User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Study Links Melasma With Comorbidities, Races, Ethnicities
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A study found significant associations between melasma and several comorbidities, including hypertension and hormonal contraception use, which were the most common.
METHODOLOGY:
- Melasma predominantly affects young women of color and often worsens in hyperestrogen states; understanding the association with comorbidities can improve surveillance and treatment strategies.
- Researchers evaluated 41,283 patients with melasma (mean age, 48.8 years; 93% women) from the TriNetX database and an equal number of matched control individuals.
- The main outcome was comorbidities including allergic rhinitis, atopic dermatitis, anticonvulsants, diabetes, hormonal contraceptives, hypothyroidism, hypertension, lupus, rosacea, skin cancer, and malignancy.
TAKEAWAY:
- Among those with melasma, 25% had hypertension and 24% used hormonal contraception, the two most commonly associated risk factors identified.
- Rosacea (odds ratio [OR], 5.1), atopic dermatitis (OR, 3.3), lupus (OR, 2.5), history of skin cancer (OR, 2.5), and history of internal malignancy (OR, 2.1) were associated with the highest risk of developing melasma (P < .01 for all).
- Asian (OR, 2.0; P < .01) and “other/unknown” races (OR, 1.7; P < .01) and Hispanic ethnicity (OR, 1.3; P < .01) were also significantly associated with melasma, while the odds were slightly lower among White, Black/African American, and “not Hispanic” groups (ORs, 0.8; P < .01 for all groups).
IN PRACTICE:
the authors wrote.
SOURCE:
The study, led by Ajay N. Sharma, MD, MBA, of the Department of Dermatology at the University of California, Irvine, was published online in Journal of Drugs in Dermatology.
LIMITATIONS:
The study limitations included the retrospective design, potential misclassification of diagnoses, and the inability to establish causality.
DISCLOSURES:
The study did not disclose any funding sources. The authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Government Accuses Health System of Paying Docs Outrageous Salaries for Patient Referrals
Strapped for cash and searching for new profits, Tennessee-based Erlanger Health System illegally paid excessive salaries to physicians in exchange for patient referrals, the US government alleged in a federal lawsuit.
Erlanger changed its compensation model to entice revenue-generating doctors, paying some two to three times the median salary for their specialty, according to the complaint.
The physicians in turn referred numerous patients to Erlanger, and the health system submitted claims to Medicare for the referred services in violation of the Stark Law, according to the suit, filed in US District Court for the Western District of North Carolina.
The government’s complaint “serves as a warning” to healthcare providers who try to boost profits through improper financial arrangements with referring physicians, said Tamala E. Miles, Special Agent in Charge for the US Department of Health and Human Services (HHS) Office of Inspector General (OIG).
In a statement provided to this news organization, Erlanger denied the allegations and said it would “vigorously” defend the lawsuit.
“Erlanger paid physicians based on amounts that outside experts advised was fair market value,” Erlanger officials said in the statement. “Erlanger did not pay for referrals. A complete picture of the facts will demonstrate that the allegations lack merit and tell a very different story than what the government now claims.”
The Erlanger case is a reminder to physicians to consult their own knowledgeable advisors when considering financial arrangements with hospitals, said William Sarraille, JD, adjunct professor for the University of Maryland Francis King Carey School of Law in Baltimore and a regulatory consultant.
“There is a tendency by physicians when contracting ... to rely on [hospitals’] perceived compliance and legal expertise,” Mr. Sarraille told this news organization. “This case illustrates the risks in doing so. Sometimes bigger doesn’t translate into more sophisticated or more effective from a compliance perspective.”
Stark Law Prohibits Kickbacks
The Stark Law prohibits hospitals from billing the Centers for Medicare & Medicaid Services (CMS) for services referred by a physician with whom the hospital has an improper financial relationship.
CMS paid Erlanger about $27.8 million for claims stemming from the improper financial arrangements, the government contends.
“HHS-OIG will continue to investigate such deals to prevent financial arrangements that could compromise impartial medical judgment, increase healthcare costs, and erode public trust in the healthcare system,” Ms. Miles said in a statement.
Suit: Health System’s Money Woes Led to Illegal Arrangements
Erlanger’s financial troubles allegedly started after a previous run-in with the US government over false claims.
In 2005, Erlanger Health System agreed to pay the government $40 million to resolve allegations that it knowingly submitted false claims to Medicare, according to the government’s complaint. At the time, Erlanger entered into a Corporate Integrity Agreement (CIA) with the OIG that required Erlanger to put controls in place to ensure its financial relationships did not violate the Stark Law.
Erlanger’s agreement with OIG ended in 2010. Over the next 3 years, the health system lost nearly $32 million and in fiscal year 2013, had only 65 days of cash on hand, according to the government’s lawsuit.
Beginning in 2013, Erlanger allegedly implemented a strategy to increase profits by employing more physicians, particularly specialists from competing hospitals whose patients would need costly hospital stays, according to the complaint.
Once hired, Erlanger’s physicians were expected to treat patients at Erlanger’s hospitals and refer them to other providers within the health system, the suit claims. Erlanger also relaxed or eliminated the oversight and controls on physician compensation put in place under the CIA. For example, Erlanger’s CEO signed some compensation contracts before its chief compliance officer could review them and no longer allowed the compliance officer to vote on whether to approve compensation arrangements, according to the complaint.
Erlanger also changed its compensation model to include large salaries for medical director and academic positions and allegedly paid such salaries to physicians without ensuring the required work was performed. As a result, Erlanger physicians with profitable referrals were among the highest paid in the nation for their specialties, the government claims. For example, according to the complaint:
- Erlanger paid an electrophysiologist an annual clinical salary of $816,701, a medical director salary of $101,080, an academic salary of $59,322, and a productivity incentive based on work relative value units (wRVUs). The medical director and academic salaries paid were near the 90th percentile of comparable salaries in the specialty.
- The health system paid a neurosurgeon a base salary of $654,735, a productivity incentive based on wRVUs, and payments for excess call coverage ranging from $400 to $1000 per 24-hour shift. In 2016, the neurosurgeon made $500,000 in excess call payments.
- Erlanger paid a cardiothoracic surgeon a base clinical salary of $1,070,000, a sign-on bonus of $150,000, a retention bonus of $100,000 (payable in the 4th year of the contract), and a program incentive of up to $150,000 per year.
In addition, Erlanger ignored patient safety concerns about some of its high revenue-generating physicians, the government claims.
For instance, Erlanger received multiple complaints that a cardiothoracic surgeon was misusing an expensive form of life support in which pumps and oxygenators take over heart and lung function. Overuse of the equipment prolonged patients’ hospital stays and increased the hospital fees generated by the surgeon, according to the complaint. Staff also raised concerns about the cardiothoracic surgeon’s patient outcomes.
But Erlanger disregarded the concerns and in 2018, increased the cardiothoracic surgeon’s retention bonus from $100,000 to $250,000, the suit alleges. A year later, the health system increased his base salary from $1,070,000 to $1,195,000.
Health care compensation and billing consultants alerted Erlanger that it was overpaying salaries and handing out bonuses based on measures that overstated the work physicians were performing, but Erlanger ignored the warnings, according to the complaint.
Administrators allegedly resisted efforts by the chief compliance officer to hire an outside consultant to review its compensation models. Erlanger fired the compliance officer in 2019.
The former chief compliance officer and another administrator filed a whistleblower lawsuit against Erlanger in 2021. The two administrators are relators in the government’s July 2024 lawsuit.
How to Protect Yourself From Illegal Hospital Deals
The Erlanger case is the latest in a series of recent complaints by the federal government involving financial arrangements between hospitals and physicians.
In December 2023, Indianapolis-based Community Health Network Inc. agreed to pay the government $345 million to resolve claims that it paid physicians above fair market value and awarded bonuses tied to referrals in violation of the Stark Law.
Also in 2023, Saginaw, Michigan–based Covenant HealthCare and two physicians paid the government $69 million to settle allegations that administrators engaged in improper financial arrangements with referring physicians and a physician-owned investment group. In another 2023 case, Massachusetts Eye and Ear in Boston agreed to pay $5.7 million to resolve claims that some of its physician compensation plans violated the Stark Law.
Before you enter into a financial arrangement with a hospital, it’s also important to examine what percentile the aggregate compensation would reflect, law professor Mr. Sarraille said. The Erlanger case highlights federal officials’ suspicion of compensation, in aggregate, that exceeds the 90th percentile and increased attention to compensation that exceeds the 75th percentile, he said.
To research compensation levels, doctors can review the Medical Group Management Association’s annual compensation report or search its compensation data.
Before signing any contracts, Mr. Sarraille suggests, physicians should also consider whether the hospital shares the same values. Ask physicians at the hospital what they have to say about the hospital’s culture, vision, and values. Have physicians left the hospital after their practices were acquired? Consider speaking with them to learn why.
Keep in mind that a doctor’s reputation could be impacted by a compliance complaint, regardless of whether it’s directed at the hospital and not the employed physician, Mr. Sarraille said.
“The [Erlanger] complaint focuses on the compensation of specific, named physicians saying they were wildly overcompensated,” he said. “The implication is that they sold their referral power in exchange for a pay day. It’s a bad look, no matter how the case evolves from here.”
Physicians could also face their own liability risk under the Stark Law and False Claims Act, depending on the circumstances. In the event of related quality-of-care issues, medical liability could come into play, Mr. Sarraille noted. In such cases, plaintiffs’ attorneys may see an opportunity to boost their claims with allegations that the patient harm was a function of “chasing compensation dollars,” Mr. Sarraille said.
“Where that happens, plaintiff lawyers see the potential for crippling punitive damages, which might not be covered by an insurer,” he said.
A version of this article appeared on Medscape.com.
Strapped for cash and searching for new profits, Tennessee-based Erlanger Health System illegally paid excessive salaries to physicians in exchange for patient referrals, the US government alleged in a federal lawsuit.
Erlanger changed its compensation model to entice revenue-generating doctors, paying some two to three times the median salary for their specialty, according to the complaint.
The physicians in turn referred numerous patients to Erlanger, and the health system submitted claims to Medicare for the referred services in violation of the Stark Law, according to the suit, filed in US District Court for the Western District of North Carolina.
The government’s complaint “serves as a warning” to healthcare providers who try to boost profits through improper financial arrangements with referring physicians, said Tamala E. Miles, Special Agent in Charge for the US Department of Health and Human Services (HHS) Office of Inspector General (OIG).
In a statement provided to this news organization, Erlanger denied the allegations and said it would “vigorously” defend the lawsuit.
“Erlanger paid physicians based on amounts that outside experts advised was fair market value,” Erlanger officials said in the statement. “Erlanger did not pay for referrals. A complete picture of the facts will demonstrate that the allegations lack merit and tell a very different story than what the government now claims.”
The Erlanger case is a reminder to physicians to consult their own knowledgeable advisors when considering financial arrangements with hospitals, said William Sarraille, JD, adjunct professor for the University of Maryland Francis King Carey School of Law in Baltimore and a regulatory consultant.
“There is a tendency by physicians when contracting ... to rely on [hospitals’] perceived compliance and legal expertise,” Mr. Sarraille told this news organization. “This case illustrates the risks in doing so. Sometimes bigger doesn’t translate into more sophisticated or more effective from a compliance perspective.”
Stark Law Prohibits Kickbacks
The Stark Law prohibits hospitals from billing the Centers for Medicare & Medicaid Services (CMS) for services referred by a physician with whom the hospital has an improper financial relationship.
CMS paid Erlanger about $27.8 million for claims stemming from the improper financial arrangements, the government contends.
“HHS-OIG will continue to investigate such deals to prevent financial arrangements that could compromise impartial medical judgment, increase healthcare costs, and erode public trust in the healthcare system,” Ms. Miles said in a statement.
Suit: Health System’s Money Woes Led to Illegal Arrangements
Erlanger’s financial troubles allegedly started after a previous run-in with the US government over false claims.
In 2005, Erlanger Health System agreed to pay the government $40 million to resolve allegations that it knowingly submitted false claims to Medicare, according to the government’s complaint. At the time, Erlanger entered into a Corporate Integrity Agreement (CIA) with the OIG that required Erlanger to put controls in place to ensure its financial relationships did not violate the Stark Law.
Erlanger’s agreement with OIG ended in 2010. Over the next 3 years, the health system lost nearly $32 million and in fiscal year 2013, had only 65 days of cash on hand, according to the government’s lawsuit.
Beginning in 2013, Erlanger allegedly implemented a strategy to increase profits by employing more physicians, particularly specialists from competing hospitals whose patients would need costly hospital stays, according to the complaint.
Once hired, Erlanger’s physicians were expected to treat patients at Erlanger’s hospitals and refer them to other providers within the health system, the suit claims. Erlanger also relaxed or eliminated the oversight and controls on physician compensation put in place under the CIA. For example, Erlanger’s CEO signed some compensation contracts before its chief compliance officer could review them and no longer allowed the compliance officer to vote on whether to approve compensation arrangements, according to the complaint.
Erlanger also changed its compensation model to include large salaries for medical director and academic positions and allegedly paid such salaries to physicians without ensuring the required work was performed. As a result, Erlanger physicians with profitable referrals were among the highest paid in the nation for their specialties, the government claims. For example, according to the complaint:
- Erlanger paid an electrophysiologist an annual clinical salary of $816,701, a medical director salary of $101,080, an academic salary of $59,322, and a productivity incentive based on work relative value units (wRVUs). The medical director and academic salaries paid were near the 90th percentile of comparable salaries in the specialty.
- The health system paid a neurosurgeon a base salary of $654,735, a productivity incentive based on wRVUs, and payments for excess call coverage ranging from $400 to $1000 per 24-hour shift. In 2016, the neurosurgeon made $500,000 in excess call payments.
- Erlanger paid a cardiothoracic surgeon a base clinical salary of $1,070,000, a sign-on bonus of $150,000, a retention bonus of $100,000 (payable in the 4th year of the contract), and a program incentive of up to $150,000 per year.
In addition, Erlanger ignored patient safety concerns about some of its high revenue-generating physicians, the government claims.
For instance, Erlanger received multiple complaints that a cardiothoracic surgeon was misusing an expensive form of life support in which pumps and oxygenators take over heart and lung function. Overuse of the equipment prolonged patients’ hospital stays and increased the hospital fees generated by the surgeon, according to the complaint. Staff also raised concerns about the cardiothoracic surgeon’s patient outcomes.
But Erlanger disregarded the concerns and in 2018, increased the cardiothoracic surgeon’s retention bonus from $100,000 to $250,000, the suit alleges. A year later, the health system increased his base salary from $1,070,000 to $1,195,000.
Health care compensation and billing consultants alerted Erlanger that it was overpaying salaries and handing out bonuses based on measures that overstated the work physicians were performing, but Erlanger ignored the warnings, according to the complaint.
Administrators allegedly resisted efforts by the chief compliance officer to hire an outside consultant to review its compensation models. Erlanger fired the compliance officer in 2019.
The former chief compliance officer and another administrator filed a whistleblower lawsuit against Erlanger in 2021. The two administrators are relators in the government’s July 2024 lawsuit.
How to Protect Yourself From Illegal Hospital Deals
The Erlanger case is the latest in a series of recent complaints by the federal government involving financial arrangements between hospitals and physicians.
In December 2023, Indianapolis-based Community Health Network Inc. agreed to pay the government $345 million to resolve claims that it paid physicians above fair market value and awarded bonuses tied to referrals in violation of the Stark Law.
Also in 2023, Saginaw, Michigan–based Covenant HealthCare and two physicians paid the government $69 million to settle allegations that administrators engaged in improper financial arrangements with referring physicians and a physician-owned investment group. In another 2023 case, Massachusetts Eye and Ear in Boston agreed to pay $5.7 million to resolve claims that some of its physician compensation plans violated the Stark Law.
Before you enter into a financial arrangement with a hospital, it’s also important to examine what percentile the aggregate compensation would reflect, law professor Mr. Sarraille said. The Erlanger case highlights federal officials’ suspicion of compensation, in aggregate, that exceeds the 90th percentile and increased attention to compensation that exceeds the 75th percentile, he said.
To research compensation levels, doctors can review the Medical Group Management Association’s annual compensation report or search its compensation data.
Before signing any contracts, Mr. Sarraille suggests, physicians should also consider whether the hospital shares the same values. Ask physicians at the hospital what they have to say about the hospital’s culture, vision, and values. Have physicians left the hospital after their practices were acquired? Consider speaking with them to learn why.
Keep in mind that a doctor’s reputation could be impacted by a compliance complaint, regardless of whether it’s directed at the hospital and not the employed physician, Mr. Sarraille said.
“The [Erlanger] complaint focuses on the compensation of specific, named physicians saying they were wildly overcompensated,” he said. “The implication is that they sold their referral power in exchange for a pay day. It’s a bad look, no matter how the case evolves from here.”
Physicians could also face their own liability risk under the Stark Law and False Claims Act, depending on the circumstances. In the event of related quality-of-care issues, medical liability could come into play, Mr. Sarraille noted. In such cases, plaintiffs’ attorneys may see an opportunity to boost their claims with allegations that the patient harm was a function of “chasing compensation dollars,” Mr. Sarraille said.
“Where that happens, plaintiff lawyers see the potential for crippling punitive damages, which might not be covered by an insurer,” he said.
A version of this article appeared on Medscape.com.
Strapped for cash and searching for new profits, Tennessee-based Erlanger Health System illegally paid excessive salaries to physicians in exchange for patient referrals, the US government alleged in a federal lawsuit.
Erlanger changed its compensation model to entice revenue-generating doctors, paying some two to three times the median salary for their specialty, according to the complaint.
The physicians in turn referred numerous patients to Erlanger, and the health system submitted claims to Medicare for the referred services in violation of the Stark Law, according to the suit, filed in US District Court for the Western District of North Carolina.
The government’s complaint “serves as a warning” to healthcare providers who try to boost profits through improper financial arrangements with referring physicians, said Tamala E. Miles, Special Agent in Charge for the US Department of Health and Human Services (HHS) Office of Inspector General (OIG).
In a statement provided to this news organization, Erlanger denied the allegations and said it would “vigorously” defend the lawsuit.
“Erlanger paid physicians based on amounts that outside experts advised was fair market value,” Erlanger officials said in the statement. “Erlanger did not pay for referrals. A complete picture of the facts will demonstrate that the allegations lack merit and tell a very different story than what the government now claims.”
The Erlanger case is a reminder to physicians to consult their own knowledgeable advisors when considering financial arrangements with hospitals, said William Sarraille, JD, adjunct professor for the University of Maryland Francis King Carey School of Law in Baltimore and a regulatory consultant.
“There is a tendency by physicians when contracting ... to rely on [hospitals’] perceived compliance and legal expertise,” Mr. Sarraille told this news organization. “This case illustrates the risks in doing so. Sometimes bigger doesn’t translate into more sophisticated or more effective from a compliance perspective.”
Stark Law Prohibits Kickbacks
The Stark Law prohibits hospitals from billing the Centers for Medicare & Medicaid Services (CMS) for services referred by a physician with whom the hospital has an improper financial relationship.
CMS paid Erlanger about $27.8 million for claims stemming from the improper financial arrangements, the government contends.
“HHS-OIG will continue to investigate such deals to prevent financial arrangements that could compromise impartial medical judgment, increase healthcare costs, and erode public trust in the healthcare system,” Ms. Miles said in a statement.
Suit: Health System’s Money Woes Led to Illegal Arrangements
Erlanger’s financial troubles allegedly started after a previous run-in with the US government over false claims.
In 2005, Erlanger Health System agreed to pay the government $40 million to resolve allegations that it knowingly submitted false claims to Medicare, according to the government’s complaint. At the time, Erlanger entered into a Corporate Integrity Agreement (CIA) with the OIG that required Erlanger to put controls in place to ensure its financial relationships did not violate the Stark Law.
Erlanger’s agreement with OIG ended in 2010. Over the next 3 years, the health system lost nearly $32 million and in fiscal year 2013, had only 65 days of cash on hand, according to the government’s lawsuit.
Beginning in 2013, Erlanger allegedly implemented a strategy to increase profits by employing more physicians, particularly specialists from competing hospitals whose patients would need costly hospital stays, according to the complaint.
Once hired, Erlanger’s physicians were expected to treat patients at Erlanger’s hospitals and refer them to other providers within the health system, the suit claims. Erlanger also relaxed or eliminated the oversight and controls on physician compensation put in place under the CIA. For example, Erlanger’s CEO signed some compensation contracts before its chief compliance officer could review them and no longer allowed the compliance officer to vote on whether to approve compensation arrangements, according to the complaint.
Erlanger also changed its compensation model to include large salaries for medical director and academic positions and allegedly paid such salaries to physicians without ensuring the required work was performed. As a result, Erlanger physicians with profitable referrals were among the highest paid in the nation for their specialties, the government claims. For example, according to the complaint:
- Erlanger paid an electrophysiologist an annual clinical salary of $816,701, a medical director salary of $101,080, an academic salary of $59,322, and a productivity incentive based on work relative value units (wRVUs). The medical director and academic salaries paid were near the 90th percentile of comparable salaries in the specialty.
- The health system paid a neurosurgeon a base salary of $654,735, a productivity incentive based on wRVUs, and payments for excess call coverage ranging from $400 to $1000 per 24-hour shift. In 2016, the neurosurgeon made $500,000 in excess call payments.
- Erlanger paid a cardiothoracic surgeon a base clinical salary of $1,070,000, a sign-on bonus of $150,000, a retention bonus of $100,000 (payable in the 4th year of the contract), and a program incentive of up to $150,000 per year.
In addition, Erlanger ignored patient safety concerns about some of its high revenue-generating physicians, the government claims.
For instance, Erlanger received multiple complaints that a cardiothoracic surgeon was misusing an expensive form of life support in which pumps and oxygenators take over heart and lung function. Overuse of the equipment prolonged patients’ hospital stays and increased the hospital fees generated by the surgeon, according to the complaint. Staff also raised concerns about the cardiothoracic surgeon’s patient outcomes.
But Erlanger disregarded the concerns and in 2018, increased the cardiothoracic surgeon’s retention bonus from $100,000 to $250,000, the suit alleges. A year later, the health system increased his base salary from $1,070,000 to $1,195,000.
Health care compensation and billing consultants alerted Erlanger that it was overpaying salaries and handing out bonuses based on measures that overstated the work physicians were performing, but Erlanger ignored the warnings, according to the complaint.
Administrators allegedly resisted efforts by the chief compliance officer to hire an outside consultant to review its compensation models. Erlanger fired the compliance officer in 2019.
The former chief compliance officer and another administrator filed a whistleblower lawsuit against Erlanger in 2021. The two administrators are relators in the government’s July 2024 lawsuit.
How to Protect Yourself From Illegal Hospital Deals
The Erlanger case is the latest in a series of recent complaints by the federal government involving financial arrangements between hospitals and physicians.
In December 2023, Indianapolis-based Community Health Network Inc. agreed to pay the government $345 million to resolve claims that it paid physicians above fair market value and awarded bonuses tied to referrals in violation of the Stark Law.
Also in 2023, Saginaw, Michigan–based Covenant HealthCare and two physicians paid the government $69 million to settle allegations that administrators engaged in improper financial arrangements with referring physicians and a physician-owned investment group. In another 2023 case, Massachusetts Eye and Ear in Boston agreed to pay $5.7 million to resolve claims that some of its physician compensation plans violated the Stark Law.
Before you enter into a financial arrangement with a hospital, it’s also important to examine what percentile the aggregate compensation would reflect, law professor Mr. Sarraille said. The Erlanger case highlights federal officials’ suspicion of compensation, in aggregate, that exceeds the 90th percentile and increased attention to compensation that exceeds the 75th percentile, he said.
To research compensation levels, doctors can review the Medical Group Management Association’s annual compensation report or search its compensation data.
Before signing any contracts, Mr. Sarraille suggests, physicians should also consider whether the hospital shares the same values. Ask physicians at the hospital what they have to say about the hospital’s culture, vision, and values. Have physicians left the hospital after their practices were acquired? Consider speaking with them to learn why.
Keep in mind that a doctor’s reputation could be impacted by a compliance complaint, regardless of whether it’s directed at the hospital and not the employed physician, Mr. Sarraille said.
“The [Erlanger] complaint focuses on the compensation of specific, named physicians saying they were wildly overcompensated,” he said. “The implication is that they sold their referral power in exchange for a pay day. It’s a bad look, no matter how the case evolves from here.”
Physicians could also face their own liability risk under the Stark Law and False Claims Act, depending on the circumstances. In the event of related quality-of-care issues, medical liability could come into play, Mr. Sarraille noted. In such cases, plaintiffs’ attorneys may see an opportunity to boost their claims with allegations that the patient harm was a function of “chasing compensation dollars,” Mr. Sarraille said.
“Where that happens, plaintiff lawyers see the potential for crippling punitive damages, which might not be covered by an insurer,” he said.
A version of this article appeared on Medscape.com.
Non-Prescription Semaglutide Purchased Online Poses Risks
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
SUNY Downstate Emergency Medicine Doc Charged With $1.5M Fraud
In a case that spotlights the importance of comprehensive financial controls in medical offices,
Michael Lucchesi, MD, who had served as chairman of Emergency Medicine at SUNY Downstate Medical Center in New York City, was arraigned on July 9 and pleaded not guilty. Dr. Lucchesi’s attorney, Earl Ward, did not respond to messages from this news organization, but he told the New York Post that “the funds he used were not stolen funds.”
Dr. Lucchesi, who’s in his late 60s, faces nine counts of first- and second-degree grand larceny, first-degree falsifying business records, and third-degree criminal tax fraud. According to a press statement from the district attorney of Kings County, which encompasses the borough of Brooklyn, Dr. Lucchesi is accused of using his clinical practice’s business card for cash advances (about $115,000), high-end pet care ($176,000), personal travel ($348,000), gym membership and personal training ($109,000), catering ($52,000), tuition payments for his children ($46,000), and other expenses such as online shopping, flowers, liquor, and electronics.
Most of the alleged pet care spending — $120,000 — went to the Green Leaf Pet Resort, which has two locations in New Jersey, including one with “56 acres of nature and lots of tail wagging.” Some of the alleged spending on gym membership was at the New York Sports Clubs chain, where monthly membership tops out at $139.99.
The alleged spending occurred between 2016 and 2023 and was discovered by SUNY Downstate during an audit. Dr. Lucchesi reportedly left his position at the hospital, where he made $399,712 in 2022 as a professor, according to public records.
“As a high-ranking doctor at this vital healthcare institution, this defendant was entrusted with access to significant funds, which he allegedly exploited, stealing more than 1 million dollars to pay for a lavish lifestyle,” District Attorney Eric Gonzalez said in a statement.
SUNY Downstate is in a fight for its life amid efforts by New York Governor Kathy Hochul to shut it down. According to The New York Times, it is the only state-run hospital in New York City.
Dr. Lucchesi, who had previously served as the hospital’s chief medical officer and acting head, was released without bail. His next court date is September 25, 2024.
Size of Alleged Theft Is ‘Very Unusual’
David P. Weber, JD, DBA, a professor and fraud specialist at Salisbury University, Salisbury, Maryland, told this news organization that the fraudulent use of a business or purchase credit card is a form of embezzlement and “one of the most frequently seen types of frauds against organizations.”
William J. Kresse, JD, MSA, CPA/CFF, who studies fraud at Governors State University in University Park, Illinois, noted in an interview with this news organization that the high amount of alleged fraud in this case is “very unusual,” as is the period it is said to have occurred (over 6 years).
Mr. Kresse highlighted a 2024 report by the Association of Certified Fraud Examiners, which found that the median fraud loss in healthcare, on the basis of 117 cases, is $100,000. The most common form of fraud in the industry is corruption (47%), followed by billing (38%), noncash theft such as inventory (22%), and expense reimbursement (21%).
The details of the current case suggest that “SUNY Downstate had weak or insufficient internal controls to prevent this type of fraud,” Salisbury University’s Mr. Weber said. “However, research also makes clear that the tenure and position of the perpetrator play a significant role in the size of the fraud. Internal controls are supposed to apply to all employees, but the higher in the organization the perpetrator is, the easier it can be to engage in fraud.”
Even Small Medical Offices Can Act to Prevent Fraud
What can be done to prevent this kind of fraud? “Each employee should be required to submit actual receipts or scanned copies, and the reimbursement requests should be reviewed and inputted by a separate department or office of the organization to ensure that the expenses are legitimate,” Mr. Weber said. “In addition, all credit card statements should be available for review by the organization either simultaneously with the bill going to the employee or available for audit or review at any time without notification to the employee. Expenses that are in certain categories should be prohibited automatically and coded to the card so such a charge is rejected by the credit card bank.”
Smaller businesses — like many medical practices — may not have the manpower to handle these roles. In that case, Mr. Weber said, “The key is segregation or separation of duties. The bookkeeper cannot be the person receiving the bank statements, the payments from patients, and the invoices from vendors. There needs to be at least one other person in the loop to have some level of control.”
One strategy, he said, “is that the practice should institute a policy that only the doctor or owner of the practice can receive the mail, not the bookkeeper. Even if the practice leader does not actually review the bank statements, simply opening them before handing them off to the bookkeeper can provide a level of deterrence [since] the employee may get caught if someone else is reviewing the bank statements.”
A version of this article first appeared on Medscape.com.
In a case that spotlights the importance of comprehensive financial controls in medical offices,
Michael Lucchesi, MD, who had served as chairman of Emergency Medicine at SUNY Downstate Medical Center in New York City, was arraigned on July 9 and pleaded not guilty. Dr. Lucchesi’s attorney, Earl Ward, did not respond to messages from this news organization, but he told the New York Post that “the funds he used were not stolen funds.”
Dr. Lucchesi, who’s in his late 60s, faces nine counts of first- and second-degree grand larceny, first-degree falsifying business records, and third-degree criminal tax fraud. According to a press statement from the district attorney of Kings County, which encompasses the borough of Brooklyn, Dr. Lucchesi is accused of using his clinical practice’s business card for cash advances (about $115,000), high-end pet care ($176,000), personal travel ($348,000), gym membership and personal training ($109,000), catering ($52,000), tuition payments for his children ($46,000), and other expenses such as online shopping, flowers, liquor, and electronics.
Most of the alleged pet care spending — $120,000 — went to the Green Leaf Pet Resort, which has two locations in New Jersey, including one with “56 acres of nature and lots of tail wagging.” Some of the alleged spending on gym membership was at the New York Sports Clubs chain, where monthly membership tops out at $139.99.
The alleged spending occurred between 2016 and 2023 and was discovered by SUNY Downstate during an audit. Dr. Lucchesi reportedly left his position at the hospital, where he made $399,712 in 2022 as a professor, according to public records.
“As a high-ranking doctor at this vital healthcare institution, this defendant was entrusted with access to significant funds, which he allegedly exploited, stealing more than 1 million dollars to pay for a lavish lifestyle,” District Attorney Eric Gonzalez said in a statement.
SUNY Downstate is in a fight for its life amid efforts by New York Governor Kathy Hochul to shut it down. According to The New York Times, it is the only state-run hospital in New York City.
Dr. Lucchesi, who had previously served as the hospital’s chief medical officer and acting head, was released without bail. His next court date is September 25, 2024.
Size of Alleged Theft Is ‘Very Unusual’
David P. Weber, JD, DBA, a professor and fraud specialist at Salisbury University, Salisbury, Maryland, told this news organization that the fraudulent use of a business or purchase credit card is a form of embezzlement and “one of the most frequently seen types of frauds against organizations.”
William J. Kresse, JD, MSA, CPA/CFF, who studies fraud at Governors State University in University Park, Illinois, noted in an interview with this news organization that the high amount of alleged fraud in this case is “very unusual,” as is the period it is said to have occurred (over 6 years).
Mr. Kresse highlighted a 2024 report by the Association of Certified Fraud Examiners, which found that the median fraud loss in healthcare, on the basis of 117 cases, is $100,000. The most common form of fraud in the industry is corruption (47%), followed by billing (38%), noncash theft such as inventory (22%), and expense reimbursement (21%).
The details of the current case suggest that “SUNY Downstate had weak or insufficient internal controls to prevent this type of fraud,” Salisbury University’s Mr. Weber said. “However, research also makes clear that the tenure and position of the perpetrator play a significant role in the size of the fraud. Internal controls are supposed to apply to all employees, but the higher in the organization the perpetrator is, the easier it can be to engage in fraud.”
Even Small Medical Offices Can Act to Prevent Fraud
What can be done to prevent this kind of fraud? “Each employee should be required to submit actual receipts or scanned copies, and the reimbursement requests should be reviewed and inputted by a separate department or office of the organization to ensure that the expenses are legitimate,” Mr. Weber said. “In addition, all credit card statements should be available for review by the organization either simultaneously with the bill going to the employee or available for audit or review at any time without notification to the employee. Expenses that are in certain categories should be prohibited automatically and coded to the card so such a charge is rejected by the credit card bank.”
Smaller businesses — like many medical practices — may not have the manpower to handle these roles. In that case, Mr. Weber said, “The key is segregation or separation of duties. The bookkeeper cannot be the person receiving the bank statements, the payments from patients, and the invoices from vendors. There needs to be at least one other person in the loop to have some level of control.”
One strategy, he said, “is that the practice should institute a policy that only the doctor or owner of the practice can receive the mail, not the bookkeeper. Even if the practice leader does not actually review the bank statements, simply opening them before handing them off to the bookkeeper can provide a level of deterrence [since] the employee may get caught if someone else is reviewing the bank statements.”
A version of this article first appeared on Medscape.com.
In a case that spotlights the importance of comprehensive financial controls in medical offices,
Michael Lucchesi, MD, who had served as chairman of Emergency Medicine at SUNY Downstate Medical Center in New York City, was arraigned on July 9 and pleaded not guilty. Dr. Lucchesi’s attorney, Earl Ward, did not respond to messages from this news organization, but he told the New York Post that “the funds he used were not stolen funds.”
Dr. Lucchesi, who’s in his late 60s, faces nine counts of first- and second-degree grand larceny, first-degree falsifying business records, and third-degree criminal tax fraud. According to a press statement from the district attorney of Kings County, which encompasses the borough of Brooklyn, Dr. Lucchesi is accused of using his clinical practice’s business card for cash advances (about $115,000), high-end pet care ($176,000), personal travel ($348,000), gym membership and personal training ($109,000), catering ($52,000), tuition payments for his children ($46,000), and other expenses such as online shopping, flowers, liquor, and electronics.
Most of the alleged pet care spending — $120,000 — went to the Green Leaf Pet Resort, which has two locations in New Jersey, including one with “56 acres of nature and lots of tail wagging.” Some of the alleged spending on gym membership was at the New York Sports Clubs chain, where monthly membership tops out at $139.99.
The alleged spending occurred between 2016 and 2023 and was discovered by SUNY Downstate during an audit. Dr. Lucchesi reportedly left his position at the hospital, where he made $399,712 in 2022 as a professor, according to public records.
“As a high-ranking doctor at this vital healthcare institution, this defendant was entrusted with access to significant funds, which he allegedly exploited, stealing more than 1 million dollars to pay for a lavish lifestyle,” District Attorney Eric Gonzalez said in a statement.
SUNY Downstate is in a fight for its life amid efforts by New York Governor Kathy Hochul to shut it down. According to The New York Times, it is the only state-run hospital in New York City.
Dr. Lucchesi, who had previously served as the hospital’s chief medical officer and acting head, was released without bail. His next court date is September 25, 2024.
Size of Alleged Theft Is ‘Very Unusual’
David P. Weber, JD, DBA, a professor and fraud specialist at Salisbury University, Salisbury, Maryland, told this news organization that the fraudulent use of a business or purchase credit card is a form of embezzlement and “one of the most frequently seen types of frauds against organizations.”
William J. Kresse, JD, MSA, CPA/CFF, who studies fraud at Governors State University in University Park, Illinois, noted in an interview with this news organization that the high amount of alleged fraud in this case is “very unusual,” as is the period it is said to have occurred (over 6 years).
Mr. Kresse highlighted a 2024 report by the Association of Certified Fraud Examiners, which found that the median fraud loss in healthcare, on the basis of 117 cases, is $100,000. The most common form of fraud in the industry is corruption (47%), followed by billing (38%), noncash theft such as inventory (22%), and expense reimbursement (21%).
The details of the current case suggest that “SUNY Downstate had weak or insufficient internal controls to prevent this type of fraud,” Salisbury University’s Mr. Weber said. “However, research also makes clear that the tenure and position of the perpetrator play a significant role in the size of the fraud. Internal controls are supposed to apply to all employees, but the higher in the organization the perpetrator is, the easier it can be to engage in fraud.”
Even Small Medical Offices Can Act to Prevent Fraud
What can be done to prevent this kind of fraud? “Each employee should be required to submit actual receipts or scanned copies, and the reimbursement requests should be reviewed and inputted by a separate department or office of the organization to ensure that the expenses are legitimate,” Mr. Weber said. “In addition, all credit card statements should be available for review by the organization either simultaneously with the bill going to the employee or available for audit or review at any time without notification to the employee. Expenses that are in certain categories should be prohibited automatically and coded to the card so such a charge is rejected by the credit card bank.”
Smaller businesses — like many medical practices — may not have the manpower to handle these roles. In that case, Mr. Weber said, “The key is segregation or separation of duties. The bookkeeper cannot be the person receiving the bank statements, the payments from patients, and the invoices from vendors. There needs to be at least one other person in the loop to have some level of control.”
One strategy, he said, “is that the practice should institute a policy that only the doctor or owner of the practice can receive the mail, not the bookkeeper. Even if the practice leader does not actually review the bank statements, simply opening them before handing them off to the bookkeeper can provide a level of deterrence [since] the employee may get caught if someone else is reviewing the bank statements.”
A version of this article first appeared on Medscape.com.
Fruits and Vegetables May Promote Kidney and Cardiovascular Health in Hypertensive Patients
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF MEDICINE
Wearables May Confirm Sleep Disruption Impact on Chronic Disease
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Ozempic Curbs Hunger – And Not Just for Food
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 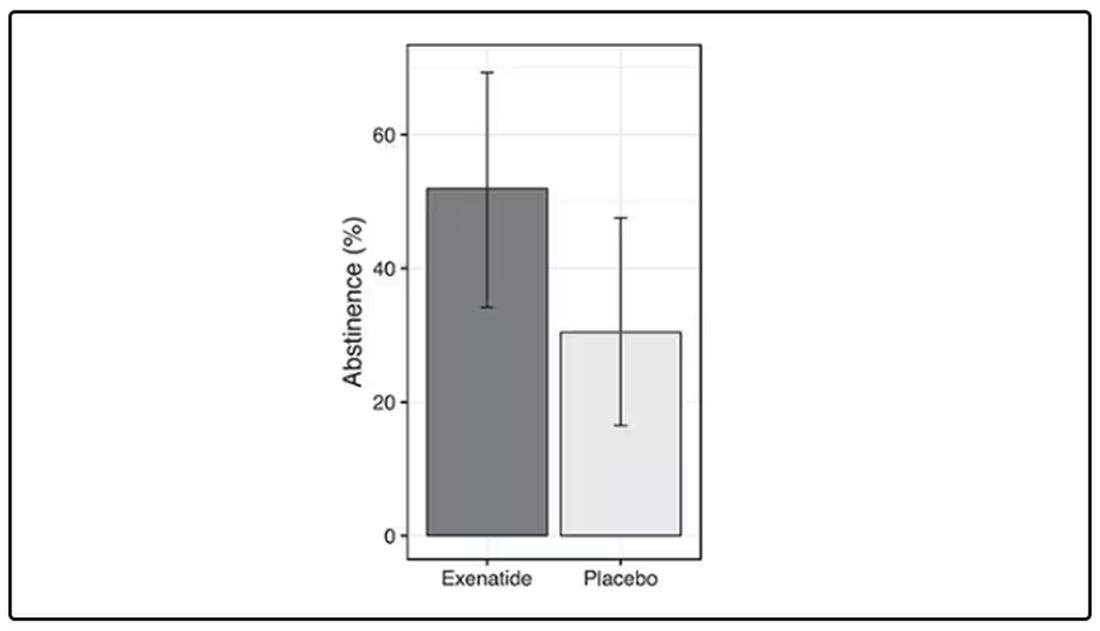
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.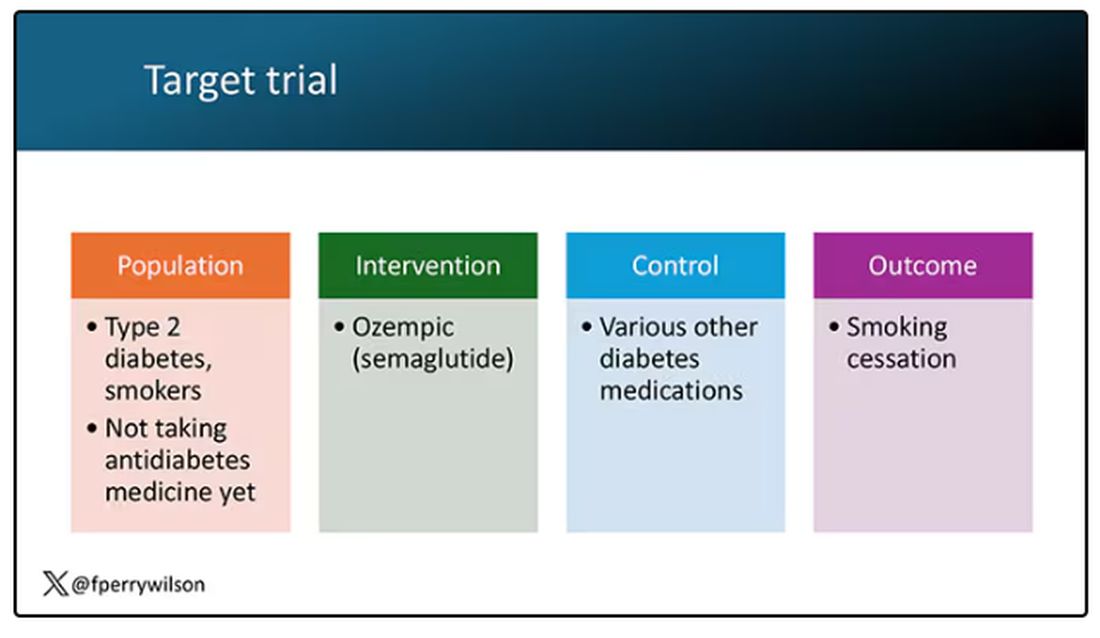
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.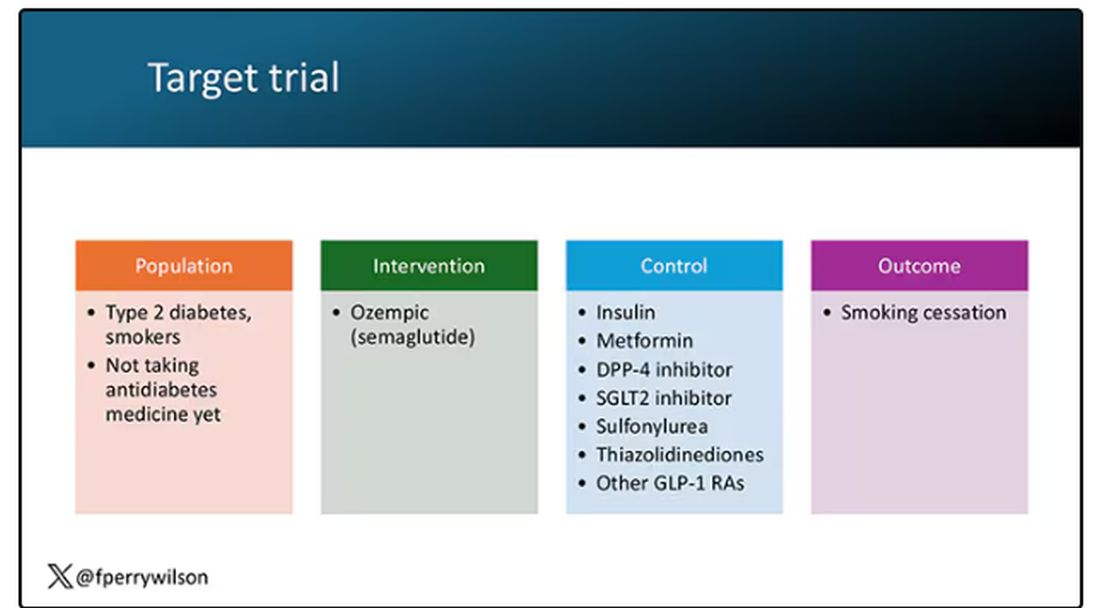
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 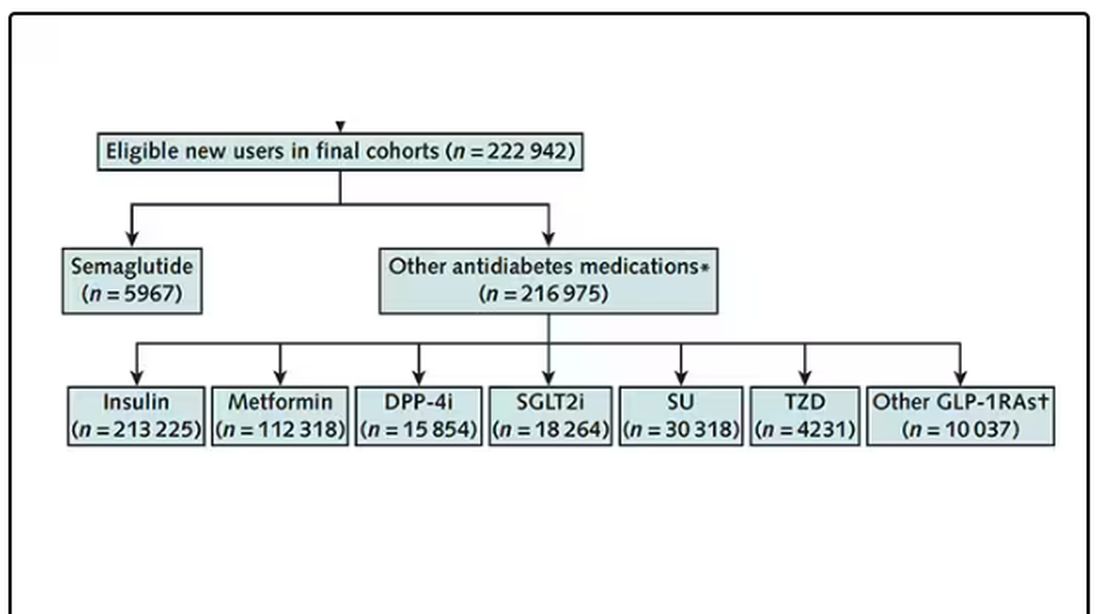
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
If you’ve been paying attention only to the headlines, when you think of “Ozempic” you’ll think of a few things: a blockbuster weight loss drug or the tip of the spear of a completely new industry — why not? A drug so popular that the people it was invented for (those with diabetes) can’t even get it.
Ozempic and other GLP-1 receptor agonists are undeniable game changers. Insofar as obesity is the number-one public health risk in the United States, antiobesity drugs hold immense promise even if all they do is reduce obesity.
In 2023, an article in Scientific Reports presented data suggesting that people on Ozempic might be reducing their alcohol intake, not just their total calories.
A 2024 article in Molecular Psychiatry found that the drug might positively impact cannabis use disorder. An article from Brain Sciences suggests that the drug reduces compulsive shopping.
A picture is starting to form, a picture that suggests these drugs curb hunger both literally and figuratively. That GLP-1 receptor agonists like Ozempic and Mounjaro are fundamentally anticonsumption drugs. In a society that — some would argue — is plagued by overconsumption, these drugs might be just what the doctor ordered.
If only they could stop people from smoking.
Oh, wait — they can.
At least it seems they can, based on a new study appearing in Annals of Internal Medicine.
Before we get too excited, this is not a randomized trial. There actually was a small randomized trial of exenatide (Byetta), which is in the same class as Ozempic but probably a bit less potent, with promising results for smoking cessation. 
But Byetta is the weaker drug in this class; the market leader is Ozempic. So how can you figure out whether Ozempic can reduce smoking without doing a huge and expensive randomized trial? You can do what Nora Volkow and colleagues from the National Institute on Drug Abuse did: a target trial emulation study.
A target trial emulation study is more or less what it sounds like. First, you decide what your dream randomized controlled trial would be and you plan it all out in great detail. You define the population you would recruit, with all the relevant inclusion and exclusion criteria. You define the intervention and the control, and you define the outcome.
But you don’t actually do the trial. You could if someone would lend you $10-$50 million, but assuming you don’t have that lying around, you do the next best thing, which is to dig into a medical record database to find all the people who would be eligible for your imaginary trial. And you analyze them.
The authors wanted to study the effect of Ozempic on smoking among people with diabetes; that’s why all the comparator agents are antidiabetes drugs. They figured out whether these folks were smoking on the basis of a medical record diagnosis of tobacco use disorder before they started one of the drugs of interest. This code is fairly specific: If a patient has it, you can be pretty sure they are smoking. But it’s not very sensitive; not every smoker has this diagnostic code. This is an age-old limitation of using EHR data instead of asking patients, but it’s part of the tradeoff for not having to spend $50 million.
After applying all those inclusion and exclusion criteria, they have a defined population who could be in their dream trial. And, as luck would have it, some of those people really were treated with Ozempic and some really were treated with those other agents. Although decisions about what to prescribe were not randomized, the authors account for this confounding-by-indication using propensity-score matching. You can find a little explainer on propensity-score matching in an earlier column here. 
It’s easy enough, using the EHR, to figure out who has diabetes and who got which drug. But how do you know who quit smoking? Remember, everyone had a diagnosis code for tobacco use disorder prior to starting Ozempic or a comparator drug. The authors decided that if the patient had a medical visit where someone again coded tobacco-use disorder, they were still smoking. If someone prescribed smoking cessation meds like a nicotine patch or varenicline, they were obviously still smoking. If someone billed for tobacco-cessation counseling, the patient is still smoking. We’ll get back to the implications of this outcome definition in a minute.
Let’s talk about the results, which are pretty intriguing. 
When Ozempic is compared with insulin among smokers with diabetes, those on Ozempic were about 30% more likely to quit smoking. They were about 18% more likely to quit smoking than those who took metformin. They were even slightly more likely to quit smoking than those on other GLP-1 receptor antagonists, though I should note that Mounjaro, which is probably the more potent GLP-1 drug in terms of weight loss, was not among the comparators.
This is pretty impressive for a drug that was not designed to be a smoking cessation drug. It speaks to this emerging idea that these drugs do more than curb appetite by slowing down gastric emptying or something. They work in the brain, modulating some of the reward circuitry that keeps us locked into our bad habits.
There are, of course, some caveats. As I pointed out, this study captured the idea of “still smoking” through the use of administrative codes in the EHR and prescription of smoking cessation aids. You could see similar results if taking Ozempic makes people less likely to address their smoking at all; maybe they shut down the doctor before they even talk about it, or there is too much to discuss during these visits to even get to the subject of smoking. You could also see results like this if people taking Ozempic had fewer visits overall, but the authors showed that that, at least, was not the case.
I’m inclined to believe that this effect is real, simply because we keep seeing signals from multiple sources. If that turns out to be the case, these new “weight loss” drugs may prove to be much more than that; they may turn out to be the drugs that can finally save us from ourselves.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vasculopathy Can Vary in Patients With Idiopathic Pulmonary Arterial Hypertension
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Approximately half of adults with idiopathic pulmonary arterial hypertension (IPAH) had nonplexiform vasculopathy characterized in part by severe pulmonary microvascular remodeling, based on data from 50 individuals.
The clinical phenotype of IPAH was historically described as a rapidly progressive rare disease in young women and characterized by plexiform lesions, wrote Esther J. Nossent, MD, of Amsterdam University Medical Centers, Amsterdam, the Netherlands, and colleagues. However, the patient population with IPAH has become older and predominantly men, and the nature of vascular phenotypes and histologic patterns in patients with contemporary IPAH has not been well studied, the researchers said.
In a cross-sectional study published in CHEST, the researchers reviewed lung histology data from 50 adults with IPAH that had been assessed by two experienced pathologists. The mean age of the patients was 52 years and 58% were women. Based on a histopathologic evaluation, 24 patients had nonplexiform vasculopathy (48%) and 26 had plexiform vasculopathy (52%). Notably, microvascular remodeling involving arterioles and venules was substantial in patients with nonplexiform vasculopathy but mild or absent in those with plexiform vasculopathy, the researchers wrote.
The researchers also compared the clinical characteristics of patients with plexiform vs nonplexiform vasculopathy. Hemodynamic parameters were similar in both patient groups. However, those with nonplexiform vasculopathy were significantly older than those with plexiform vasculopathy (60 years vs 44 years), were more likely to be men (67% vs 20%), and had a lower diffusing capacity of the lungs for carbon monoxide (DLCO) at diagnosis (all P < .001). Patients with nonplexiform vasculopathy also were significantly more likely than those with plexiform vasculopathy to have a history of smoking (P = .03). Genetic testing revealed no mutations in established PAH genes in the nonplexiform group.
Low DLCO has been associated with worse outcomes regardless of hemodynamic response, the researchers noted. In the current study, “a DLCO of < 45% almost perfectly identified patients with nonplexiform vasculopathy with prominent pulmonary microvascular disease,” they said.
The findings were limited by several factors, including the small study population and the higher frequency of surgical lung biopsies in the nonplexiform group vs the plexiform group, which is not part of the general workup of patients with IPAH, the researchers noted.
, they said. However, the results suggest that differences between patients with IPAH with plexiform vasculopathy and those with nonplexiform vasculopathy could ultimately inform targeted treatment strategies.
“Recognizing these clinical phenotypes allows revisiting current datasets to understand better the potential future clinical consequences of the vascular phenotypes for treatment response and clinical outcome,” the researchers concluded.
Findings May Inform More Targeted Therapy
“Any investigation that adds substantive insight into a complex disease that can translate into a better understanding of clinical patient phenotypes and eventually into improved treatments and patient outcomes has relevance at any time,” Paul Forfia, MD, professor of medicine at the Lewis Katz School of Medicine at Temple University, Philadelphia, said in an interview.
“There is focus on the antiproliferative forms of pulmonary arterial hypertension–specific therapy, and the results of the current study may have implications to these therapies,” said Dr. Forfia, who was not involved in the current study.
“In the current study, the investigators show that 48% of patients that were traditionally categorized as IPAH had a vascular phenotype that is not considered ‘typical’ or classic for IPAH,” Dr. Forfia told this news organization. “These findings highlight a significant heterogeneity of the pulmonary vascular phenotype within IPAH, which raises the question of whether the nonplexiform patient would be less responsive to the novel, antiproliferative forms of therapy,” he said.
The new findings are quite interesting but not surprising, Dr. Forfia said. “The World Symposia diagnostic groupings for pulmonary hypertension are a very important and necessary form of categorization and differentiation amongst forms of PH [pulmonary hypertension], and these groupings make a best attempt based on available evidence to separate patients of varying PH pathophysiology, both in terms of diagnosis and in how PH patients are treated,” he explained.
“However, clinical experts in PH have known that subphenotypes of PH pathophysiology exist within group I PAH, as well as in PH related to left heart disease (group 2), chronic respiratory disease (group 3), and chronic thromboembolic disease (group 4),” he said.
Findings from the current study reinforce the importance of clinical and physiological phenotyping of each patient, which can help in terms of therapy selection and in managing expectations in response to therapy, Dr. Forfia added.
“Perhaps the most evident and important clinical implication from the current study is to remind clinicians treating patients with PH that heterogeneity exists within the vascular phenotype and clinical makeup of patients even within the same type of PAH,” Dr. Forfia said. “With this insight, clinicians are more informed and thus more apt to consider nuances in the diagnosis, treatment, and expectations for treatment response within PAH,” he said.
Dr. Forfia also highlighted the potential implications of the association between cigarette smoking and the nonplexiform vascular phenotype. “This association was present in the absence of radiographic evidence of emphysema and raises the provocative notion that cigarette smoking may lead to pulmonary vascular abnormalities, perhaps even PAH, in patients without a diagnosis of emphysema,” he said.
“An important limitation from the current study is that the vascular phenotypes observed within their cohort of IPAH patients were obtained from histopathology specimens at the time of autopsy, explant at the time of lung transplantation, and surgical lung biopsy spanning over a 22-year period,” Dr. Forfia noted. Additional research is needed to explore how vascular phenotypic differences can be appreciated in the absence of histopathology and how these differences could impact therapy selection and patient outcomes, he said.
The study received no outside funding. Dr. Nossent disclosed receiving speaker fees from Janssen, MSD, and United Therapeutics/Ferrer and consulting fees from Janssen and United Therapeutics/Ferrer. Dr. Forfia had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Insurers’ Rules and AI for Preauthorization: ‘Ethically Nuts,’ Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
New Study Says Your Sedentary Lifestyle Is Killing You
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.