the space and interaction randomized trial. Health Environ Res Design. 2009;3(1):41–78.
- Maslow A, Mintz N. Effects of esthetic surroundings: I. Initial effects of three esthetic conditions upon perceiving “energy” and “well-being” in faces. J Psychology. 1956;41(2):247–254.
- Siegrist RB. The patient experience. In: Sanfilippo JS, Bieber E, Javich D, Siegrist R, eds. MBA for Healthcare. New York, NY: Oxford Press;2016:227–236.
- Press I. Patient satisfaction: Understanding and managing the experience of care. 2nd ed. Chicago, IL: Health Administration Press; 2005:66–78.
- Piper L, Tallman E. Hospital consumer assessment of healthcare providers and systems: An ethical leadership dilemma to satisfy patients. Health Care Manag (Frederick). 2016;35(2):151–155.
- Giordano L, Elliott M, Goldstein E, Lehrman W, Spencer P. Development, implementation and public reporting of HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- Jones KE. Helping the health profession help others: Applying business principles to the medical world. University of Tennessee, Knoxville Honors Thesis Projects. http://trace.tennessee.edu/cgi/viewcontent.cgi?article=1560&context=utk_chanhonoproj. Published 2002. Accessed August 14, 2017.
- Baum N. Marketing your practice: ethically, effectively and economically. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:123–154.
- Baum NH. Four pillars of a successful practice: 1. Keep your current patients happy. OBG Manag. 2013;25(3):49–56.
- Baum NH. Four pillars of a successful practice: 4. Motivate your staff. OBG Manag. 2013;25(8):29–33.
Independent of the Affordable Care Act or any upcoming changes in health care, the focus of an ObGyn practice remains paramount: the patient comes first.
The “recipe” for creating patient satisfaction and service excellence is predicated upon the mission of your practice and creating a shared vision with your employees. An action plan that is created and “visited/revisited”on a regular basis will serve to keep all abreast of the latest information to enhance the quality of patient care. It goes without saying, the ObGyn must first “lead by example” and always strive for satisfied patients who will tell their friends about your practice.
Start with the right tools
To organize a practice well, you need the right tools, which ideally include mission and vision statements and an action plan with goals and objectives.
Mission statement
A mission statement can be developed by the ObGyn(s) in your office or in concert with your staff. It should include:
- the “here and now” focus on the current approach to patient care
- why the practice exists (Develop a brief description of your practice, including the desired patient population.)
- the products and services offered and why and how those services are provided.
Here is an example of a mission statement for an ObGyn practice: “Our mission is to provide excellent, exceptional, personalized care for women of all ages in a warm and friendly environment. We incorporate leading-edge technology in our practice and continue to be a leader in obstetrics and gynecology.”
Vision statement
A vision statement should be developed in concert with your staff. It should include:
- the “then and there” focus on the historic perspective of your practice
- the ObGyn(s) and staff vision of the future
- what the ObGyn(s) and staff want to create.
The vision statement should energize and excite your personnel, create a shared and meaningful purpose, inspire passion and interest, and convey the values you want to share in your practice.
Here is an example of a vision statement for an ObGyn practice: “We aim to become the premier obstetrics and gynecology pro-vider to residents of (location) community.”
Action plan: Setting goals
To succeed, an ObGyn practice needs to:
- develop targets and challenges reflecting periodic (quarterly) meetings with staff and new entity development in the practice
- establish benchmarks and measurable parameters (How do you compare with other local practices? Set criteria/metrics to assess your progress.)
- ensure that the objectives support the goals (Develop goals and objectives over a defined period of time.)
- revisit the goals (Have they have been met? Do they need revision?)
Goals and objectives are essential for the continued health of your practice. This is all predicated upon developing a competitive advantage and then maintaining it.
Read about how to make a positive first impression on a new patient.
Is the environment welcoming?
When we examine a practice from the patient’s point of view, a good starting place is with the front desk. Have you looked at your front desk “from the outside in?” In one sense, this is the showcase of your practice.
Related article:
Four pillars of a successful practice: 2. Attract new patients
The first impression: Appointment scheduling
The first impression a patient receives about your practice occurs when she attempts to set up an appointment. Perhaps you might ask someone to call in to schedule an appointment. Is the caller immediately put on hold? Are your personnel courteous on the phone? Can she be seen quickly if she has a problem? How long is the wait for an annual exam? A test run can be very revealing.
Walk in the front door
When a patient walks in the door, does the physical office space radiate a friendly, relaxed atmosphere? Walk through the waiting room, then consultation and exam rooms as if you are a patient seeing it for the first time. Have you created an environment in which patients sense a well-organized office and the esprit de corps of the personnel? Does it look and smell fresh and clean? This all sends a loud and clear positive message about your practice.1–3
Here are some suggestions for making a waiting room more inviting:
- Provide a seating arrangement that is “patient centered.” For example, semi- circular arrangements allow easy viewing of any monitors in the waiting room.
- WiFi is a great addition. Post several signs with the user name and password.
- Offer computers for patients to use to complete registration
- Set up a fish tank. If well-maintained, it can be soothing to many people.
- Display medical information pamphlets, even if they are rarely taken.
- Provide a big screen television that offers information about your practice, including personnel and procedures.
Streaming ads for physician offices are available. One platform, Outcome Health (https://www.outcomehealth.com), provides flat-screen TVs and tablets that show patient education videos.4 Another vendor, Patient Point (http://patientpoint.com), offers waiting room networks, editorials, and other communications designed to support “the goals of improving healthcare.”5 Other available media include channel news and music programming to relax patients.6
Wait times. A patient’s perceived wait time and the actual wait time are often quite different. How long she waits to see the ObGyn is “numero uno” with regard to patient satisfaction and can be a key source of annoyance, irritability, stress, and anger.
Does someone inform waiting patients that the ObGyn is running late? Does staff at the front desk or perhaps your medical assistant inquire, “Can I get you anything? The doctor is running late,” or “Dr. Jones has just finished delivering a baby. He’ll be here in 10 minutes. He’ll see you first.”
Consultation and exam rooms
Suggestions to develop a relaxing environment in your consultation and exam rooms are7:
- decorate the walls with soft, pastel colors
- use “spa aesthetics” to create a colorful atmosphere with appropriate lighting, artwork, and modern furnishings
- present a few magazines neatly and update them periodically
- stock and appropriately maintain the patients rooms with medical supplies
- remember, “Subjects perceive people more positively in beautiful rooms than in ugly rooms.”5
Read about how to keep your patients satisfied and your business stable.
Set the lead example
The need for open and supportive communication between you and your office staff cannot be overly emphasized. An ideal office staff member understands and shares in the vision, is aware of stated goals and objectives, is responsive to patient needs, and wants to create a win-win environment.
Frequently discuss your expectations with your staff. Expect them to be responsive, courteous, competent, have good communication skills, and be influenced by the appearance of the physical environ-ment. Provide support and educational tools to help them successfully perform their work.
Related article:
Four pillars of a successful practice: 1. Keep your current patients happy
Discover your patients’ vision of customer service
Formal measurement of patient satisfaction began with Professor Irwin Press at the University of Notre Dame. Rod Ganey, a sociologist and statistician, then developed the Press Ganey Patient Satisfaction Survey. These points earlier conveyed by Maslow and Mintz8 addressed the “effects of esthetic surroundings.” Color and art proved to be preferences in an esthetically pleasing environment. Additional historical information has been provided by Siegrist, who addressed “the patient experience.”9 He cites the myth that patients do not fill out satisfaction surveys. Indeed they do. Patient satisfaction is not a personality contest but rather a reflection of the health care provider’s investment of time and effort to offer patient-centered care. Siegrist also notes that the patient’s family plays a key role in how a patient perceives her experience with her health care professional.9
The federal government has been actively involved in assessing patient satisfaction in the hospital setting since 2002. This is reflected in the Centers for Medicare and Medicaid Services, the Agency for Healthcare Research and Quality, and Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys. The HCAHPS is a 27-question survey randomly administered to adult inpatients after discharge.10–12
The following metrics are often included in patient satisfaction surveys9,10:
- rating of hospital care from 0 (lowest) to 10 (highest)
- percentage of patients who would recommend a practice to family and friends
- number of patients who say their health care providers always communicate well
- the number of patients who report that the office is always clean and friendly.
Use of search engines focused on health care patient surveys can provide a number of options for clinicians to use in their practice.
Tips on patient satisfaction
Several interesting tips from the busi-ness world can be applied to an ObGyn’s practice14:
- You will only hear from 4% of unhappy customers.
- One dissatisfied customer tells 9.
- 95% of customers with resolved issues will do business with you again.
- If a problem is not addressed, that patient will tell 10 others.
- Resolve the problem and 5 people will know about it.
- It costs 5 times as much effort to gain 1 new customer.
- Loyal customers in 1 area of service are good prospects for other (new) services.
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
Tell stories about good, satisfied patients
Sharing the stories of satisfied patients motivates others to consider coming to your practice. To develop these stories, offer a “suggestion box” where patients can leave compliments or comments about their experiences. Ask patients to record their positive reviews (be sure to obtain written consent before recording and publishing). Show the videos on the big-screen TVs in your waiting room and include patient reviews (written, audio, and video) on your website.15
Related article:
Four pillars of a successful practice: 4. Motivate your staff
Reevaluate periodically
Encouraging team spirit makes good business sense. Offer staff members bonuses for coming up with improved processes. Provide educational programs for staff on patient care, technology, etc. If a difficult experience occurs, discuss it openly with staff members without accusing, asking them for suggestions to improve the situation.16
To assess the monetary value of your practice, you need to know what contributes to your profit margin and overhead. What investments are the most profitable? Then monitor each segment of the office practice.
Should you proceed with a purchase? Should you take on a new hire? Let's look at one excellent model from the Boston Consulting Group (FIGURE) that provides insight into "low and high performance" aspects of business or practice.1
In the matrix, Stars use large amounts of cash and are leaders in cash generation. Stars lead to development of a Cash Cow, which are entities that generate profits and cash with low investment prerequisites. Dogs are segments of product and service line(s) that should be carefully reevaluated. A decision must be made to liquidate if the problem cannot be corrected. Question Marks have the worst cash characteristics of all and are associated with high demands and low profit margin(s).1

SWOT analysis
A SWOT analysis is most helpful when assessing a practice in real time. The basic tenets are2:
Strengths:
- prestigious reputation
- technological expertise
Weaknesses:
- antiquated computer system
- lack of experience in specific areas
Opportunities:
- growing market demand for a specific product or procedure
- provision of unique services
Threats:
- changing demographics
- competitive practices
- changes in health care third-party payers.
The American College of Obstetricians and Gynecologists (ACOG) has developed an "ACOG Medical Home Toolkit" to allow ObGyns to assess how significant the changes regarding payers will be to their practice. Sections include the patient/practice partnership support; clinical care information; community resources; care delivery management; performance measurement and improvement; and payment and finance.3 The toolkit is available for download from the ACOG website.
References
- Morrison A, Wensley R. Boxing up or boxed in? A short history of the Boston Consulting Group Share/Growth Matrix. J Market Manag. 1993;7(2):105-129. http://www.tandfonline.com/doi/abs/10.1080/0267257X.1991.9964145.
- Klasko SK, Toub DB. It's not a plan without a business plan. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:36-37.
- American Congress of Obstetricians and Gynecologists. ACOG Medical Home Toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Practice-Management-and-Managed-Care/ACOG-Medical-Home-Toolkit. Accessed August 14, 2017.
Bottom line
Ensuring that your patients have an outstanding experience is a smart business strategy. A unified approach that includes team members’ involvement to create a patient-centered environment will provide a quality experience and encourage patients to recommend your ObGyn practice to others.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Independent of the Affordable Care Act or any upcoming changes in health care, the focus of an ObGyn practice remains paramount: the patient comes first.
The “recipe” for creating patient satisfaction and service excellence is predicated upon the mission of your practice and creating a shared vision with your employees. An action plan that is created and “visited/revisited”on a regular basis will serve to keep all abreast of the latest information to enhance the quality of patient care. It goes without saying, the ObGyn must first “lead by example” and always strive for satisfied patients who will tell their friends about your practice.
Start with the right tools
To organize a practice well, you need the right tools, which ideally include mission and vision statements and an action plan with goals and objectives.
Mission statement
A mission statement can be developed by the ObGyn(s) in your office or in concert with your staff. It should include:
- the “here and now” focus on the current approach to patient care
- why the practice exists (Develop a brief description of your practice, including the desired patient population.)
- the products and services offered and why and how those services are provided.
Here is an example of a mission statement for an ObGyn practice: “Our mission is to provide excellent, exceptional, personalized care for women of all ages in a warm and friendly environment. We incorporate leading-edge technology in our practice and continue to be a leader in obstetrics and gynecology.”
Vision statement
A vision statement should be developed in concert with your staff. It should include:
- the “then and there” focus on the historic perspective of your practice
- the ObGyn(s) and staff vision of the future
- what the ObGyn(s) and staff want to create.
The vision statement should energize and excite your personnel, create a shared and meaningful purpose, inspire passion and interest, and convey the values you want to share in your practice.
Here is an example of a vision statement for an ObGyn practice: “We aim to become the premier obstetrics and gynecology pro-vider to residents of (location) community.”
Action plan: Setting goals
To succeed, an ObGyn practice needs to:
- develop targets and challenges reflecting periodic (quarterly) meetings with staff and new entity development in the practice
- establish benchmarks and measurable parameters (How do you compare with other local practices? Set criteria/metrics to assess your progress.)
- ensure that the objectives support the goals (Develop goals and objectives over a defined period of time.)
- revisit the goals (Have they have been met? Do they need revision?)
Goals and objectives are essential for the continued health of your practice. This is all predicated upon developing a competitive advantage and then maintaining it.
Read about how to make a positive first impression on a new patient.
Is the environment welcoming?
When we examine a practice from the patient’s point of view, a good starting place is with the front desk. Have you looked at your front desk “from the outside in?” In one sense, this is the showcase of your practice.
Related article:
Four pillars of a successful practice: 2. Attract new patients
The first impression: Appointment scheduling
The first impression a patient receives about your practice occurs when she attempts to set up an appointment. Perhaps you might ask someone to call in to schedule an appointment. Is the caller immediately put on hold? Are your personnel courteous on the phone? Can she be seen quickly if she has a problem? How long is the wait for an annual exam? A test run can be very revealing.
Walk in the front door
When a patient walks in the door, does the physical office space radiate a friendly, relaxed atmosphere? Walk through the waiting room, then consultation and exam rooms as if you are a patient seeing it for the first time. Have you created an environment in which patients sense a well-organized office and the esprit de corps of the personnel? Does it look and smell fresh and clean? This all sends a loud and clear positive message about your practice.1–3
Here are some suggestions for making a waiting room more inviting:
- Provide a seating arrangement that is “patient centered.” For example, semi- circular arrangements allow easy viewing of any monitors in the waiting room.
- WiFi is a great addition. Post several signs with the user name and password.
- Offer computers for patients to use to complete registration
- Set up a fish tank. If well-maintained, it can be soothing to many people.
- Display medical information pamphlets, even if they are rarely taken.
- Provide a big screen television that offers information about your practice, including personnel and procedures.
Streaming ads for physician offices are available. One platform, Outcome Health (https://www.outcomehealth.com), provides flat-screen TVs and tablets that show patient education videos.4 Another vendor, Patient Point (http://patientpoint.com), offers waiting room networks, editorials, and other communications designed to support “the goals of improving healthcare.”5 Other available media include channel news and music programming to relax patients.6
Wait times. A patient’s perceived wait time and the actual wait time are often quite different. How long she waits to see the ObGyn is “numero uno” with regard to patient satisfaction and can be a key source of annoyance, irritability, stress, and anger.
Does someone inform waiting patients that the ObGyn is running late? Does staff at the front desk or perhaps your medical assistant inquire, “Can I get you anything? The doctor is running late,” or “Dr. Jones has just finished delivering a baby. He’ll be here in 10 minutes. He’ll see you first.”
Consultation and exam rooms
Suggestions to develop a relaxing environment in your consultation and exam rooms are7:
- decorate the walls with soft, pastel colors
- use “spa aesthetics” to create a colorful atmosphere with appropriate lighting, artwork, and modern furnishings
- present a few magazines neatly and update them periodically
- stock and appropriately maintain the patients rooms with medical supplies
- remember, “Subjects perceive people more positively in beautiful rooms than in ugly rooms.”5
Read about how to keep your patients satisfied and your business stable.
Set the lead example
The need for open and supportive communication between you and your office staff cannot be overly emphasized. An ideal office staff member understands and shares in the vision, is aware of stated goals and objectives, is responsive to patient needs, and wants to create a win-win environment.
Frequently discuss your expectations with your staff. Expect them to be responsive, courteous, competent, have good communication skills, and be influenced by the appearance of the physical environ-ment. Provide support and educational tools to help them successfully perform their work.
Related article:
Four pillars of a successful practice: 1. Keep your current patients happy
Discover your patients’ vision of customer service
Formal measurement of patient satisfaction began with Professor Irwin Press at the University of Notre Dame. Rod Ganey, a sociologist and statistician, then developed the Press Ganey Patient Satisfaction Survey. These points earlier conveyed by Maslow and Mintz8 addressed the “effects of esthetic surroundings.” Color and art proved to be preferences in an esthetically pleasing environment. Additional historical information has been provided by Siegrist, who addressed “the patient experience.”9 He cites the myth that patients do not fill out satisfaction surveys. Indeed they do. Patient satisfaction is not a personality contest but rather a reflection of the health care provider’s investment of time and effort to offer patient-centered care. Siegrist also notes that the patient’s family plays a key role in how a patient perceives her experience with her health care professional.9
The federal government has been actively involved in assessing patient satisfaction in the hospital setting since 2002. This is reflected in the Centers for Medicare and Medicaid Services, the Agency for Healthcare Research and Quality, and Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys. The HCAHPS is a 27-question survey randomly administered to adult inpatients after discharge.10–12
The following metrics are often included in patient satisfaction surveys9,10:
- rating of hospital care from 0 (lowest) to 10 (highest)
- percentage of patients who would recommend a practice to family and friends
- number of patients who say their health care providers always communicate well
- the number of patients who report that the office is always clean and friendly.
Use of search engines focused on health care patient surveys can provide a number of options for clinicians to use in their practice.
Tips on patient satisfaction
Several interesting tips from the busi-ness world can be applied to an ObGyn’s practice14:
- You will only hear from 4% of unhappy customers.
- One dissatisfied customer tells 9.
- 95% of customers with resolved issues will do business with you again.
- If a problem is not addressed, that patient will tell 10 others.
- Resolve the problem and 5 people will know about it.
- It costs 5 times as much effort to gain 1 new customer.
- Loyal customers in 1 area of service are good prospects for other (new) services.
Related article:
Using the Internet in your practice. Part 2: Generating new patients using social media
Tell stories about good, satisfied patients
Sharing the stories of satisfied patients motivates others to consider coming to your practice. To develop these stories, offer a “suggestion box” where patients can leave compliments or comments about their experiences. Ask patients to record their positive reviews (be sure to obtain written consent before recording and publishing). Show the videos on the big-screen TVs in your waiting room and include patient reviews (written, audio, and video) on your website.15
Related article:
Four pillars of a successful practice: 4. Motivate your staff
Reevaluate periodically
Encouraging team spirit makes good business sense. Offer staff members bonuses for coming up with improved processes. Provide educational programs for staff on patient care, technology, etc. If a difficult experience occurs, discuss it openly with staff members without accusing, asking them for suggestions to improve the situation.16
To assess the monetary value of your practice, you need to know what contributes to your profit margin and overhead. What investments are the most profitable? Then monitor each segment of the office practice.
Should you proceed with a purchase? Should you take on a new hire? Let's look at one excellent model from the Boston Consulting Group (FIGURE) that provides insight into "low and high performance" aspects of business or practice.1
In the matrix, Stars use large amounts of cash and are leaders in cash generation. Stars lead to development of a Cash Cow, which are entities that generate profits and cash with low investment prerequisites. Dogs are segments of product and service line(s) that should be carefully reevaluated. A decision must be made to liquidate if the problem cannot be corrected. Question Marks have the worst cash characteristics of all and are associated with high demands and low profit margin(s).1

SWOT analysis
A SWOT analysis is most helpful when assessing a practice in real time. The basic tenets are2:
Strengths:
- prestigious reputation
- technological expertise
Weaknesses:
- antiquated computer system
- lack of experience in specific areas
Opportunities:
- growing market demand for a specific product or procedure
- provision of unique services
Threats:
- changing demographics
- competitive practices
- changes in health care third-party payers.
The American College of Obstetricians and Gynecologists (ACOG) has developed an "ACOG Medical Home Toolkit" to allow ObGyns to assess how significant the changes regarding payers will be to their practice. Sections include the patient/practice partnership support; clinical care information; community resources; care delivery management; performance measurement and improvement; and payment and finance.3 The toolkit is available for download from the ACOG website.
References
- Morrison A, Wensley R. Boxing up or boxed in? A short history of the Boston Consulting Group Share/Growth Matrix. J Market Manag. 1993;7(2):105-129. http://www.tandfonline.com/doi/abs/10.1080/0267257X.1991.9964145.
- Klasko SK, Toub DB. It's not a plan without a business plan. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:36-37.
- American Congress of Obstetricians and Gynecologists. ACOG Medical Home Toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Practice-Management-and-Managed-Care/ACOG-Medical-Home-Toolkit. Accessed August 14, 2017.
Bottom line
Ensuring that your patients have an outstanding experience is a smart business strategy. A unified approach that includes team members’ involvement to create a patient-centered environment will provide a quality experience and encourage patients to recommend your ObGyn practice to others.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Ulrich RS. Evidence-based environmental design for improving medical outcomes: Paper Delivered at a Conference Entitled Healing by Design: Building for Health Care in the 21st Century. Montreal: McGill University Health Centre; 2000. http://www.brikbase.org/sites/default/files/Evidence%20Based%20Environmental%20Design%20for%20Improving%20Medical.pdf. Accessed August 15, 2017.
- Becker F, Douglass S. The ecology of the patient visit: physical attractiveness, waiting times and perceived quality of care. J Ambul Care Manag. 2008;31(2):128–141.
- Becker F, Sweeney B, Parsons K. Ambulatory facility design and patients’ perceptions of healthcare quality. HERD. 2008;1(4):35–54.
- Outcome Health Website. https://www.outcomehealth.com/. Accessed August 14, 2017.
- Mazer SE. The waiting room: Where suffering begins. Healing Healthcare Systems website. http://www.healinghealth.com/waiting-room-suffering-begins/. Published November 7, 2014. Accessed August 14, 2017.
- Patient Point Programs Website. http://patientpoint.com/. Accessed August 14, 2017.
- Almquist J, Kelly C, Bromberg J, Bryant S, Christianson T, Montori V. Consultation room design and the clinical encounter: the space and interaction randomized trial. Health Environ Res Design. 2009;3(1):41–78.
- Maslow A, Mintz N. Effects of esthetic surroundings: I. Initial effects of three esthetic conditions upon perceiving “energy” and “well-being” in faces. J Psychology. 1956;41(2):247–254.
- Siegrist RB. The patient experience. In: Sanfilippo JS, Bieber E, Javich D, Siegrist R, eds. MBA for Healthcare. New York, NY: Oxford Press;2016:227–236.
- Press I. Patient satisfaction: Understanding and managing the experience of care. 2nd ed. Chicago, IL: Health Administration Press; 2005:66–78.
- Piper L, Tallman E. Hospital consumer assessment of healthcare providers and systems: An ethical leadership dilemma to satisfy patients. Health Care Manag (Frederick). 2016;35(2):151–155.
- Giordano L, Elliott M, Goldstein E, Lehrman W, Spencer P. Development, implementation and public reporting of HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- Jones KE. Helping the health profession help others: Applying business principles to the medical world. University of Tennessee, Knoxville Honors Thesis Projects. http://trace.tennessee.edu/cgi/viewcontent.cgi?article=1560&context=utk_chanhonoproj. Published 2002. Accessed August 14, 2017.
- Baum N. Marketing your practice: ethically, effectively and economically. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:123–154.
- Baum NH. Four pillars of a successful practice: 1. Keep your current patients happy. OBG Manag. 2013;25(3):49–56.
- Baum NH. Four pillars of a successful practice: 4. Motivate your staff. OBG Manag. 2013;25(8):29–33.
- Ulrich RS. Evidence-based environmental design for improving medical outcomes: Paper Delivered at a Conference Entitled Healing by Design: Building for Health Care in the 21st Century. Montreal: McGill University Health Centre; 2000. http://www.brikbase.org/sites/default/files/Evidence%20Based%20Environmental%20Design%20for%20Improving%20Medical.pdf. Accessed August 15, 2017.
- Becker F, Douglass S. The ecology of the patient visit: physical attractiveness, waiting times and perceived quality of care. J Ambul Care Manag. 2008;31(2):128–141.
- Becker F, Sweeney B, Parsons K. Ambulatory facility design and patients’ perceptions of healthcare quality. HERD. 2008;1(4):35–54.
- Outcome Health Website. https://www.outcomehealth.com/. Accessed August 14, 2017.
- Mazer SE. The waiting room: Where suffering begins. Healing Healthcare Systems website. http://www.healinghealth.com/waiting-room-suffering-begins/. Published November 7, 2014. Accessed August 14, 2017.
- Patient Point Programs Website. http://patientpoint.com/. Accessed August 14, 2017.
- Almquist J, Kelly C, Bromberg J, Bryant S, Christianson T, Montori V. Consultation room design and the clinical encounter: the space and interaction randomized trial. Health Environ Res Design. 2009;3(1):41–78.
- Maslow A, Mintz N. Effects of esthetic surroundings: I. Initial effects of three esthetic conditions upon perceiving “energy” and “well-being” in faces. J Psychology. 1956;41(2):247–254.
- Siegrist RB. The patient experience. In: Sanfilippo JS, Bieber E, Javich D, Siegrist R, eds. MBA for Healthcare. New York, NY: Oxford Press;2016:227–236.
- Press I. Patient satisfaction: Understanding and managing the experience of care. 2nd ed. Chicago, IL: Health Administration Press; 2005:66–78.
- Piper L, Tallman E. Hospital consumer assessment of healthcare providers and systems: An ethical leadership dilemma to satisfy patients. Health Care Manag (Frederick). 2016;35(2):151–155.
- Giordano L, Elliott M, Goldstein E, Lehrman W, Spencer P. Development, implementation and public reporting of HCAHPS survey. Med Care Res Rev. 2010;67(1):27–37.
- Jones KE. Helping the health profession help others: Applying business principles to the medical world. University of Tennessee, Knoxville Honors Thesis Projects. http://trace.tennessee.edu/cgi/viewcontent.cgi?article=1560&context=utk_chanhonoproj. Published 2002. Accessed August 14, 2017.
- Baum N. Marketing your practice: ethically, effectively and economically. In: Sanfilippo JS, Nolan TE, Whiteside BH, eds. MBA Handbook for Healthcare Professionals. New York, NY: Parthenon Publishing Group; 2002:123–154.
- Baum NH. Four pillars of a successful practice: 1. Keep your current patients happy. OBG Manag. 2013;25(3):49–56.
- Baum NH. Four pillars of a successful practice: 4. Motivate your staff. OBG Manag. 2013;25(8):29–33.