User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Serious Mental Illness Tied to Multiple Physical Illnesses
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
Serious mental illness (SMI), including bipolar disorder or schizophrenia spectrum disorders, is associated with a twofold increased risk for comorbid physical illness, results of a new meta-analysis showed.
“Although treatment of physical and mental health remains siloed in many health services globally, the high prevalence of physical multimorbidity attests to the urgent need for integrated care models that address both physical and mental health outcomes in people with severe mental illness,” the authors, led by Sean Halstead, MD, of The University of Queensland Medical School in Brisbane, Australia, wrote.
The findings were published online in The Lancet Psychiatry.
Shorter Lifespan?
SMI is associated with reduced life expectancy, and experts speculate that additional chronic illnesses — whether physical or psychiatric — may underlie this association.
While previous research has paired SMI with comorbid physical illnesses, the researchers noted that this study is the first to focus on both physical and psychiatric multimorbidity in individuals with SMI.
The investigators conducted a meta-analysis of 82 observational studies comprising 1.6 million individuals with SMI and 13.2 million control subjects to determine the risk for physical or psychiatric multimorbidity.
Studies were included if participants were diagnosed with either a schizophrenia spectrum disorder or bipolar disorder, and the study assessed either physical multimorbidity (at least two physical health conditions) or psychiatric multimorbidity (at least three psychiatric conditions), including the initial SMI.
Investigators found that individuals with SMI had more than a twofold increased risk for physical multimorbidity than those without SMI (odds ratio [OR], 2.40; 95% CI, 1.57-3.65; P = .0009).
Physical multimorbidity, which included cardiovascular, endocrine, neurological rental, gastrointestinal, musculoskeletal, and infectious disorders, was prevalent at similar rates in both schizophrenia spectrum disorder and bipolar disorder.
The ratio of physical multimorbidity was about four times higher in younger populations with SMI (mean age ≤ 40; OR, 3.99; 95% CI, 1.43-11.10) than in older populations (mean age > 40; OR, 1.55; 95% CI, 0.96-2.51; subgroup differences, P = .0013).
In terms of absolute prevalence, 25% of those with SMI had a physical multimorbidity, and 14% had a psychiatric multimorbidity, which were primarily anxiety and substance use disorders.
Investigators speculated that physical multimorbidity in SMI could stem from side effects of psychotropic medications, which are known to cause rapid cardiometabolic changes, including weight gain. In addition, lifestyle factors or nonmodifiable risk factors could also contribute to physical multimorbidity.
The study’s limitations included its small sample sizes for subgroup analyses and insufficient analysis for significant covariates, including smoking rates and symptom severity.
“While health services and treatment guidelines often operate on the assumption that individuals have a single principal diagnosis, these results attest to the clinical complexity many people with severe mental illness face in relation to burden of chronic disease,” the investigators wrote. They added that a greater understanding of the epidemiological manifestations of multimorbidity in SMI is “imperative.”
There was no source of funding for this study. Dr. Halstead is supported by the Australian Research Training Program scholarship. Other disclosures were noted in the original article.
A version of this article appeared on Medscape.com .
FROM THE LANCET PSYCHIATRY
CPAP Underperforms: The Sequel
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
Why Cardiac Biomarkers Don’t Help Predict Heart Disease
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.
Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
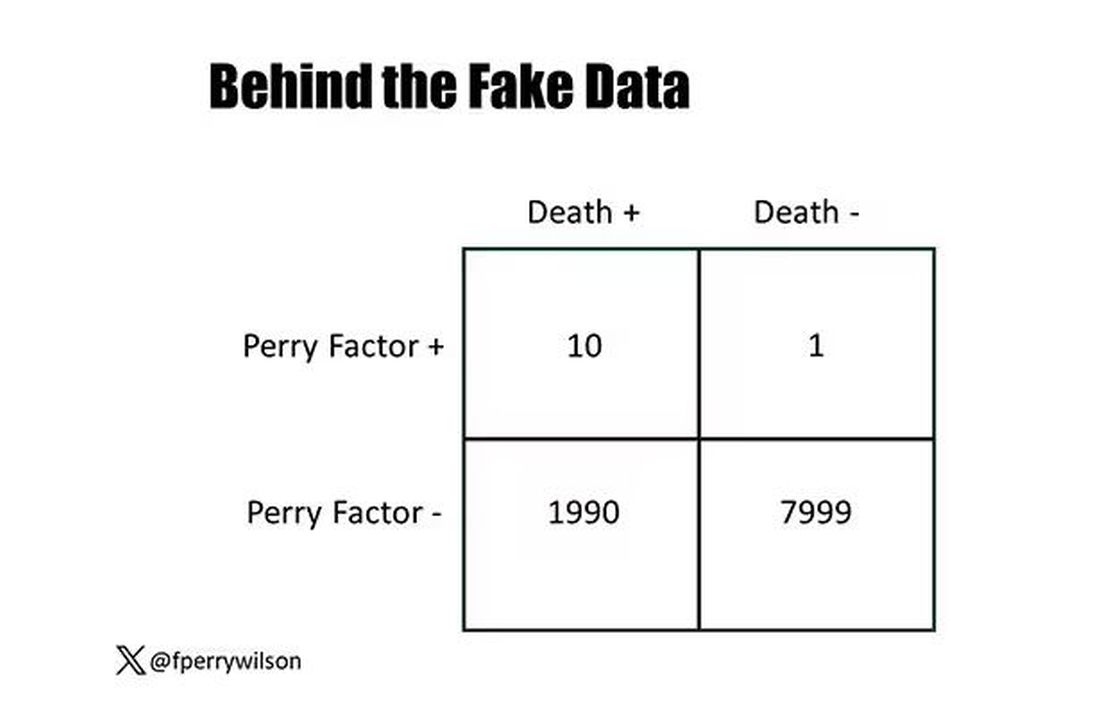
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
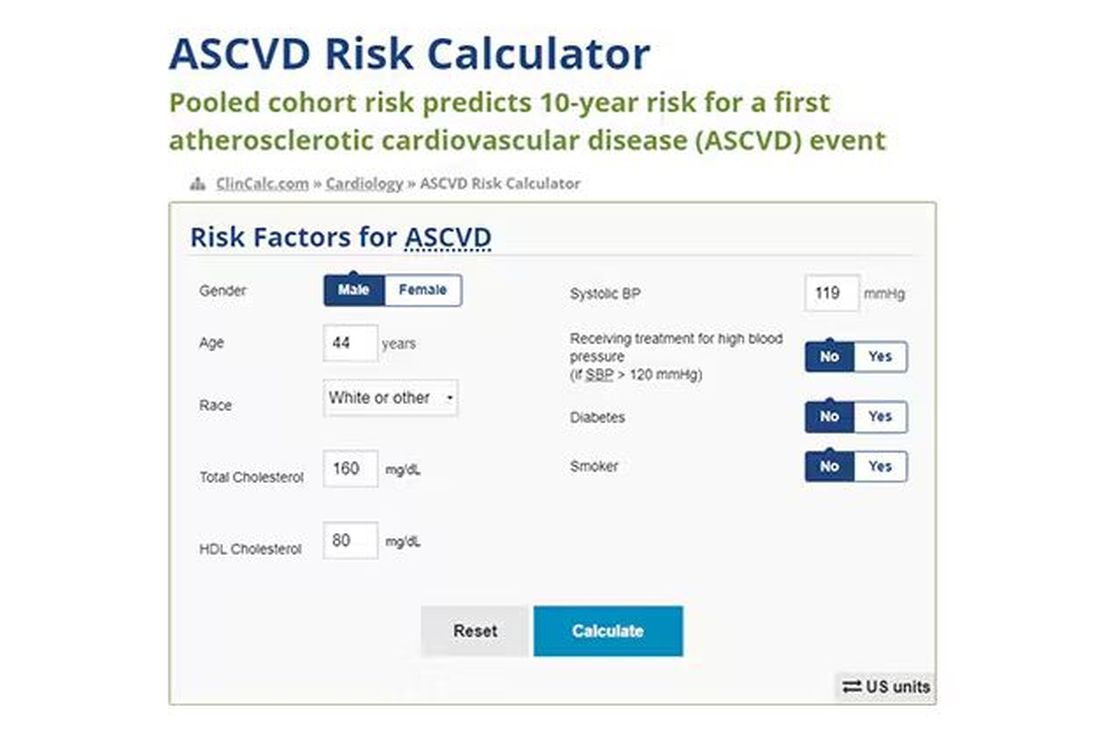
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
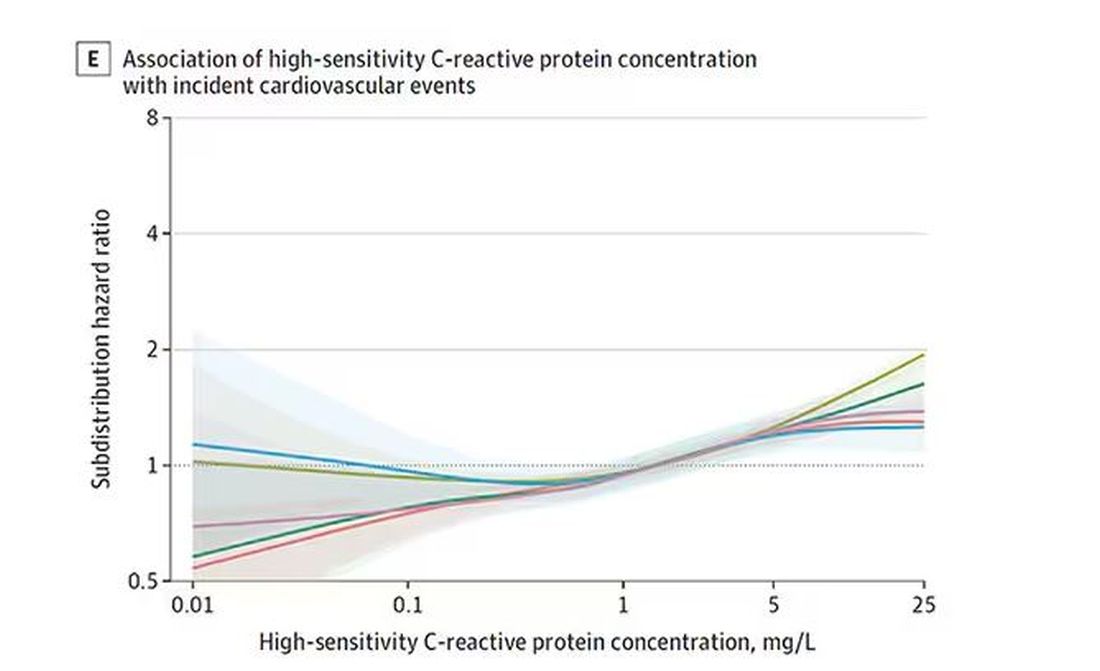
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
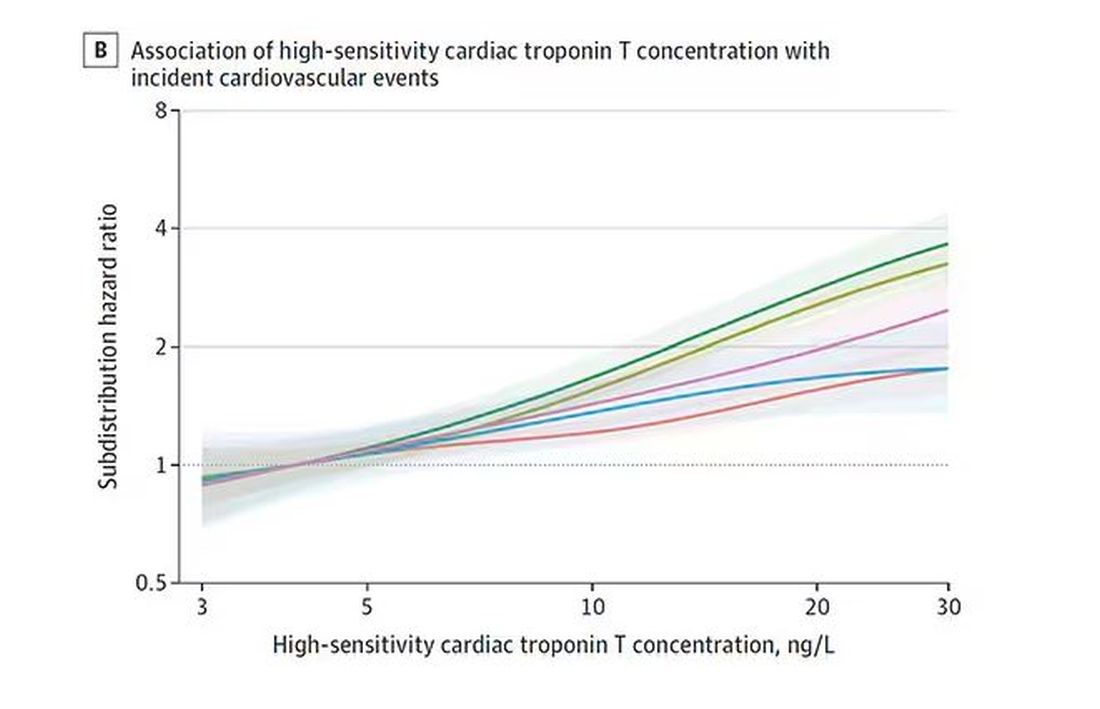
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
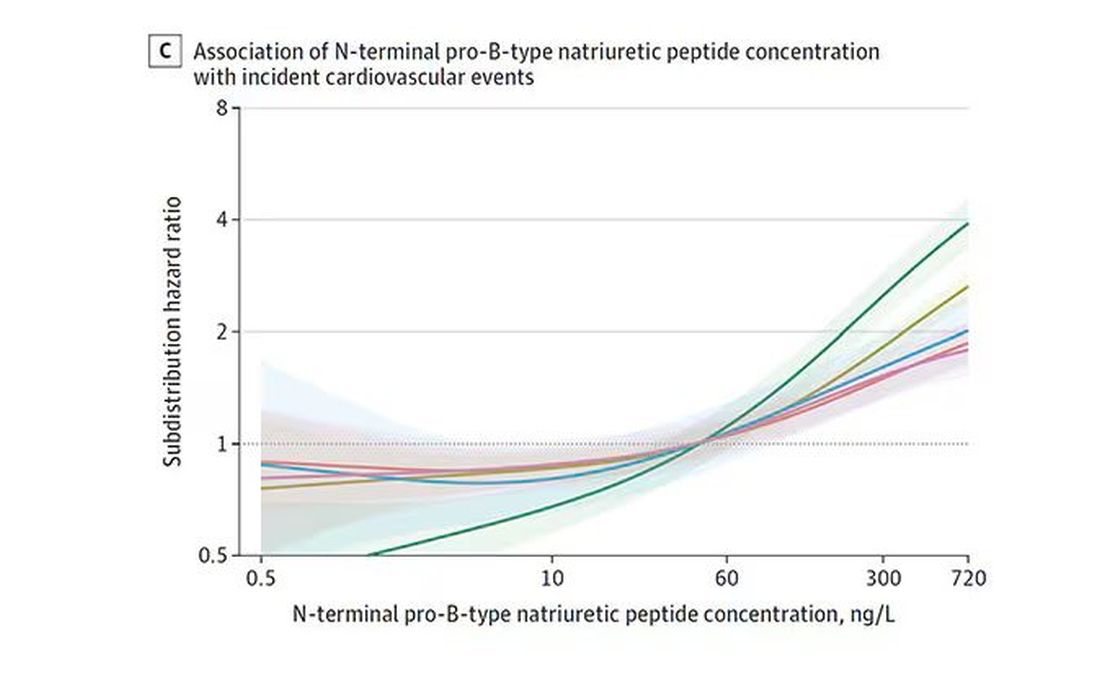
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
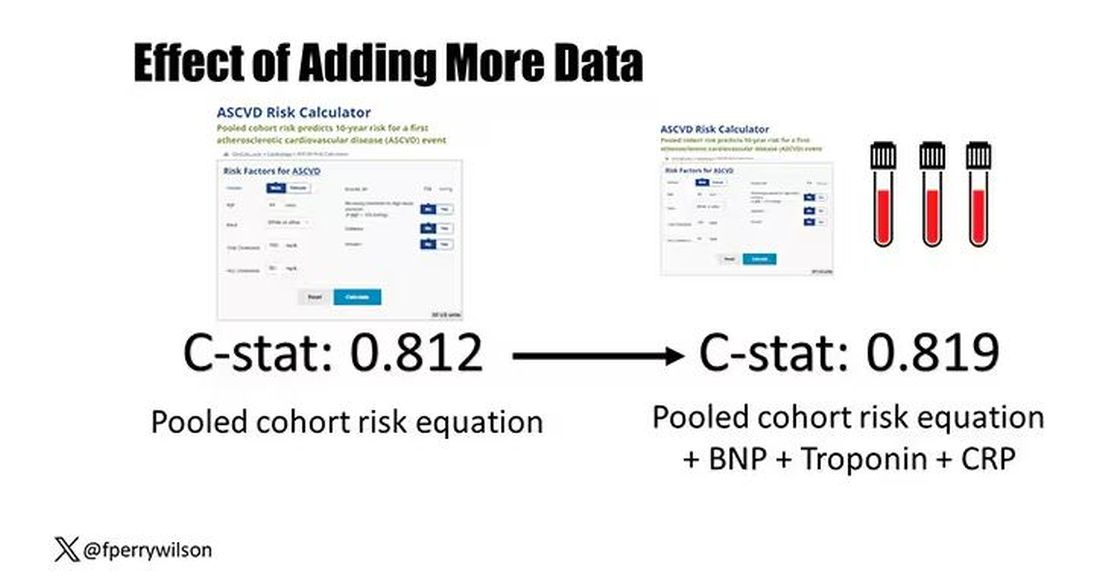
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.
Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.
Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Why Incorporating Obstetric History Matters for CVD Risk Management in Autoimmune Diseases
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.
NEW YORK — Systemic autoimmune disease is well-recognized as a major risk factor for cardiovascular disease (CVD), but less recognized as a cardiovascular risk factor is a history of pregnancy complications, including preeclampsia, and cardiologists and rheumatologists need to include an obstetric history when managing patients with autoimmune diseases, a specialist in reproductive health in rheumatology told attendees at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
“Autoimmune diseases, lupus in particular, increase the risk for both cardiovascular disease and maternal placental syndromes,” Lisa R. Sammaritano, MD, a professor at Hospital for Special Surgery in New York City and a specialist in reproductive health issues in rheumatology patients, told attendees. “For those patients who have complications during pregnancy, it further increases their already increased risk for later cardiovascular disease.”
CVD Risk Double Whammy
A history of systemic lupus erythematosus (SLE) and problematic pregnancy can be a double whammy for CVD risk. Dr. Sammaritano cited a 2022 meta-analysis that showed patients with SLE had a 2.5 times greater risk for stroke and almost three times greater risk for myocardial infarction than people without SLE.
Maternal placental syndromes include pregnancy loss, restricted fetal growth, preeclampsia, premature membrane rupture, placental abruption, and intrauterine fetal demise, Dr. Sammaritano said. Hypertensive disorders of pregnancy, formerly called adverse pregnancy outcomes, she noted, include gestational hypertension, preeclampsia, and eclampsia.
Pregnancy complications can have an adverse effect on the mother’s postpartum cardiovascular health, Dr. Sammaritano noted, a fact borne out by the cardiovascular health after maternal placental syndromes population-based retrospective cohort study and a 2007 meta-analysis that found a history of preeclampsia doubles the risk for venous thromboembolism, stroke, and ischemic heart disease up to 15 years after pregnancy.
“It is always important to obtain a reproductive health history from patients with autoimmune diseases,” Dr. Sammaritano told this news organization in an interview. “This is an integral part of any medical history. In the usual setting, this includes not only pregnancy history but also use of contraception in reproductive-aged women. Unplanned pregnancy can lead to adverse outcomes in the setting of active or severe autoimmune disease or when teratogenic medications are used.”
Pregnancy history can be a factor in a woman’s cardiovascular health more than 15 years postpartum, even if a woman is no longer planning a pregnancy or is menopausal. “As such, this history is important in assessing every woman’s risk profile for CVD in addition to usual traditional risk factors,” Dr. Sammaritano said.
“It is even more important for women with autoimmune disorders, who have been shown to have an already increased risk for CVD independent of their pregnancy history, likely related to a chronic inflammatory state and other autoimmune-related factors such as presence of antiphospholipid antibodies [aPL] or use of corticosteroids.”
Timing of disease onset is also an issue, she said. “In patients with SLE, for example, onset of CVD is much earlier than in the general population,” Dr. Sammaritano said. “As a result, these patients should likely be assessed for risk — both traditional and other risk factors — earlier than the general population, especially if an adverse obstetric history is present.”
At the younger end of the age continuum, women with autoimmune disease, including SLE and antiphospholipid syndrome, who are pregnant should be put on guideline-directed low-dose aspirin preeclampsia prophylaxis, Dr. Sammaritano said. “Whether every patient with SLE needs this is still uncertain, but certainly, those with a history of renal disease, hypertension, or aPL antibody clearly do,” she added.
The evidence supporting hydroxychloroquine (HCQ) in these patients is controversial, but Dr. Sammaritano noted two meta-analyses, one in 2022 and the other in 2023, that showed that HCQ lowered the risk for preeclampsia in women.
“The clear benefit of HCQ in preventing maternal disease complications, including flare, means we recommend it regardless for all patients with SLE at baseline and during pregnancy [if tolerated],” Dr. Sammaritano said. “The benefit or optimal use of these medications in other autoimmune diseases is less studied and less certain.”
Dr. Sammaritano added in her presentation, “We really need better therapies and, hopefully, those will be on the way, but I think the takeaway message, particularly for practicing rheumatologists and cardiologists, is to ask the question about obstetric history. Many of us don’t. It doesn’t seem relevant in the moment, but it really is in terms of the patient’s long-term risk for cardiovascular disease.”
The Case for Treatment During Pregnancy
Prophylaxis against pregnancy complications in patients with autoimmune disease may be achievable, Taryn Youngstein, MBBS, consultant rheumatologist and codirector of the Centre of Excellence in Vasculitis Research, Imperial College London, London, England, told this news organization after Dr. Sammaritano’s presentation. At the 2023 American College of Rheumatology Annual Meeting, her group reported the safety and effectiveness of continuing tocilizumab in pregnant women with Takayasu arteritis, a large-vessel vasculitis predominantly affecting women of reproductive age.
“What traditionally happens is you would stop the biologic particularly before the third trimester because of safety and concerns that the monoclonal antibody is actively transported across the placenta, which means the baby gets much more concentration of the drug than the mum,” Dr. Youngstein said.
It’s a situation physicians must monitor closely, she said. “The mum is donating their immune system to the baby, but they’re also donating drug.”
“In high-risk patients, we would share decision-making with the patient,” Dr. Youngstein continued. “We have decided it’s too high of a risk for us to stop the drug, so we have been continuing the interleukin-6 [IL-6] inhibitor throughout the entire pregnancy.”
The data from Dr. Youngstein’s group showed that pregnant women with Takayasu arteritis who continued IL-6 inhibition therapy all carried to term with healthy births.
“We’ve shown that it’s relatively safe to do that, but you have to be very careful in monitoring the baby,” she said. This includes not giving the infant any live vaccines at birth because it will have the high levels of IL-6 inhibition, she said.
Dr. Sammaritano and Dr. Youngstein had no relevant financial relationships to disclose.
A version of this article appeared on Medscape.com.
CVD Risk Rises With Higher NSAID Doses in Ankylosing Spondylitis
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Self-Monitoring Better Than Usual Care Among Patients With Hypertension
TOPLINE:
Blood pressure (BP) self-monitoring and medication management may be better than usual care for controlling hypertension, a new study published in JAMA Network Open suggested.
METHODOLOGY:
- The secondary analysis of a randomized, unblinded clinical trial included patients aged ≥ 40 years with uncontrolled hypertension in Valencia, Spain, between 2017 and 2020.
- The 111 patients in the intervention group received educational materials and instructions for self-monitoring of BP with a home monitor and medication adjustment as needed without contacting their healthcare clinicians.
- The 108 patients in the control group received usual care, including education on BP control.
- After 24 months, researchers recorded BP levels, the number of people who achieved a target BP (systolic BP < 140 mm Hg and diastolic BP < 90 mm Hg), adverse events, quality of life, behavioral changes, and health service use.
TAKEAWAY:
- Patients in the intervention group had a lower average systolic BP reading at 24 months than patients who received usual care (adjusted mean difference, -3.4 mm Hg).
- Patients in the intervention group also had a lower average diastolic BP reading than usual care (adjusted mean difference, -2.5 mm Hg).
- The percentage of people who achieved the target BP was similar in both groups (64% in the intervention group compared with 54% in the control group).
- Researchers found no difference between groups in terms of adverse events, use of health services, behavioral changes such as smoking status or body weight, or quality of life.
IN PRACTICE:
“These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.”
SOURCE:
The study was led by Gabriel Sanfélix-Gimeno, PhD, Pharm D, head of the Health Services Research & Pharmacoepidemiology Unit at Fisabio Research Institute in Valencia, Spain.
LIMITATIONS:
Some study participants were lost to follow-up due to COVID-19 restrictions. The trial was unblinded, which may have led to biases among patients and clinicians. Clinicians treated both the control and intervention groups. The results may not be extrapolated to those with controlled hypertension, very high BP, or people who are pregnant because they were not included in the study.
DISCLOSURES:
Various authors reported receiving grants from RTI Health Solutions or personal fees from GSK and MSD outside the submitted work. No other disclosures were reported. The study was funded by the Instituto de Salud Carlos III at the Spanish Ministry of Research, Innovation and Universities, the European Regional Development Fund, and Spanish Clinical Research Network.
A version of this article appeared on Medscape.com.
TOPLINE:
Blood pressure (BP) self-monitoring and medication management may be better than usual care for controlling hypertension, a new study published in JAMA Network Open suggested.
METHODOLOGY:
- The secondary analysis of a randomized, unblinded clinical trial included patients aged ≥ 40 years with uncontrolled hypertension in Valencia, Spain, between 2017 and 2020.
- The 111 patients in the intervention group received educational materials and instructions for self-monitoring of BP with a home monitor and medication adjustment as needed without contacting their healthcare clinicians.
- The 108 patients in the control group received usual care, including education on BP control.
- After 24 months, researchers recorded BP levels, the number of people who achieved a target BP (systolic BP < 140 mm Hg and diastolic BP < 90 mm Hg), adverse events, quality of life, behavioral changes, and health service use.
TAKEAWAY:
- Patients in the intervention group had a lower average systolic BP reading at 24 months than patients who received usual care (adjusted mean difference, -3.4 mm Hg).
- Patients in the intervention group also had a lower average diastolic BP reading than usual care (adjusted mean difference, -2.5 mm Hg).
- The percentage of people who achieved the target BP was similar in both groups (64% in the intervention group compared with 54% in the control group).
- Researchers found no difference between groups in terms of adverse events, use of health services, behavioral changes such as smoking status or body weight, or quality of life.
IN PRACTICE:
“These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.”
SOURCE:
The study was led by Gabriel Sanfélix-Gimeno, PhD, Pharm D, head of the Health Services Research & Pharmacoepidemiology Unit at Fisabio Research Institute in Valencia, Spain.
LIMITATIONS:
Some study participants were lost to follow-up due to COVID-19 restrictions. The trial was unblinded, which may have led to biases among patients and clinicians. Clinicians treated both the control and intervention groups. The results may not be extrapolated to those with controlled hypertension, very high BP, or people who are pregnant because they were not included in the study.
DISCLOSURES:
Various authors reported receiving grants from RTI Health Solutions or personal fees from GSK and MSD outside the submitted work. No other disclosures were reported. The study was funded by the Instituto de Salud Carlos III at the Spanish Ministry of Research, Innovation and Universities, the European Regional Development Fund, and Spanish Clinical Research Network.
A version of this article appeared on Medscape.com.
TOPLINE:
Blood pressure (BP) self-monitoring and medication management may be better than usual care for controlling hypertension, a new study published in JAMA Network Open suggested.
METHODOLOGY:
- The secondary analysis of a randomized, unblinded clinical trial included patients aged ≥ 40 years with uncontrolled hypertension in Valencia, Spain, between 2017 and 2020.
- The 111 patients in the intervention group received educational materials and instructions for self-monitoring of BP with a home monitor and medication adjustment as needed without contacting their healthcare clinicians.
- The 108 patients in the control group received usual care, including education on BP control.
- After 24 months, researchers recorded BP levels, the number of people who achieved a target BP (systolic BP < 140 mm Hg and diastolic BP < 90 mm Hg), adverse events, quality of life, behavioral changes, and health service use.
TAKEAWAY:
- Patients in the intervention group had a lower average systolic BP reading at 24 months than patients who received usual care (adjusted mean difference, -3.4 mm Hg).
- Patients in the intervention group also had a lower average diastolic BP reading than usual care (adjusted mean difference, -2.5 mm Hg).
- The percentage of people who achieved the target BP was similar in both groups (64% in the intervention group compared with 54% in the control group).
- Researchers found no difference between groups in terms of adverse events, use of health services, behavioral changes such as smoking status or body weight, or quality of life.
IN PRACTICE:
“These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.”
SOURCE:
The study was led by Gabriel Sanfélix-Gimeno, PhD, Pharm D, head of the Health Services Research & Pharmacoepidemiology Unit at Fisabio Research Institute in Valencia, Spain.
LIMITATIONS:
Some study participants were lost to follow-up due to COVID-19 restrictions. The trial was unblinded, which may have led to biases among patients and clinicians. Clinicians treated both the control and intervention groups. The results may not be extrapolated to those with controlled hypertension, very high BP, or people who are pregnant because they were not included in the study.
DISCLOSURES:
Various authors reported receiving grants from RTI Health Solutions or personal fees from GSK and MSD outside the submitted work. No other disclosures were reported. The study was funded by the Instituto de Salud Carlos III at the Spanish Ministry of Research, Innovation and Universities, the European Regional Development Fund, and Spanish Clinical Research Network.
A version of this article appeared on Medscape.com.
Testosterone/CVD Risk Debate Revived by New Meta-Analysis
A new systematic literature review adds complexity to the controversy over testosterone’s relationship to risk for myocardial infarction, stroke, cardiovascular death, and all-cause mortality.
Last year, the TRAVERSE (Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy ResponSE in Hypogonadal Men) trial was the first randomized, placebo-controlled study designed and powered to determine whether testosterone therapy increased risk for major cardiovascular events in men (ages 45-80 years). Its conclusions provided reassurance that modest use of testosterone therapy short term does not increase CVD risk.
But other studies have had different conclusions and TRAVERSE left unanswered questions, so Bu B. Yeap, MBBS, PhD, an endocrinologist at the University of Western Australia in Crawley, and colleagues completed a literature review with 11 prospective cohort studies of community-dwelling men with sex steroid levels measured with mass spectrometry. Nine of the studies provided individual participation data (IPD); two used aggregate data, and all had at least 5 years of follow-up.
The findings were published in Annals of Internal Medicine .
Dr. Yeap’s team concluded that certain groups of men have higher risk for CVD events. In this study, men with very low testosterone, high luteinizing hormone (LH), or very low estradiol concentrations had higher all-cause mortality. Sex hormone–binding globulin (SHBG) concentration was positively associated and dihydrotestosterone (DHT) levels were nonlinearly associated with all-cause mortality and CVD mortality.
The testosterone level below which men had higher risk of death from any cause was 7.4 nmol/L (213 ng/dL), regardless of LH concentration, the researchers concluded, writing, “This adds to information on reference ranges based on distributions of testosterone in selected samples of healthy men.”
The link between higher SHBG concentrations and higher all-cause mortality “may be related to its role as the major binding protein for sex steroids in the circulation,” the authors wrote. “We found a U-shaped association of DHT with all-cause and CVD-related mortality risks, which were higher at lower and very high DHT concentrations. Men with very low DHT concentrations also had increased risk for incident CVD events. Further investigation into potential underlying mechanisms for these associations is warranted.”
Rigorous Methodology Adds Value
Bradley D. Anawalt, MD, with the University of Washington School of Medicine in Seattle, pointed out in an accompanying editorial that the study’s findings are particularly valuable because of the team’s rigorous methodology. The team measured testosterone with the gold standard, mass spectrometry, which can also measure DHT and estradiol more accurately than widely available commercial immunoassays, which “are inaccurate for measurement of these sex steroids in men, who typically have low serum concentrations of these two metabolites of testosterone,” Dr. Anawalt said.
Also, the researchers obtained raw data from the nine IPD studies and reanalyzed the combined data, which allows for more sophisticated analysis when combining data from multiple studies, Dr. Anawalt explained.
The main finding from the Yeap et al. study, he wrote, is that high testosterone concentrations at baseline were not linked with increased deaths from CVD or from all causes “but very low serum total testosterone concentrations at baseline were.
“It is tempting to hypothesize that testosterone therapy might have cardiovascular benefits solely in patients with very low concentrations of serum total testosterone,” Dr. Anawalt wrote.
He pointed out as particularly interesting the findings for DHT and estradiol.
“The finding that a low serum estradiol concentration is associated with higher all-cause mortality adds another reason (in addition to the adverse effects on body fat and bone health) to avoid aromatase inhibitors that are commonly taken by persons who use anabolic steroids,” he wrote. “The prospect of a U-shaped curve for the relationship between serum DHT and higher cardiovascular risk warrants further study.”
The work is funded by the Government of Western Australia and Lawley Pharmaceuticals. The authors’ and editorial writer’s conflicts of interest are listed in the full study.
A new systematic literature review adds complexity to the controversy over testosterone’s relationship to risk for myocardial infarction, stroke, cardiovascular death, and all-cause mortality.
Last year, the TRAVERSE (Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy ResponSE in Hypogonadal Men) trial was the first randomized, placebo-controlled study designed and powered to determine whether testosterone therapy increased risk for major cardiovascular events in men (ages 45-80 years). Its conclusions provided reassurance that modest use of testosterone therapy short term does not increase CVD risk.
But other studies have had different conclusions and TRAVERSE left unanswered questions, so Bu B. Yeap, MBBS, PhD, an endocrinologist at the University of Western Australia in Crawley, and colleagues completed a literature review with 11 prospective cohort studies of community-dwelling men with sex steroid levels measured with mass spectrometry. Nine of the studies provided individual participation data (IPD); two used aggregate data, and all had at least 5 years of follow-up.
The findings were published in Annals of Internal Medicine .
Dr. Yeap’s team concluded that certain groups of men have higher risk for CVD events. In this study, men with very low testosterone, high luteinizing hormone (LH), or very low estradiol concentrations had higher all-cause mortality. Sex hormone–binding globulin (SHBG) concentration was positively associated and dihydrotestosterone (DHT) levels were nonlinearly associated with all-cause mortality and CVD mortality.
The testosterone level below which men had higher risk of death from any cause was 7.4 nmol/L (213 ng/dL), regardless of LH concentration, the researchers concluded, writing, “This adds to information on reference ranges based on distributions of testosterone in selected samples of healthy men.”
The link between higher SHBG concentrations and higher all-cause mortality “may be related to its role as the major binding protein for sex steroids in the circulation,” the authors wrote. “We found a U-shaped association of DHT with all-cause and CVD-related mortality risks, which were higher at lower and very high DHT concentrations. Men with very low DHT concentrations also had increased risk for incident CVD events. Further investigation into potential underlying mechanisms for these associations is warranted.”
Rigorous Methodology Adds Value
Bradley D. Anawalt, MD, with the University of Washington School of Medicine in Seattle, pointed out in an accompanying editorial that the study’s findings are particularly valuable because of the team’s rigorous methodology. The team measured testosterone with the gold standard, mass spectrometry, which can also measure DHT and estradiol more accurately than widely available commercial immunoassays, which “are inaccurate for measurement of these sex steroids in men, who typically have low serum concentrations of these two metabolites of testosterone,” Dr. Anawalt said.
Also, the researchers obtained raw data from the nine IPD studies and reanalyzed the combined data, which allows for more sophisticated analysis when combining data from multiple studies, Dr. Anawalt explained.
The main finding from the Yeap et al. study, he wrote, is that high testosterone concentrations at baseline were not linked with increased deaths from CVD or from all causes “but very low serum total testosterone concentrations at baseline were.
“It is tempting to hypothesize that testosterone therapy might have cardiovascular benefits solely in patients with very low concentrations of serum total testosterone,” Dr. Anawalt wrote.
He pointed out as particularly interesting the findings for DHT and estradiol.
“The finding that a low serum estradiol concentration is associated with higher all-cause mortality adds another reason (in addition to the adverse effects on body fat and bone health) to avoid aromatase inhibitors that are commonly taken by persons who use anabolic steroids,” he wrote. “The prospect of a U-shaped curve for the relationship between serum DHT and higher cardiovascular risk warrants further study.”
The work is funded by the Government of Western Australia and Lawley Pharmaceuticals. The authors’ and editorial writer’s conflicts of interest are listed in the full study.
A new systematic literature review adds complexity to the controversy over testosterone’s relationship to risk for myocardial infarction, stroke, cardiovascular death, and all-cause mortality.
Last year, the TRAVERSE (Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy ResponSE in Hypogonadal Men) trial was the first randomized, placebo-controlled study designed and powered to determine whether testosterone therapy increased risk for major cardiovascular events in men (ages 45-80 years). Its conclusions provided reassurance that modest use of testosterone therapy short term does not increase CVD risk.
But other studies have had different conclusions and TRAVERSE left unanswered questions, so Bu B. Yeap, MBBS, PhD, an endocrinologist at the University of Western Australia in Crawley, and colleagues completed a literature review with 11 prospective cohort studies of community-dwelling men with sex steroid levels measured with mass spectrometry. Nine of the studies provided individual participation data (IPD); two used aggregate data, and all had at least 5 years of follow-up.
The findings were published in Annals of Internal Medicine .
Dr. Yeap’s team concluded that certain groups of men have higher risk for CVD events. In this study, men with very low testosterone, high luteinizing hormone (LH), or very low estradiol concentrations had higher all-cause mortality. Sex hormone–binding globulin (SHBG) concentration was positively associated and dihydrotestosterone (DHT) levels were nonlinearly associated with all-cause mortality and CVD mortality.
The testosterone level below which men had higher risk of death from any cause was 7.4 nmol/L (213 ng/dL), regardless of LH concentration, the researchers concluded, writing, “This adds to information on reference ranges based on distributions of testosterone in selected samples of healthy men.”
The link between higher SHBG concentrations and higher all-cause mortality “may be related to its role as the major binding protein for sex steroids in the circulation,” the authors wrote. “We found a U-shaped association of DHT with all-cause and CVD-related mortality risks, which were higher at lower and very high DHT concentrations. Men with very low DHT concentrations also had increased risk for incident CVD events. Further investigation into potential underlying mechanisms for these associations is warranted.”
Rigorous Methodology Adds Value
Bradley D. Anawalt, MD, with the University of Washington School of Medicine in Seattle, pointed out in an accompanying editorial that the study’s findings are particularly valuable because of the team’s rigorous methodology. The team measured testosterone with the gold standard, mass spectrometry, which can also measure DHT and estradiol more accurately than widely available commercial immunoassays, which “are inaccurate for measurement of these sex steroids in men, who typically have low serum concentrations of these two metabolites of testosterone,” Dr. Anawalt said.
Also, the researchers obtained raw data from the nine IPD studies and reanalyzed the combined data, which allows for more sophisticated analysis when combining data from multiple studies, Dr. Anawalt explained.
The main finding from the Yeap et al. study, he wrote, is that high testosterone concentrations at baseline were not linked with increased deaths from CVD or from all causes “but very low serum total testosterone concentrations at baseline were.
“It is tempting to hypothesize that testosterone therapy might have cardiovascular benefits solely in patients with very low concentrations of serum total testosterone,” Dr. Anawalt wrote.
He pointed out as particularly interesting the findings for DHT and estradiol.
“The finding that a low serum estradiol concentration is associated with higher all-cause mortality adds another reason (in addition to the adverse effects on body fat and bone health) to avoid aromatase inhibitors that are commonly taken by persons who use anabolic steroids,” he wrote. “The prospect of a U-shaped curve for the relationship between serum DHT and higher cardiovascular risk warrants further study.”
The work is funded by the Government of Western Australia and Lawley Pharmaceuticals. The authors’ and editorial writer’s conflicts of interest are listed in the full study.
FROM ANNALS OF INTERNAL MEDICINE
It Would Be Nice if Olive Oil Really Did Prevent Dementia
This transcript has been edited for clarity.
As you all know by now, I’m always looking out for lifestyle changes that are both pleasurable and healthy. They are hard to find, especially when it comes to diet. My kids complain about this all the time: “When you say ‘healthy food,’ you just mean yucky food.” And yes, French fries are amazing, and no, we can’t have them three times a day.
So, when I saw an article claiming that olive oil reduces the risk for dementia, I was interested. I love olive oil; I cook with it all the time. But as is always the case in the world of nutritional epidemiology, we need to be careful. There are a lot of reasons to doubt the results of this study — and one reason to believe it’s true.
The study I’m talking about is “Consumption of Olive Oil and Diet Quality and Risk of Dementia-Related Death,” appearing in JAMA Network Open and following a well-trod formula in the nutritional epidemiology space.
Nearly 100,000 participants, all healthcare workers, filled out a food frequency questionnaire every 4 years with 130 questions touching on all aspects of diet: How often do you eat bananas, bacon, olive oil? Participants were followed for more than 20 years, and if they died, the cause of death was flagged as being dementia-related or not. Over that time frame there were around 38,000 deaths, of which 4751 were due to dementia.
The rest is just statistics. The authors show that those who reported consuming more olive oil were less likely to die from dementia — about 50% less likely, if you compare those who reported eating more than 7 grams of olive oil a day with those who reported eating none.
Is It What You Eat, or What You Don’t Eat?
And we could stop there if we wanted to; I’m sure big olive oil would be happy with that. Is there such a thing as “big olive oil”? But no, we need to dig deeper here because this study has the same problems as all nutritional epidemiology studies. Number one, no one is sitting around drinking small cups of olive oil. They consume it with other foods. And it was clear from the food frequency questionnaire that people who consumed more olive oil also consumed less red meat, more fruits and vegetables, more whole grains, more butter, and less margarine. And those are just the findings reported in the paper. I suspect that people who eat more olive oil also eat more tomatoes, for example, though data this granular aren’t shown. So, it can be really hard, in studies like this, to know for sure that it’s actually the olive oil that is helpful rather than some other constituent in the diet.
The flip side of that coin presents another issue. The food you eat is also a marker of the food you don’t eat. People who ate olive oil consumed less margarine, for example. At the time of this study, margarine was still adulterated with trans-fats, which a pretty solid evidence base suggests are really bad for your vascular system. So perhaps it’s not that olive oil is particularly good for you but that something else is bad for you. In other words, simply adding olive oil to your diet without changing anything else may not do anything.
The other major problem with studies of this sort is that people don’t consume food at random. The type of person who eats a lot of olive oil is simply different from the type of person who doesn›t. For one thing, olive oil is expensive. A 25-ounce bottle of olive oil is on sale at my local supermarket right now for $11.00. A similar-sized bottle of vegetable oil goes for $4.00.
Isn’t it interesting that food that costs more money tends to be associated with better health outcomes? (I’m looking at you, red wine.) Perhaps it’s not the food; perhaps it’s the money. We aren’t provided data on household income in this study, but we can see that the heavy olive oil users were less likely to be current smokers and they got more physical activity.
Now, the authors are aware of these limitations and do their best to account for them. In multivariable models, they adjust for other stuff in the diet, and even for income (sort of; they use census tract as a proxy for income, which is really a broad brush), and still find a significant though weakened association showing a protective effect of olive oil on dementia-related death. But still — adjustment is never perfect, and the small effect size here could definitely be due to residual confounding.
Evidence More Convincing
Now, I did tell you that there is one reason to believe that this study is true, but it’s not really from this study.
It’s from the PREDIMED randomized trial.
This is nutritional epidemiology I can get behind. Published in 2018, investigators in Spain randomized around 7500 participants to receive a liter of olive oil once a week vs mixed nuts, vs small nonfood gifts, the idea here being that if you have olive oil around, you’ll use it more. And people who were randomly assigned to get the olive oil had a 30% lower rate of cardiovascular events. A secondary analysis of that study found that the rate of development of mild cognitive impairment was 65% lower in those who were randomly assigned to olive oil. That’s an impressive result.
So, there might be something to this olive oil thing, but I’m not quite ready to add it to my “pleasurable things that are still good for you” list just yet. Though it does make me wonder: Can we make French fries in the stuff?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
As you all know by now, I’m always looking out for lifestyle changes that are both pleasurable and healthy. They are hard to find, especially when it comes to diet. My kids complain about this all the time: “When you say ‘healthy food,’ you just mean yucky food.” And yes, French fries are amazing, and no, we can’t have them three times a day.
So, when I saw an article claiming that olive oil reduces the risk for dementia, I was interested. I love olive oil; I cook with it all the time. But as is always the case in the world of nutritional epidemiology, we need to be careful. There are a lot of reasons to doubt the results of this study — and one reason to believe it’s true.
The study I’m talking about is “Consumption of Olive Oil and Diet Quality and Risk of Dementia-Related Death,” appearing in JAMA Network Open and following a well-trod formula in the nutritional epidemiology space.
Nearly 100,000 participants, all healthcare workers, filled out a food frequency questionnaire every 4 years with 130 questions touching on all aspects of diet: How often do you eat bananas, bacon, olive oil? Participants were followed for more than 20 years, and if they died, the cause of death was flagged as being dementia-related or not. Over that time frame there were around 38,000 deaths, of which 4751 were due to dementia.
The rest is just statistics. The authors show that those who reported consuming more olive oil were less likely to die from dementia — about 50% less likely, if you compare those who reported eating more than 7 grams of olive oil a day with those who reported eating none.
Is It What You Eat, or What You Don’t Eat?
And we could stop there if we wanted to; I’m sure big olive oil would be happy with that. Is there such a thing as “big olive oil”? But no, we need to dig deeper here because this study has the same problems as all nutritional epidemiology studies. Number one, no one is sitting around drinking small cups of olive oil. They consume it with other foods. And it was clear from the food frequency questionnaire that people who consumed more olive oil also consumed less red meat, more fruits and vegetables, more whole grains, more butter, and less margarine. And those are just the findings reported in the paper. I suspect that people who eat more olive oil also eat more tomatoes, for example, though data this granular aren’t shown. So, it can be really hard, in studies like this, to know for sure that it’s actually the olive oil that is helpful rather than some other constituent in the diet.
The flip side of that coin presents another issue. The food you eat is also a marker of the food you don’t eat. People who ate olive oil consumed less margarine, for example. At the time of this study, margarine was still adulterated with trans-fats, which a pretty solid evidence base suggests are really bad for your vascular system. So perhaps it’s not that olive oil is particularly good for you but that something else is bad for you. In other words, simply adding olive oil to your diet without changing anything else may not do anything.
The other major problem with studies of this sort is that people don’t consume food at random. The type of person who eats a lot of olive oil is simply different from the type of person who doesn›t. For one thing, olive oil is expensive. A 25-ounce bottle of olive oil is on sale at my local supermarket right now for $11.00. A similar-sized bottle of vegetable oil goes for $4.00.
Isn’t it interesting that food that costs more money tends to be associated with better health outcomes? (I’m looking at you, red wine.) Perhaps it’s not the food; perhaps it’s the money. We aren’t provided data on household income in this study, but we can see that the heavy olive oil users were less likely to be current smokers and they got more physical activity.
Now, the authors are aware of these limitations and do their best to account for them. In multivariable models, they adjust for other stuff in the diet, and even for income (sort of; they use census tract as a proxy for income, which is really a broad brush), and still find a significant though weakened association showing a protective effect of olive oil on dementia-related death. But still — adjustment is never perfect, and the small effect size here could definitely be due to residual confounding.
Evidence More Convincing
Now, I did tell you that there is one reason to believe that this study is true, but it’s not really from this study.
It’s from the PREDIMED randomized trial.
This is nutritional epidemiology I can get behind. Published in 2018, investigators in Spain randomized around 7500 participants to receive a liter of olive oil once a week vs mixed nuts, vs small nonfood gifts, the idea here being that if you have olive oil around, you’ll use it more. And people who were randomly assigned to get the olive oil had a 30% lower rate of cardiovascular events. A secondary analysis of that study found that the rate of development of mild cognitive impairment was 65% lower in those who were randomly assigned to olive oil. That’s an impressive result.
So, there might be something to this olive oil thing, but I’m not quite ready to add it to my “pleasurable things that are still good for you” list just yet. Though it does make me wonder: Can we make French fries in the stuff?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
As you all know by now, I’m always looking out for lifestyle changes that are both pleasurable and healthy. They are hard to find, especially when it comes to diet. My kids complain about this all the time: “When you say ‘healthy food,’ you just mean yucky food.” And yes, French fries are amazing, and no, we can’t have them three times a day.
So, when I saw an article claiming that olive oil reduces the risk for dementia, I was interested. I love olive oil; I cook with it all the time. But as is always the case in the world of nutritional epidemiology, we need to be careful. There are a lot of reasons to doubt the results of this study — and one reason to believe it’s true.
The study I’m talking about is “Consumption of Olive Oil and Diet Quality and Risk of Dementia-Related Death,” appearing in JAMA Network Open and following a well-trod formula in the nutritional epidemiology space.
Nearly 100,000 participants, all healthcare workers, filled out a food frequency questionnaire every 4 years with 130 questions touching on all aspects of diet: How often do you eat bananas, bacon, olive oil? Participants were followed for more than 20 years, and if they died, the cause of death was flagged as being dementia-related or not. Over that time frame there were around 38,000 deaths, of which 4751 were due to dementia.
The rest is just statistics. The authors show that those who reported consuming more olive oil were less likely to die from dementia — about 50% less likely, if you compare those who reported eating more than 7 grams of olive oil a day with those who reported eating none.
Is It What You Eat, or What You Don’t Eat?
And we could stop there if we wanted to; I’m sure big olive oil would be happy with that. Is there such a thing as “big olive oil”? But no, we need to dig deeper here because this study has the same problems as all nutritional epidemiology studies. Number one, no one is sitting around drinking small cups of olive oil. They consume it with other foods. And it was clear from the food frequency questionnaire that people who consumed more olive oil also consumed less red meat, more fruits and vegetables, more whole grains, more butter, and less margarine. And those are just the findings reported in the paper. I suspect that people who eat more olive oil also eat more tomatoes, for example, though data this granular aren’t shown. So, it can be really hard, in studies like this, to know for sure that it’s actually the olive oil that is helpful rather than some other constituent in the diet.
The flip side of that coin presents another issue. The food you eat is also a marker of the food you don’t eat. People who ate olive oil consumed less margarine, for example. At the time of this study, margarine was still adulterated with trans-fats, which a pretty solid evidence base suggests are really bad for your vascular system. So perhaps it’s not that olive oil is particularly good for you but that something else is bad for you. In other words, simply adding olive oil to your diet without changing anything else may not do anything.
The other major problem with studies of this sort is that people don’t consume food at random. The type of person who eats a lot of olive oil is simply different from the type of person who doesn›t. For one thing, olive oil is expensive. A 25-ounce bottle of olive oil is on sale at my local supermarket right now for $11.00. A similar-sized bottle of vegetable oil goes for $4.00.
Isn’t it interesting that food that costs more money tends to be associated with better health outcomes? (I’m looking at you, red wine.) Perhaps it’s not the food; perhaps it’s the money. We aren’t provided data on household income in this study, but we can see that the heavy olive oil users were less likely to be current smokers and they got more physical activity.
Now, the authors are aware of these limitations and do their best to account for them. In multivariable models, they adjust for other stuff in the diet, and even for income (sort of; they use census tract as a proxy for income, which is really a broad brush), and still find a significant though weakened association showing a protective effect of olive oil on dementia-related death. But still — adjustment is never perfect, and the small effect size here could definitely be due to residual confounding.
Evidence More Convincing
Now, I did tell you that there is one reason to believe that this study is true, but it’s not really from this study.
It’s from the PREDIMED randomized trial.
This is nutritional epidemiology I can get behind. Published in 2018, investigators in Spain randomized around 7500 participants to receive a liter of olive oil once a week vs mixed nuts, vs small nonfood gifts, the idea here being that if you have olive oil around, you’ll use it more. And people who were randomly assigned to get the olive oil had a 30% lower rate of cardiovascular events. A secondary analysis of that study found that the rate of development of mild cognitive impairment was 65% lower in those who were randomly assigned to olive oil. That’s an impressive result.
So, there might be something to this olive oil thing, but I’m not quite ready to add it to my “pleasurable things that are still good for you” list just yet. Though it does make me wonder: Can we make French fries in the stuff?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Traffic Noise Negatively Impacts Health
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New research by Thomas Münzel, MD, senior professor of cardiology at Johannes Gutenberg University Mainz in Mainz, Germany, and colleagues again emphasized the harmful effects of noise on the heart and blood vessels. An analysis of current epidemiologic data provided strong indications that transportation noise is closely related to cardiovascular and cerebrovascular diseases, according to a statement on the data analysis. The results were published in Circulation Research.
Morbidity and Mortality
Epidemiologic studies have shown that road, rail, or air traffic noise increases the risk for cardiovascular morbidity and mortality, with strong evidence for ischemic heart disease, heart failure, and stroke, according to the scientists. These factors could favor vascular (endothelial) dysfunction, inflammation, and hypertension, thereby increasing cardiovascular risk.
Consequences and Pathomechanisms
In the current publication, the authors provided an overview of epidemiologic research on the effects of transportation noise on cardiovascular risk factors and diseases, discussed mechanistic insights from the latest clinical and experimental studies, and proposed new risk markers to address noise-induced cardiovascular effects in the general population. An integrated analysis in the article demonstrated that for every 10 dB(A) increase, the risk for cardiovascular diseases such as heart attack, stroke, and heart failure significantly increases by 3.2%.
The authors also explained the possible effects of noise on changes in gene networks, epigenetic pathways, circadian rhythms, signal transmission along the neuronal-cardiovascular axis, oxidative stress, inflammation, and metabolism. Finally, current and future noise protection strategies are described, and the existing evidence on noise as a cardiovascular risk factor is discussed.
Confirmed Cardiovascular Risk Factor
“As an increasing proportion of the population is exposed to harmful traffic noise, efforts to reduce noise and laws for noise reduction are of great importance for future public health,” said Dr. Münzel. “It is also important for us that due to the strong evidence, traffic noise is finally recognized as a risk factor for cardiovascular diseases.”
Heart Attack Outcomes
Dr. Münzel and other researchers from Mainz have been studying the cardiovascular consequences of air pollution and traffic noise for several years. For example, they found that heart attacks in people and animals exposed to high noise levels earlier in life healed poorly. These results were published last year in Cardiovascular Research. According to the authors, the findings suggest that traffic noise may play a significant role in the development and course of coronary heart disease, such as after a heart attack.
The scientists initially found in animal experiments that exposure to aircraft noise for 4 days led to increased inflammation in the vessels. Compared with mice not exposed to aircraft noise, the noise-exposed animals showed an increase in free radicals; these animals exhibited a significant inflammatory response and had impaired vessel function.
The researchers explained that the experimental data showed aircraft noise alone triggers a proinflammatory transcription program that promotes the infiltration of immune cells into cardiovascular tissue in animals with acute myocardial infarction. They noted an increased infiltration of CD45+ cells into the vessels and heart, dominated by neutrophils in vessel tissue and Ly6Chigh monocytes in heart tissue. This infiltration creates a proinflammatory milieu that adversely affects the outcome after myocardial infarction by predisposing the heart tissue to greater ischemic damage and functional impairment. Exposure of animals to aircraft noise before induction of myocardial infarction by left anterior descending (LAD) coronary artery ligation impaired left ventricular function and increased infarct size after cardiac ischemia. In addition, noise exposure exacerbated infarct-induced endothelial dysfunction of peripheral vessels as early as 24 hours after LAD ligation.
Clinical Confirmation
These experimental results were confirmed by observations in the population-based Gutenberg Health Study. The researchers analyzed data from 100 patients with heart attack. The lead and senior authors of the study Michael Molitor, MD, and Philip Wenzel, MD, of the University of Mainz, explained, “From our studies, we have learned that exposure to aircraft noise before a heart attack significantly amplifies subsequent cardiovascular inflammation and exacerbates ischemic heart failure, which is favored by inflammation-promoting vascular conditioning. Our translational results show that people who have been exposed to noise in the past have a worse course if they experience a heart attack later in life.”
Study participants who had experienced a heart attack in their medical history had elevated levels of C-reactive protein if they had been exposed to aircraft noise in the past and subsequently developed noise annoyance reactions (0.305 vs 1.5; P = .0094). In addition, left ventricular ejection fraction in these patients after a heart attack was worse than that in patients with infarction without noise exposure in their medical history (62.5 vs 65.6; P = .0053).
The results suggest that measures to reduce environmental noise could help improve the clinical outcomes of heart attack patients, according to the authors.
Mental Health Effects
Traffic noise also may be associated with an increased risk for depression and anxiety disorders, as reported 2 years ago by the German Society for Psychosomatic Medicine and Medical Psychotherapy. Evolution has programmed the human organism to perceive noises as indicators of potential sources of danger — even during sleep. “Noise puts the body on alert,” explained Manfred E. Beutel, MD, director of the Clinic for Psychosomatic Medicine and Psychotherapy at the University of Mainz. As a result, the autonomic nervous system activates stress hormones such as adrenaline and cortisol, leading to an increase in heart rate and blood pressure. If noise becomes chronic, chronic diseases can develop. “Indeed, observational and experimental studies have shown that persistent noise annoyance promotes incident hypertension, cardiovascular diseases, and type 2 diabetes,” said Dr. Beutel.
Depression Risk Doubled
Among the negative effects of noise annoyance are also mental illnesses, as has become increasingly clear. “Noise annoyance disrupts daily activities and interferes with feelings and thoughts, sleep, and recovery,” said Dr. Beutel. The interruptions trigger negative emotional reactions such as anger, distress, exhaustion, flight impulses, and stress symptoms. “Such conditions promote the development of depression over time,” said Dr. Beutel. This observation was confirmed by the large-scale Gutenberg Health Study using the example of the Mainz population, which suffers to a large extent from noise annoyance because of the nearby Frankfurt Airport. “With increasing noise annoyance, the rates of depression and anxiety disorders steadily increased, until the risks eventually doubled with extreme annoyance,” said Dr. Beutel. Other studies point in the same direction. For example, a meta-analysis found a 12% increase in the risk for depression per 10-dB increase in noise. Another study found an association between nocturnal noise annoyance and the use of antidepressants.
Fine Particulate Matter
According to an evaluation of the Gutenberg Study, people perceive noise annoyance from aircraft noise as the most pronounced, followed by road, neighborhood, industrial, and railway noise. Noise occurs most frequently in urban areas that also produce air pollution such as fine particulate matter. “Fine particulate matter is also suspected of promoting anxiety and depression,” said Dr. Beutel, “because the small particles of fine particulate matter can enter the bloodstream and trigger inflammatory processes there, which in turn are closely related to depression.”
This story was translated from Univadis Germany, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is Red Meat Healthy? Multiverse Analysis Has Lessons Beyond Meat
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.