User login
Breast density and optimal screening for breast cancer
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
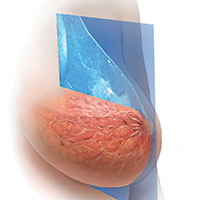
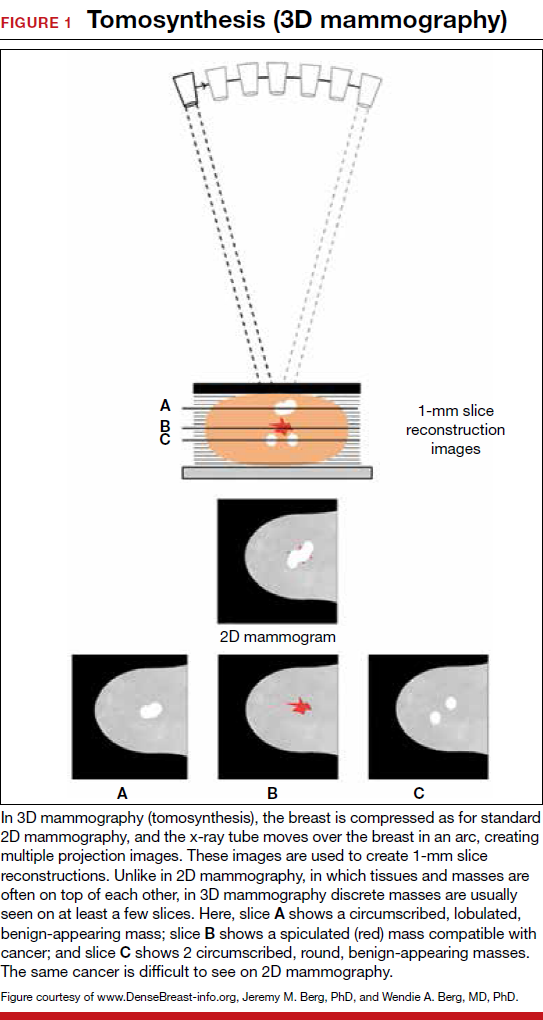
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.
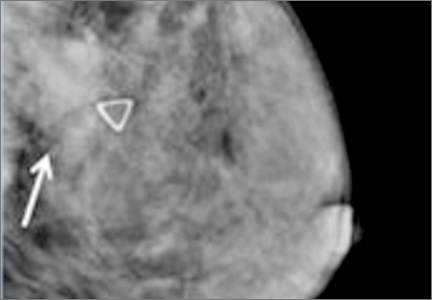
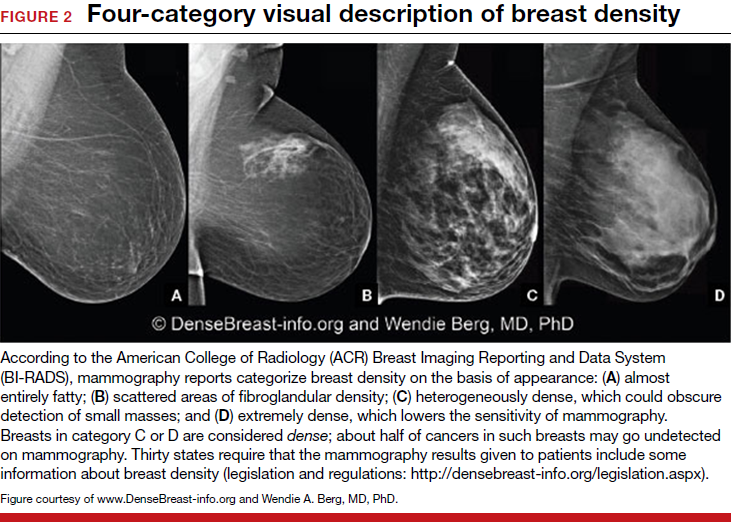
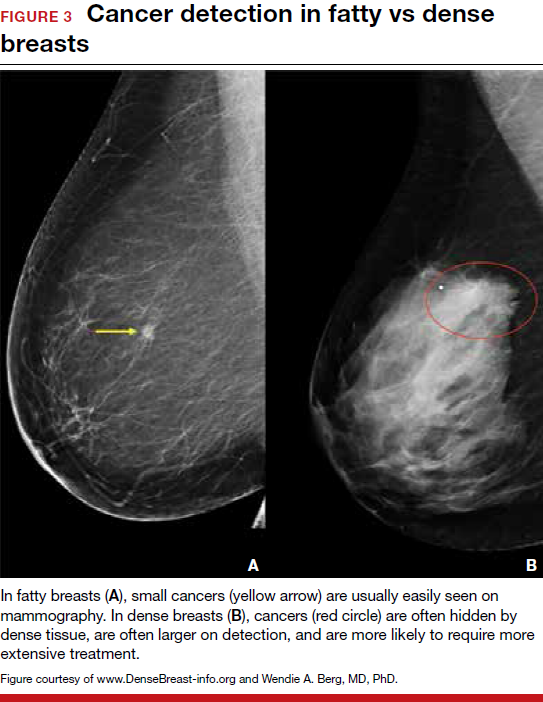
Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).
3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts
MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
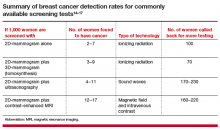
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

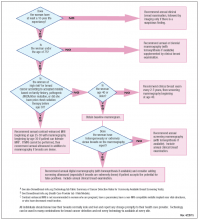
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.

FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.

Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
Get smart about dense breasts
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
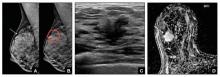
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
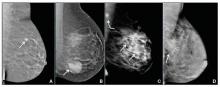
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
It’s a movement that shows no signs of abating. Women in 24 states, encompassing 67% of American women, now receive some level of notification after their mammogram about breast density. Individual patient advocates continue to push for notification, and states are likely to continue to draft bills. On the national level, a federal standard is being pursued through both federal legislation and federal regulation. Clinicians practicing in states with an “inform” law, either already in effect or imminent, will be tasked with engaging in new patient conversations arising from density notification. Are you ready to answer your patients’ questions?
Navigating inconsistent data and expert comments about density and discerning which patients may benefit from additional screening can create challenges in addressing a patient’s questions about the implications of her dense tissue. If you feel less than equipped to address these issues, you are not alone. A recent survey of clinicians, con- ducted after California’s breast density notification law went into effect, showed that only 6% were comfortable answering patients’ questions relating to breast density. Seventy-five percent of respondents indicated they wanted more education on the topic.1
For women having mammography, dense breast tissue is most important because it can mask detection of cancers, and women may want to have additional screening beyond mammography. Women with dense breasts are also at increased risk for developing breast cancer. For clinicians who are on the front lines of care for women undergoing screening, the most important action items are:
- identifying who needs more screening
- weighing the risks and benefits of such additional screening.
To assist you in informing patient discussions, in this article we answer some of the most frequently asked questions of ObGyns.
Which breasts are considered dense?
The American College of Radiology recommends that density be reported in 1 of 4 categories depending on the relative amounts of fat and fibroglandular tissue2:
- almost entirely fatty—on mammography most of the tissue appears dark gray while small amounts of dense (or fibroglandular) tissue display as light gray or white.
- scattered fibroglandular density—scattered areas of dense tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers sometimes can be missed when they resemble areas of normal tissue or are within an area of denser tissue.
- heterogeneously dense—there are large portions of the breast where dense tissue could hide masses.
- extremely dense—most of the breast appears to consist of dense tissue, creating a “white out” situation and making it extremely difficult to see through.
Breasts that are either heterogeneously dense or extremely dense are considered “dense.” About 40% of women older than age 40 have dense breasts.3
Case study: Imaging of a cancerous breast mass in a 48-year-old woman with dense breasts
This patient has heterogeneously dense breast tissue. Standard 2D mediolateral oblique (MLO) digital mammogram (A) and MLO tomosynthesis 1-mm slice (B) reveal subtle possible distortion (arrow) in the upper right breast. On tomosynthesis, the distortion is better seen, as is the underlying irregular mass (red circle).
Ultrasound (US) directed to the mass (C) shows an irregular hypoechoic (dark gray) mass (marked by calipers), compatible with cancer. US-guided core needle biopsy revealed grade 2 invasive ductal cancer with associated ductal carcinoma in situ.
Axial magnetic resonance imaging of the right breast obtained after contrast injection, and after computer subtraction of nonenhanced image (D), shows irregular spiculated enhancing (white) mass (arrow) due to the known carcinoma.
Images: Courtesy Wendie Berg, MD, PhD
Who needs more screening?
The FIGURE is a screening decision support tool representing the consensus opinion of several medical experts based on the best available scientific evidence to optimize breast cancer detection.
Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breasts are a risk factor for breast cancer. According to the American Cancer Society’s Breast Cancer Facts & Figures 2013−2014, “The risk of breast cancer in-creases with increasing breast density; women with very high breast density have a 4- to 6-fold increased risk of breast cancer compared to women with the least dense breasts.”4,5
There are several reasons that dense tissue increases risk. First, the glands tend to be made up of relatively actively dividing cells that can mutate and become cancerous (the more glandular tissue present, the greater the risk). Second, the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this seems to occur more in fibrous tissue than in fatty tissue.
Most women have breast density somewhere in the middle range, with their risk for breast cancer falling in between those with extremely dense breasts and those with fatty breasts.6 The risk for developing breast cancer is influenced by a combination of many different factors, including age, family history of cancer (particularly breast or ovarian cancer), and prior atypical breast biopsies. There currently is no reliable way to fully account for the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. Importantly, more than half of all women who develop breast cancer have no known risk factors other than being female and aging.
Is your medical support staff “density ready?”
We’re all familiar with the adage that a picture is worth a thousand words. While the medical support personnel in your office are likely quite familiar with imaging reports and the terminology used in describing dense breasts, they may be quite unfamiliar with what a fatty versus dense breast actually looks like on a mammogram, and how cancer may display in each. Illustrated examples, as seen here, are useful for reference.
In the fatty breast (A), a small cancer (arrow) is seen easily. In a breast categorized as scattered fibroglandular density (B), a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden in areas with normal glandular tissue.
In a breast categorized as heterogeneously dense (C), a 4-cm (about 1.5-inch) cancer (arrows) is hidden by the dense breast tissue. This cancer also has spread to a lymph node under the arm (curved arrow).
In an extremely dense breast (D), a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
Image: Courtesy of Dr. Regina Hooley and DenseBreast-info.org
Are screening mammography outcomes different for women with dense versus fatty breasts?
Yes. Cancer is more likely to be clinically detected in the interval between mammography screens (defined as interval cancer) in women with dense breasts. Such interval cancers tend to be more aggressive and have worse outcomes. Compared with those in fatty breasts, cancers found in dense breasts more often7:
- are locally advanced (stage IIb and III)
- are multifocal or multicentric
- require a mastectomy (rather than a lumpectomy).
Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials with mortality as an endpoint. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes.8
No randomized controlled trial has yet been reported on any other imaging screening modality, but it is expected that other screening tests that increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized controlled trial with 15 to 20 years of follow-up. Given the speed of technologic developments, any results likely would be obsolete by the trial’s conclusion. What we do know is that women at high risk for breast cancer who undergo annual magnetic resonance imaging (MRI) screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI.9
We also know that average-risk women who are screened with ultrasonography in addition to mammography are unlikely to have palpable cancer in the interval between screens,10,11 with the rates of such interval cancers similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size, approximately 1 cm) that are mostly node negative.12,13 MRI also finds some ductal carcinoma in situ (DCIS).
These results suggest that there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, as with mammography, some of the cancers found with supplemental screening are slow growing and may never have caused a woman harm even if left untreated.
Dense breasts: Medically sourced resources
Educational Web site
DenseBreast-info.org. This site is a collaborative, multidisciplinary educational resource. It features content for both patients and health care providers with separate data streams for each and includes:
a comprehensive list of FAQs; screening flow charts; a Patient Risk Checklist; an explanation of risks, risk assessment, and links to risk assessment tools; an illustrated round-up of technologies commonly used in screening; and state-by-state legislative analysis of density inform laws across the country.
State-specific Web sites
BreastDensity.info. This site was created by the California Breast Density Information Group (CBDIG), a working group of breast radiologists and breast cancer risk specialists. The content is primarily for health care providers and features screening scenarios as well as FAQs about breast density, breast cancer risk, and the breast density notification law in California.
MIdensebreasts.org. This is a Web-based education resource created for primary care providers by the University of Michigan Health System and the Michigan Department of Health and Human Services. It includes continuing medical education credit.
Medical society materials
American Cancer Society offers Breast Density and Your Mammogram Report for patients: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-039989.pdf
American College of Obstetricians and Gynecologists’ 2015 Density Policy statement is available online: http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Management-of-Women-With-Dense-Breasts-Diagnosed-by-Mammography
American College of Radiology patient brochure details basic information about breast density and can be customized with your center’s information: http://www.acr.org/News-Publications/~/media/180321AF51AF4EA38FEC091461F5B695.pdf
What additional screening tests are available after a 2D mammogram for a woman with dense breasts?
Depending on the patient’s age, risk level, and breast density, additional screening tools—such as tomosynthesis (also known as 3D mammography), ultrasonography, or MRI—may be recommended in addition to mammography. Indeed, in some centers, tomosynthesis is performed alone and the radiologist also reviews computer-generated 2D mammograms.
The addition of another imaging tool after a mammogram will find more cancers than mammography alone (TABLE).14−17 Women at high risk for breast cancer, such as those with pathogenic BRCA mutations, and those who were treated with radiation therapy to their chest (typically for Hodgkin disease) before age 30 and at least 8 years earlier, should be referred for annual MRI in addition to mammography (see Screening Decision Support Tool FIGURE above). If tomosynthesis is performed, the added benefit of ultrasound will be lower; further study on the actual benefit of supplemental ultrasound screening after 3D mammography is needed.
Will insurance cover supplemental screening beyond mammography?
The answer depends on the type of screening, the patient’s insurance and risk factors, the state in which you practice, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, a woman with dense breasts can receive a screening ultrasound without a copay or deductible if it is ordered by a physician. In Connecticut, an ultrasound copay for screening dense breasts cannot exceed $20. Generally, in other states, an ultrasound will be covered if ordered by a physician, but it is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts.
Regardless of state, an MRI generally will be covered by insurance (subject to copay and deductible) if the patient meets high-risk criteria. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost that matches the copay and deductible of a screening mammogram. It is important for patients to check with their insurance carrier prior to having an MRI.
Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women (except for those who are pregnant or breastfeeding, in which case ultrasound can be performed but is usually deferred until several months after the patient is no longer pregnant or breastfeeding). While additional screening may be recommended for women with dense breasts, and for women at high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Wherever possible, women with dense breasts should have digital mammography rather than film mammography, due to slightly improved cancer detection using digital mammography.18
Does tomosynthesis solve the problem of screening dense breasts?
Tomosynthesis (3D mammography) slightly improves detection of cancers compared with standard digital mammography, but some cancers will remain hidden by overlapping dense tissue. We do not yet know the benefit of annual screening tomosynthesis. Without question, women at high risk for breast cancer still should have MRI if they are able to tolerate it, even if they have had tomosynthesis.
If a patient with dense breasts undergoes screening tomosynthesis, will she also need a screening ultrasound?
Preliminary studies not yet published suggest that, for women with dense breasts, ultrasound does find another 2 to 3 invasive cancers per 1,000 women screened that are not found on tomosynthesis, but further study of this issue is needed.
If recommended for additional screening with ultrasound or MRI, will a patient need that screening every year?
Usually, yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations. Therefore, screening recommendations may change from one year to the next. With technology advancements and evolving guidelines, additional screening recommendations will change in the future.
Prepare yourself and your patients will benefit
The foundation of a successful screening program involves buy-in from both patient and clinician. Patients confused as to what their density notification means may have little understanding as to what next steps should be considered. To allay confusion, your patient will be best served by being provided understandable and actionable information. Advanced preparation for these conversations about the implications of dense tissue will make for more effective patient engagement.
Acknowledgment
Much of the material within this article has been sourced from the educational Web site DenseBreast-info.org. For comprehensive lists of both patient and health care provider frequently asked questions, visit http://www.DenseBreast-info.org.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
- Khong KA, Hargreaves J, Aminololama-Shakeri S, Lindfors KK. Impact of the California breast density law on primary care physicians. J Am Coll Radiol. 2015;12(3):256–260.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS Mammography. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- American Cancer Society. Breast Cancer Facts & Figures 2013–2014. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042725.pdf. Published 2013. Accessed September 15, 2015.
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837.
- Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol. 2010;17(suppl 3):211–218.
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806, v.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7): 1021–1026.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234–240.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:2–19.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–43.
- Berg WA. Screening MRI. In: Berg WA, Yang WT, eds. Diagnostic Imaging: Breast. 2nd ed. Salt Lake City, UT: Amirsys; 2014:9–49.
- Hooley R. Tomosynthesis. In: Berg WA, Yang WT, eds.Berg WA. Screening Ultrasound. In: Berg WA, Yang WT, eds.Berg WA. Screening MRI. In: Berg WA, Yang WT, eds.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783.
In this Article
- Breast mass imaging case study
- Screening decision support tool
- Is your support staff “density” ready?