User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Healthy habits lower T2D microvascular risks: Cohort study
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
People with diabetes who adhere to a healthy diet, exercise regularly, and follow other healthy lifestyle practices have a significantly lower risk of microvascular complications from the disease, such as diabetic neuropathy, retinopathy, and nephropathy, as well as foot disorders, than counterparts with diabetes who don’t, a prospective cohort study of more than 7,000 patients with type 2 diabetes has found.
“We believe this is one of the first large-scale analyses among diabetes patients that specifically examined an overall healthy lifestyle in relation to the risk of developing microvascular complications,” senior study author Qi Sun, MD, ScD, said in an interview. “The results are not surprising that the healthy lifestyle is associated with lower risk of developing these complications and the enhanced adherence to the healthy lifestyle is associated with lower risk as well. And these findings bear lots of public health significance as they suggest the important role of living a healthy lifestyle in the prevention of diabetes complications, on top of the clinical treatment.”
Dr. Sun is an associate professor of nutrition and epidemiology at the Harvard T.H. Chan School of Public Health, Boston.
The study stated that the findings “lend support” for the American Diabetes Association guidelines for healthy lifestyle practices in people with diabetes.
The study used a cohort from two large prospective cohort studies, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), comprising 4,982 women and 2,095 men who were diagnosed with type 2 diabetes during follow-up. They had no cardiovascular disease or cancer at the time of their diabetes diagnosis. Both NHS and HPFS used validated questionnaires to gather information on diet, lifestyle, medical history, and newly diagnosed diseases every 2-4 years. The latter study included NHS and HPFS participants who also completed supplementary questionnaires about their diabetes.
The latest study took into account five modifiable lifestyle-related factors: diet, body weight, smoking status, alcohol, and physical activity. For diet, both large studies used the 2010 Alternate Healthy Eating Index to assess diet quality; those in the upper 40th percentile of the study population were defined as healthy diet. Healthy body weight was defined at a body mass index of 18.5-25 kg/m2.
Among the latter study cohort, 2,878 incident cases of diabetic microvascular complications were documented during follow-up. Patients who adhered to a healthy lifestyle before their diabetes diagnosis, defined as having four or more low-risk lifestyle factors, had a 27% lower relative risk of developing any microvascular complication than counterparts with no low-risk lifestyle factors (relative risk, 0.73; 95% confidence interval, 0.35-1; P = .006).
The study found similar outcomes for those who adopted a healthy lifestyle after their diabetes diagnosis, with a 32% reduction in relative risk compared with those who didn’t adopt any healthy lifestyle practices (RR, 0.68; 95% CI, 0.55-0.83; P < .001).
Dr. Sun noted what was noteworthy about his group’s cohort study. “The unique design is truly the prospective follow-up over time so that we could examine the lifestyle at diabetes diagnosis as well as changes in lifestyle before and after diabetes in relation to the future risk of developing the complications,” he said.
A randomized trial would be a more rigorous way to evaluate the impact of a healthy lifestyle, he added, “although it’s much more expensive than a cohort study like what we did with this investigation.”
As for future research, Dr. Sun said, “It will be interesting to understand mechanisms underlying these observations. It’s also critical to understand why certain diabetes patients may not benefit from a healthy lifestyle, since some of them, even when living a healthy lifestyle, still develop the complications.”
This trial shows in a new light the benefits of healthy lifestyle practices on microvascular complications of type 2 diabetes, Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., and a professor at the University of Miami, said in a comment. “These benefits have always been surmised and demonstrated in a limited way in previous trials, but not subject to the level of analysis seen in this prospective cohort trial.”
He called the study design “excellent,” adding, “ ‘Validated’ self-reported questionnaires were used widely, although minimal detail is provided about the validation process.” One limitation, he noted, was “the homogeneity of the participants; all were health professionals.”
The study “affirms” and “quantitates” the benefits of a healthy lifestyle in type 2 diabetes. “The issue is not unawareness but rather application,” Dr. Jellinger said. “Modifying long-held lifestyle habits is a real challenge. Perhaps by ‘quantitating’ the benefit, as shown in this trial and hopefully additional studies, impetus will be provided to refocus on this approach, which is too often simply given lip service.”
The National Institutes of Health provided funding for the study. Dr. Sun has no relevant disclosures. Dr. Jellinger disclosed relationships with Amgen and Esperion.
FROM JAMA NETWORK OPEN
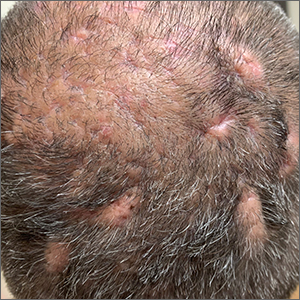
Scalp ridges

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002
Angioedema risk jumps when switching HF meds
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New renin-angiotensin-system (RAS) inhibitor therapy using sacubitril-valsartan (Entresto) is no more likely to cause angioedema than starting out with an ACE inhibitor or angiotensin receptor blocker (ARB).
But the risk climbs when such patients start on an ACE inhibitor or ARB and then switch to sacubitril-valsartan, compared with those prescribed the newer drug, the only available angiotensin receptor-neprilysin inhibitor (ARNI), in the first place.
Those findings and others from a large database analysis, by researchers at the Food and Drug Administration and Harvard Medical School, may clarify and help alleviate a residual safety concern about the ARNI – that it might promote angioedema – that persists after the drug’s major HF trials.
The angioedema risk increased the most right after the switch to the ARNI from one of the older RAS inhibitors. For example, the overall risk doubled for patients who started with an ARB then switched to sacubitril-valsartan, compared with those who started on the newer drug. But it went up about 2.5 times during the first 14 days after the switch.
A similar pattern emerged for ACE inhibitors, but the increased angioedema risk reached significance only within 2 weeks of the switch from an ACE inhibitor to sacubitril-valsartan compared to starting on the latter.
The analysis, based on data from the FDA’s Sentinel adverse event reporting system, was published in the Journal of the American College of Cardiology.
A rare complication, but ...
Angioedema was rare overall in the study, with an unadjusted rate of about 6.75 per 1,000 person-years for users of ACE inhibitors, less than half that rate for ARB users, and only one-fifth that rate for sacubitril-valsartan recipients.
But even a rare complication can be a worry for drugs as widely used as RAS inhibitors. And it’s not unusual for patients cautiously started on an ACE inhibitor or ARB to be switched to sacubitril-valsartan, which is only recently a core guideline–recommended therapy for HF with reduced ejection fraction.
Such patients transitioning to the ARNI, the current study suggests, should probably be watched closely for signs of angioedema for 2 weeks but especially during the first few days. Indeed, the study’s event curves show most of the extra risk “popping up” right after the switch to sacubitril-valsartan, lead author Efe Eworuke, PhD, told this news organization.
The ARNI’s labeling, which states the drug should follow ACE inhibitors only after 36-hour washout period, “has done justice to this issue,” she said. But “whether clinicians are adhering to that, we can’t tell.”
Potentially, patients who miss the 36-hour washout between ACE inhibitors or ARBs and sacubitril-valsartan may account for the excess angioedema risk seen in the analysis, said Dr. Eworuke, with the FDA’s Center for Drug Evaluation and Research, Silver Spring, Md.
But the analysis doesn’t nail down the window of excess risk to only 36 hours. It suggests that patients switching to the ARNI – even those pausing for 36 hours in between drugs – should probably be monitored “2 weeks or longer,” she said. “They could still have angioedema after the washout period.”
Indeed, the “timing of the switch may be critical,” according to an editorial accompanying the report. “Perhaps a longer initial exposure period of ACE inhibitor or ARB,” beyond 2 weeks, “should be considered before switching to an ARNI,” contended Robert L. Page II, PharmD, MSPH, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora.
Moreover, he wrote, the study suggests that “initiation of an ARNI de novo may be safer compared with trialing an ACE inhibitor or ARB then switching to an ARNI,” and “should be a consideration when beginning guideline-directed medical therapy for patients with HF.”
New RAS inhibition with ARNI ‘protective’
Compared with ARNI “new users” who had not received any RAS inhibitor in the prior 6 months, patients in the study who switched from an ACE inhibitor to ARNI (41,548 matched pairs) showed a hazard ratio (HR) for angioedema of 1.62 (95% confidence interval [CI], 0.91-2.89), that is, only a “trend,” the report states.
But that trend became significant when the analysis considered only angioedema cases in the first 14 days after the drug switch: HR, 1.98 (95% CI, 1.11-3.53).
Those switching from an ARB to ARNI, compared with ARNI new users (37,893 matched pairs), showed a significant HR for angioedema of 2.03 (95% CI, 1.16-3.54). The effect was more pronounced when considering only angioedema arising in the first 2 weeks: HR, 2.45 (95% CI, 1.36-4.43).
Compared with new use of ACE inhibitors, new ARNI use (41,998 matched pairs) was “protective,” the report states, with an HR for angioedema of 0.18 (95% CI, 0.11-0.29). So was a switch from ACE inhibitors to the ARNI (69,639 matched pairs), with an HR of 0.31 (95% CI, 0.23-0.43).
But compared with starting with an ARB, ARNI new use (43,755 matched pairs) had a null effect on angioedema risk, HR, 0.59 (95% CI, 0.35-1.01); as did switching from an ARB to ARNI (49,137 matched pairs), HR, 0.85 (95% CI, 0.58-1.26).
The analysis has limitations, Dr. Eworuke acknowledged. The comparator groups probably differed in unknown ways given the limits of propensity matching, for example, and because the FDA’s Sentinel system data can reflect only cases that are reported, the study probably underestimates the true prevalence of angioedema.
For example, a patient may see a clinician for a milder case that resolves without a significant intervention, she noted. But “those types of angioedema would not have been captured by our study.”
Dr. Eworuke disclosed that her comments reflect her views and are not those of the Food and Drug Administration; she and the other authors, as well as editorialist Dr. Page, report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Novel nomogram distinguishes pneumonias
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
A model incorporating factors such as lymphocytes and lung lesions differentiated adenovirus pneumonias from Chlamydia psittaci (CPP) in a multicenter study of nearly 200 individuals.
Symptoms of pneumonia caused by CPP are often confused with other respiratory infections, particularly adenovirus pneumonia (AVP), which can delay correct diagnosis and impact treatment, Yi Li, MD, of Xiangya Hospital, Central South University, Changsha, China, and colleagues wrote. Detailed comparisons of the two conditions are lacking.
In a retrospective study published in the International Journal of Infectious Diseases, the researchers examined laboratory, clinical, and radiological differences and created a nomogram to distinguish CPP from AVP. The study population included 78 adults with CPP and 102 with AVP who were seen at a single center in China. The mean ages of the CPP and AVP patients were 61.0 years and 38.5 years, and 57.7% men and 91.2% men, respectively. Patients with CPP were significantly more likely to have hypertension and diabetes at baseline, compared with the AVP group.
The primary outcome was 30-day mortality after hospital admission, which was 10.3% and 14.7% for the CPP and AVP patients, respectively (P = 0.376). However, the incidence of cardiac injury was significantly higher in AVP patients versus those with CPP (48.0% vs. 11.5%; P < 0.001).
In a multivariate analysis, age, sex, nervous system symptoms, lymphocyte count, C-reactive protein level (CRP), and bilateral lung lesions were risk factors for CPP. The researchers combined these factors into a nomogram that showed a concordance value of 0.949 for differentiating between the CPP and AVP groups.
Overall, CPP patients were older, had more nervous system symptoms, and had higher CRP levels, compared with patients with AVP, who were more likely to be men and to have higher lymphocyte percentages and more bilateral lung lesions on chest imaging.
The current study is the first known to provide a way to distinguish CPP and AVP, the researchers wrote. “The antibiotic treatments, prognoses, and life support measures of CPP and AVP are considerably different. Therefore, differentiating the two diseases through early identification of specific clinical characteristics is vital.”
The findings were limited by several factors including the small sample size, retrospective design, and the use of mNGS to diagnose CPP in the absence of standard clinical diagnostic kits, which may have resulted in underestimated CPP incidence, the researchers noted.
However, “the nomogram we established combines patient data on age, sex, and readily available laboratory results to reasonably predict CPP, thus making rapid and direct diagnosis possible,” they said.
The study was supported by the Key R&D Program of Hunan Province, Project Program of National Clinical Research Center for Geriatric Disorders, National Natural Science Foundation of China, Hunan Natural Science Youth Foundation, and the national key clinical specialist construction programs of China. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Even one head injury boosts all-cause mortality risk
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An analysis of more than 13,000 adult participants in the Atherosclerosis Risk in Communities (ARIC) study showed a dose-response pattern in which one head injury was linked to a 66% increased risk for all-cause mortality, and two or more head injuries were associated with twice the risk in comparison with no head injuries.
These findings underscore the importance of preventing head injuries and of swift clinical intervention once a head injury occurs, lead author Holly Elser, MD, PhD, department of neurology, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
“Clinicians should counsel patients who are at risk for falls about head injuries and ensure patients are promptly evaluated in the hospital setting if they do have a fall – especially with loss of consciousness or other symptoms, such as headache or dizziness,” Dr. Elser added.
The findings were published online in JAMA Neurology.
Consistent evidence
There is “pretty consistent evidence” that mortality rates are increased in the short term after head injury, predominantly among hospitalized patients, Dr. Elser noted.
“But there’s less evidence about the long-term mortality implications of head injuries and less evidence from adults living in the community,” she added.
The analysis included 13,037 participants in the ARIC study, an ongoing study involving adults aged 45-65 years who were recruited from four geographically and racially diverse U.S. communities. The mean age at baseline (1987-1989) was 54 years; 57.7% were women; and 27.9% were Black.
Study participants are followed at routine in-person visits and semiannually via telephone.
Data on head injuries came from hospital diagnostic codes and self-reports. These reports included information on the number of injuries and whether the injury required medical care and involved loss of consciousness.
During the 27-year follow-up, 18.4% of the study sample had at least one head injury. Injuries occurred more frequently among women, which may reflect the predominance of women in the study population, said Dr. Elser.
Overall, about 56% of participants died during the study period. The estimated median amount of survival time after head injury was 4.7 years.
The most common causes of death were neoplasm, cardiovascular disease, and neurologic disorders. Regarding specific neurologic causes of death, the researchers found that 62.2% of deaths were due to neurodegenerative disease among individuals with head injury, vs. 51.4% among those without head injury.
This, said Dr. Elser, raises the possibility of reverse causality. “If you have a neurodegenerative disorder like Alzheimer’s disease dementia or Parkinson’s disease that leads to difficulty walking, you may be more likely to fall and have a head injury. The head injury in turn may lead to increased mortality,” she noted.
However, she stressed that the data on cause-specific mortality are exploratory. “Our research motivates future studies that really examine this time-dependent relationship between neurodegenerative disease and head injuries,” Dr. Elser said.
Dose-dependent response
In the unadjusted analysis, the hazard ratio of mortality among individuals with head injury was 2.21 (95% confidence interval, 2.09-2.34) compared with those who did not have head injury.
The association remained significant with adjustment for sociodemographic factors (HR, 1.99; 95% CI, 1.88-2.11) and with additional adjustment for vascular risk factors (HR, 1.92; 95% CI, 1.81-2.03).
The findings also showed a dose-response pattern in the association of head injuries with mortality. Compared with participants who did not have head injury, the HR was 1.66 (95% CI, 1.56-1.77) for those with one head injury and 2.11 (95% CI, 1.89-2.37) for those with two or more head injuries.
“It’s not as though once you’ve had one head injury, you’ve accrued all the damage you possibly can. We see pretty clearly here that recurrent head injury further increased the rate of deaths from all causes,” said Dr. Elser.
Injury severity was determined from hospital diagnostic codes using established algorithms. Results showed that mortality rates were increased with even mild head injury.
Interestingly, the association between head injury and all-cause mortality was weaker among those whose injuries were self-reported. One possibility is that these injuries were less severe, Dr. Elser noted.
“If you have head injury that’s mild enough that you don’t need to go to the hospital, it’s probably going to confer less long-term health risks than one that’s severe enough that you needed to be examined in an acute care setting,” she said.
Results were similar by race and for sex. “Even though there were more women with head injuries, the rate of mortality associated with head injury doesn’t differ from the rate among men,” Dr. Elser reported.
However, the association was stronger among those younger than 54 years at baseline (HR, 2.26) compared with older individuals (HR, 2.0) in the model that adjusted for demographics and lifestyle factors.
This may be explained by the reference group (those without a head injury) – the mortality rate was in general higher for the older participants, said Dr. Elser. It could also be that younger adults are more likely to have severe head injuries from, for example, motor vehicle accidents or violence, she added.
These new findings underscore the importance of public health measures, such as seatbelt laws, to reduce head injuries, the investigators note.
They add that clinicians with patients at risk for head injuries may recommend steps to lessen the risk of falls, such as having access to durable medical equipment, and ensuring driver safety.
Shorter life span
Commenting for this news organization, Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology in Port St. Lucie and past president of the Florida Society of Neurology, said the large number of participants “adds validity” to the finding that individuals with head injury are likely to have a shorter life span than those who do not suffer head trauma – and that this “was not purely by chance or from other causes.”
However, patients may not have accurately reported head injuries, in which case the rate of injury in the self-report subgroup would not reflect the actual incidence, noted Dr. Conidi, who was not involved with the research.
“In my practice, most patients have little knowledge as to the signs and symptoms of concussion and traumatic brain injury. Most think there needs to be some form of loss of consciousness to have a head injury, which is of course not true,” he said.
Dr. Conidi added that the finding of a higher incidence of death from neurodegenerative disorders supports the generally accepted consensus view that about 30% of patients with traumatic brain injury experience progression of symptoms and are at risk for early dementia.
The ARIC study is supported by the National Heart, Lung, and Blood Institute. Dr. Elser and Dr. Conidi have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
Long COVID affecting more than one-third of college students, faculty
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
With a median age of 23 years, the study is unique for evaluating mostly healthy, young adults and for its rare look at long COVID in a university community.
The more symptoms during a bout with COVID, the greater the risk for long COVID, the researchers found. That lines up with previous studies. Also, the more vaccinations and booster shots against SARS-CoV-2, the virus that causes COVID, the lower the long COVID risk.
Women were more likely than men to be affected. Current or prior smoking, seeking medical care for COVID, and receiving antibody treatment also were linked to higher chances for developing long COVID.
Lead author Megan Landry, DrPH, MPH, and colleagues were already assessing students, staff, and faculty at George Washington University, Washington, who tested positive for COVID. Then they started seeing symptoms that lasted 28 days or more after their 10-day isolation period.
“We were starting to recognize that individuals ... were still having symptoms longer than the typical isolation period,” said Dr. Landry. So they developed a questionnaire to figure out the how long these symptoms last and how many people are affected by them.
The list of potential symptoms was long and included trouble thinking, fatigue, loss of smell or taste, shortness of breath, and more.
The study was published online in Emerging Infectious Diseases. Results are based on records and responses from 1,388 students, faculty, and staff from July 2021 to March 2022.
People had a median of four long COVID symptoms, about 63% were women, and 56% were non-Hispanic White. About three-quarters were students and the remainder were faculty and staff.
The finding that 36% of people with a history of COVID reported long COVID symptoms did not surprise Dr. Landry.
“Based on the literature that’s currently out there, it ranges from a 10% to an 80% prevalence of long COVID,” she said. “We kind of figured that we would fall somewhere in there.”
In contrast, that figure seemed high to Eric Topol, MD.
“That’s really high,” said Dr. Topol, founder and director of the Scripps Research Translational Institute in La Jolla, Calif. He added most studies estimate that about 10% of people with a history of acute infection develop long COVID.
Even at 10%, which could be an underestimate, that’s a lot of affected people globally.
“At least 65 million individuals around the world have long COVID, based on a conservative estimated incidence of 10% of infected people and more than 651 million documented COVID-19 cases worldwide; the number is likely much higher due to many undocumented cases,” Dr. Topol and colleagues wrote in a long COVID review article published in Nature Reviews Microbiology.
About 30% of study participants were fully vaccinated with an initial vaccine series, 42% had received a booster dose, and 29% were not fully vaccinated at the time of their first positive test for COVID. Those who were not fully vaccinated were significantly more likely to report symptoms of long COVID.
“I know a lot of people wish they could put COVID on the back burner or brush it under the rug, but COVID is still a real thing. We need to continue supporting vaccines and boosters and make sure people are up to date. Not only for COVID, but for flu as well,” Dr. Topol said
Research continues
“Long COVID is still evolving and we continue to learn more about it every day,” Landry said. “It’s just so new and there are still a lot of unknowns. That’s why it’s important to get this information out.”
People with long COVID often have a hard time with occupational, educational, social, or personal activities, compared with before COVID, with effects that can last for more than 6 months, the authors noted.
“I think across the board, universities in general need to consider the possibility of folks on their campuses are having symptoms of long COVID,” Dr. Landry said.
Moving forward, Dr. Landry and colleagues would like to continue investigating long COVID. For example, in the current study, they did not ask about severity of symptoms or how the symptoms affected daily functioning.
“I would like to continue this and dive deeper into how disruptive their symptoms of long COVID are to their everyday studying, teaching, or their activities to keeping a university running,” Dr. Landry said.
A version of this article originally appeared on WebMD.com.
FROM EMERGING INFECTIOUS DISEASES
New cancer data spark outcry from patient advocates
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
More type 2 diabetes deaths from cancer than heart disease
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.
In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer appears to have overtaken cardiovascular disease (CVD) as a leading cause of death in adults with type 2 diabetes, a 20-year population study in England suggests.
The researchers found that, from 1998 to 2018, in more than 130,000 adults aged 35 and older with type 2 diabetes, all-cause mortality declined for all ages, but cancer mortality increased for those aged 75 and older; people with type 2 diabetes who were smokers had higher and steadily increasing cancer mortality rates; and people with type 2 diabetes had more than twice the rate of colorectal, pancreatic, liver, and endometrial cancer mortality than age- and sex-matched individuals in the general population.
The findings suggest that “cancer prevention strategies therefore deserve at least a similar level of attention as cardiovascular disease prevention, particularly in older people and for some cancers such as liver, colorectal, and pancreatic cancer,” the researchers wrote.
Tailored cancer prevention and early-detection strategies are needed to address persistent inequalities in the older population, the most deprived, and smokers, they added.
Breast cancer rates in younger women with type 2 diabetes rising
According to the researchers, “early cancer detection through changes to existing screening [programs], or more in-depth investigations for suspected/nonspecific symptoms, may reduce the number of avoidable cancer deaths in people with type 2 diabetes.”
Moreover, breast cancer rates in younger women with type 2 diabetes are rising by 4.1% per year, they wrote, which suggests such women are high risk and should be screened at a younger age, but screening age would need to be determined in cost-effectiveness analyses.
The study by Suping Ling, PhD, and colleagues was published online in Diabetologia.
Results challenge belief that preventing CVD is priority in type 2 diabetes
“The prevention of cardiovascular disease has been, and is still considered, a priority in people with diabetes,” the researchers wrote.
“Our results challenge this view by showing that cancer may have overtaken cardiovascular disease as a leading cause of death in people with type 2 diabetes.”
“The proportion of cancer deaths out of all-cause deaths remains high (> 30%) in young ages, and it was steadily increasing in older ages,” Dr. Ling, from the department of noncommunicable disease epidemiology, London School of Hygiene & Tropical Medicine, said in a comment.
“Combined with previous studies reporting decreasing CVD mortality rates,” she said, “we concluded that cancer might have overtaken CVD as the leading cause of death in people with type 2 diabetes.”
Many evidence-based cancer-prevention strategies related to lifestyle (such as being physically active, being a healthy weight, eating a better diet, stopping smoking, as summarized by the World Cancer Research Fund), are helpful for preventing both cancer and CVD, Ling observed.
However, in the medical community, many additional efforts were made for monitoring, early detection, and innovating medications for CVD, she noted. “Therefore, we would like to propose a similar level of attention and effort for cancer in people with type 2 diabetes.”
Deaths from cancer vs. all causes in patients with diabetes
The researchers identified 137,804 patients aged 35 and older who were newly diagnosed with type 2 diabetes from 1998 to 2018 in general practices in the UK that were part of the Clinical Practice Research Datalink.
Patients were a median age of 64 years and 45% were women. Most (83%) were White, followed by South Asian (3.5%), Black (2.0%), and other (3%); 8.4% had missing information for race. Patients had a median body mass index (BMI) of 30.6 kg/m2.
Researchers divided patients into socioeconomic quintiles of most to least deprived based on income, employment, education, and other factors. During a median follow-up of 8.4 years, there were 39,212 deaths (28.5%).
Cancer mortality in subgroups of patients with type 2 diabetes
Researchers analyzed annual deaths from cancer and from all causes over 20 years in subgroups of patients with type 2 diabetes.

In adults with type 2 diabetes, the average percentage change in cancer mortality per year, from 1998 to 2018 decreased in people aged 55 and 65 (–1.4% and –0.2%, respectively), but increased in people aged 75 and 85 (1.2% and 1.6%, respectively); increased more in women than in men (1.5% vs 1.0%), although women had lower cancer mortality than men; and increased more in the least deprived (wealthiest) individuals than in the most deprived (1.5% vs 1.0%). Cancer mortality rates were consistently higher in the most deprived individuals, Dr. Ling noted.
Cancer mortality also increased more in people with class III obesity (BMI ≥ 35) versus normal weight (5.8% vs 0.7%) and versus other weights. In addition, there was an upward trend in cancer mortality in people who were White or former/current smokers.
Deaths from specific cancers in diabetes vs. general population
Next, researchers determined cancer mortality ratios – the cancer mortality of the patients with diabetes divided by the cancer mortality of the general population.
They determined this for all cancers, the four most common cancers in the United Kingdom (lung, colorectal, breast, and prostate), and cancers caused by type 2 diabetes (pancreatic, liver, gallbladder, and endometrial cancer), standardized by sex and age.
Mortality from all cancer was 18% higher in patients with type 2 diabetes, compared with the general population.
Overall, mortality from colorectal cancer, pancreatic cancer, and liver cancer was 2.4 times, 2.12 times, and 2.13 times higher, respectively, in patients with type 2 diabetes than in the general population.
Mortality from breast cancer was 9% higher and mortality from endometrial cancer was 2.08 times higher in women with type 2 diabetes than in women in the general population.
There was a constant upward trend for mortality rates for pancreatic, liver, and lung cancer at all ages, colorectal cancer at most ages, breast cancer at younger ages, and prostate and endometrial cancer at older ages.
The study was funded by Hope Against Cancer. Dr. Ling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA