User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
What Would ‘Project 2025’ Mean for Health and Healthcare?
The Heritage Foundation sponsored and developed Project 2025 for the explicit, stated purpose of building a conservative victory through policy, personnel, and training with a 180-day game plan after a sympathetic new President of the United States takes office. To date, Project 2025 has not been formally endorsed by any presidential campaign.
Chapter 14 of the “Mandate for Leadership” is an exhaustive proposed overhaul of the Department of Health and Human Services (HHS), one of the major existing arms of the executive branch of the US government.
The mandate’s sweeping recommendations, if implemented, would impact the lives of all Americans and all healthcare workers, as outlined in the following excerpts.
Healthcare-Related Excerpts From Project 2025
- “From the moment of conception, every human being possesses inherent dignity and worth, and our humanity does not depend on our age, stage of development, race, or abilities. The Secretary must ensure that all HHS programs and activities are rooted in a deep respect for innocent human life from day one until natural death: Abortion and euthanasia are not health care.”
- “Unfortunately, family policies and programs under President Biden’s HHS are fraught with agenda items focusing on ‘LGBTQ+ equity,’ subsidizing single motherhood, disincentivizing work, and penalizing marriage. These policies should be repealed and replaced by policies that support the formation of stable, married, nuclear families.”
- “The next Administration should guard against the regulatory capture of our public health agencies by pharmaceutical companies, insurers, hospital conglomerates, and related economic interests that these agencies are meant to regulate. We must erect robust firewalls to mitigate these obvious financial conflicts of interest.”
- “All National Institutes of Health, Centers for Disease Control and Prevention, and Food and Drug Administration regulators should be entirely free from private biopharmaceutical funding. In this realm, ‘public–private partnerships’ is a euphemism for agency capture, a thin veneer for corporatism. Funding for agencies and individual government researchers must come directly from the government with robust congressional oversight.”
- “The CDC [Centers for Disease Control and Prevention] operates several programs related to vaccine safety including the Vaccine Adverse Event Reporting System (VAERS); Vaccine Safety Datalink (VSD); and Clinical Immunization Safety Assessment (CISA) Project. Those functions and their associated funding should be transferred to the FDA [Food and Drug Administration], which is responsible for post-market surveillance and evaluation of all other drugs and biological products.”
- “Because liberal states have now become sanctuaries for abortion tourism, HHS should use every available tool, including the cutting of funds, to ensure that every state reports exactly how many abortions take place within its borders, at what gestational age of the child, for what reason, the mother’s state of residence, and by what method. It should also ensure that statistics are separated by category: spontaneous miscarriage; treatments that incidentally result in the death of a child (such as chemotherapy); stillbirths; and induced abortion. In addition, CDC should require monitoring and reporting for complications due to abortion and every instance of children being born alive after an abortion.”
- “The CDC should immediately end its collection of data on gender identity, which legitimizes the unscientific notion that men can become women (and vice versa) and encourages the phenomenon of ever-multiplying subjective identities.”
- “A test developed by a lab in accordance with the protocols developed by another lab (non-commercial sharing) currently constitutes a ‘new’ laboratory-developed test because the lab in which it will be used is different from the initial developing lab. To encourage interlaboratory collaboration and discourage duplicative test creation (and associated regulatory and logistical burdens), the FDA should introduce mechanisms through which laboratory-developed tests can easily be shared with other laboratories without the current regulatory burdens.”
- “[FDA should] Reverse its approval of chemical abortion drugs because the politicized approval process was illegal from the start. The FDA failed to abide by its legal obligations to protect the health, safety, and welfare of girls and women.”
- “[FDA should] Stop promoting or approving mail-order abortions in violation of long-standing federal laws that prohibit the mailing and interstate carriage of abortion drugs.”
- “[HHS should] Promptly restore the ethics advisory committee to oversee abortion-derived fetal tissue research, and Congress should prohibit such research altogether.”
- “[HHS should] End intramural research projects using tissue from aborted children within the NIH, which should end its human embryonic stem cell registry.”
- “Under Francis Collins, NIH became so focused on the #MeToo movement that it refused to sponsor scientific conferences unless there were a certain number of women panelists, which violates federal civil rights law against sex discrimination. This quota practice should be ended, and the NIH Office of Equity, Diversity, and Inclusion, which pushes such unlawful actions, should be abolished.”
- “Make Medicare Advantage [MA] the default enrollment option.”
- “[Legislation reforming legacy (non-MA) Medicare should] Repeal harmful health policies enacted under the Obama and Biden Administrations such as the Medicare Shared Savings Program and Inflation Reduction Act.”
- “…the next Administration should] Add work requirements and match Medicaid benefits to beneficiary needs. Because Medicaid serves a broad and diverse group of individuals, it should be flexible enough to accommodate different designs for different groups.”
- “The No Surprises Act should scrap the dispute resolution process in favor of a truth-in-advertising approach that will protect consumers and free doctors, insurers, and arbiters from confused and conflicting standards for resolving disputes that the disputing parties can best resolve themselves.”
- “Prohibit abortion travel funding. Providing funding for abortions increases the number of abortions and violates the conscience and religious freedom rights of Americans who object to subsidizing the taking of life.”
- “Prohibit Planned Parenthood from receiving Medicaid funds. During the 2020–2021 reporting period, Planned Parenthood performed more than 383,000 abortions.”
- “Protect faith-based grant recipients from religious liberty violations and maintain a biblically based, social science–reinforced definition of marriage and family. Social science reports that assess the objective outcomes for children raised in homes aside from a heterosexual, intact marriage are clear.”
- “Allocate funding to strategy programs promoting father involvement or terminate parental rights quickly.”
- “Eliminate the Head Start program.”
- “Support palliative care. Physician-assisted suicide (PAS) is legal in 10 states and the District of Columbia. Legalizing PAS is a grave mistake that endangers the weak and vulnerable, corrupts the practice of medicine and the doctor–patient relationship, compromises the family and intergenerational commitments, and betrays human dignity and equality before the law.”
- “Eliminate men’s preventive services from the women’s preventive services mandate. In December 2021, HRSA [Health Resources and Services Administration] updated its women’s preventive services guidelines to include male condoms.”
- “Prioritize funding for home-based childcare, not universal day care.”
- “ The Office of the Secretary should eliminate the HHS Reproductive Healthcare Access Task Force and install a pro-life task force to ensure that all of the department’s divisions seek to use their authority to promote the life and health of women and their unborn children.”
- “The ASH [Assistant Secretary for Health] and SG [Surgeon General] positions should be combined into one four-star position with the rank, responsibilities, and authority of the ASH retained but with the title of Surgeon General.”
- “OCR [Office for Civil Rights] should withdraw its Health Insurance Portability and Accountability Act (HIPAA) guidance on abortion.”
Dr. Lundberg is Editor in Chief, Cancer Commons, and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Heritage Foundation sponsored and developed Project 2025 for the explicit, stated purpose of building a conservative victory through policy, personnel, and training with a 180-day game plan after a sympathetic new President of the United States takes office. To date, Project 2025 has not been formally endorsed by any presidential campaign.
Chapter 14 of the “Mandate for Leadership” is an exhaustive proposed overhaul of the Department of Health and Human Services (HHS), one of the major existing arms of the executive branch of the US government.
The mandate’s sweeping recommendations, if implemented, would impact the lives of all Americans and all healthcare workers, as outlined in the following excerpts.
Healthcare-Related Excerpts From Project 2025
- “From the moment of conception, every human being possesses inherent dignity and worth, and our humanity does not depend on our age, stage of development, race, or abilities. The Secretary must ensure that all HHS programs and activities are rooted in a deep respect for innocent human life from day one until natural death: Abortion and euthanasia are not health care.”
- “Unfortunately, family policies and programs under President Biden’s HHS are fraught with agenda items focusing on ‘LGBTQ+ equity,’ subsidizing single motherhood, disincentivizing work, and penalizing marriage. These policies should be repealed and replaced by policies that support the formation of stable, married, nuclear families.”
- “The next Administration should guard against the regulatory capture of our public health agencies by pharmaceutical companies, insurers, hospital conglomerates, and related economic interests that these agencies are meant to regulate. We must erect robust firewalls to mitigate these obvious financial conflicts of interest.”
- “All National Institutes of Health, Centers for Disease Control and Prevention, and Food and Drug Administration regulators should be entirely free from private biopharmaceutical funding. In this realm, ‘public–private partnerships’ is a euphemism for agency capture, a thin veneer for corporatism. Funding for agencies and individual government researchers must come directly from the government with robust congressional oversight.”
- “The CDC [Centers for Disease Control and Prevention] operates several programs related to vaccine safety including the Vaccine Adverse Event Reporting System (VAERS); Vaccine Safety Datalink (VSD); and Clinical Immunization Safety Assessment (CISA) Project. Those functions and their associated funding should be transferred to the FDA [Food and Drug Administration], which is responsible for post-market surveillance and evaluation of all other drugs and biological products.”
- “Because liberal states have now become sanctuaries for abortion tourism, HHS should use every available tool, including the cutting of funds, to ensure that every state reports exactly how many abortions take place within its borders, at what gestational age of the child, for what reason, the mother’s state of residence, and by what method. It should also ensure that statistics are separated by category: spontaneous miscarriage; treatments that incidentally result in the death of a child (such as chemotherapy); stillbirths; and induced abortion. In addition, CDC should require monitoring and reporting for complications due to abortion and every instance of children being born alive after an abortion.”
- “The CDC should immediately end its collection of data on gender identity, which legitimizes the unscientific notion that men can become women (and vice versa) and encourages the phenomenon of ever-multiplying subjective identities.”
- “A test developed by a lab in accordance with the protocols developed by another lab (non-commercial sharing) currently constitutes a ‘new’ laboratory-developed test because the lab in which it will be used is different from the initial developing lab. To encourage interlaboratory collaboration and discourage duplicative test creation (and associated regulatory and logistical burdens), the FDA should introduce mechanisms through which laboratory-developed tests can easily be shared with other laboratories without the current regulatory burdens.”
- “[FDA should] Reverse its approval of chemical abortion drugs because the politicized approval process was illegal from the start. The FDA failed to abide by its legal obligations to protect the health, safety, and welfare of girls and women.”
- “[FDA should] Stop promoting or approving mail-order abortions in violation of long-standing federal laws that prohibit the mailing and interstate carriage of abortion drugs.”
- “[HHS should] Promptly restore the ethics advisory committee to oversee abortion-derived fetal tissue research, and Congress should prohibit such research altogether.”
- “[HHS should] End intramural research projects using tissue from aborted children within the NIH, which should end its human embryonic stem cell registry.”
- “Under Francis Collins, NIH became so focused on the #MeToo movement that it refused to sponsor scientific conferences unless there were a certain number of women panelists, which violates federal civil rights law against sex discrimination. This quota practice should be ended, and the NIH Office of Equity, Diversity, and Inclusion, which pushes such unlawful actions, should be abolished.”
- “Make Medicare Advantage [MA] the default enrollment option.”
- “[Legislation reforming legacy (non-MA) Medicare should] Repeal harmful health policies enacted under the Obama and Biden Administrations such as the Medicare Shared Savings Program and Inflation Reduction Act.”
- “…the next Administration should] Add work requirements and match Medicaid benefits to beneficiary needs. Because Medicaid serves a broad and diverse group of individuals, it should be flexible enough to accommodate different designs for different groups.”
- “The No Surprises Act should scrap the dispute resolution process in favor of a truth-in-advertising approach that will protect consumers and free doctors, insurers, and arbiters from confused and conflicting standards for resolving disputes that the disputing parties can best resolve themselves.”
- “Prohibit abortion travel funding. Providing funding for abortions increases the number of abortions and violates the conscience and religious freedom rights of Americans who object to subsidizing the taking of life.”
- “Prohibit Planned Parenthood from receiving Medicaid funds. During the 2020–2021 reporting period, Planned Parenthood performed more than 383,000 abortions.”
- “Protect faith-based grant recipients from religious liberty violations and maintain a biblically based, social science–reinforced definition of marriage and family. Social science reports that assess the objective outcomes for children raised in homes aside from a heterosexual, intact marriage are clear.”
- “Allocate funding to strategy programs promoting father involvement or terminate parental rights quickly.”
- “Eliminate the Head Start program.”
- “Support palliative care. Physician-assisted suicide (PAS) is legal in 10 states and the District of Columbia. Legalizing PAS is a grave mistake that endangers the weak and vulnerable, corrupts the practice of medicine and the doctor–patient relationship, compromises the family and intergenerational commitments, and betrays human dignity and equality before the law.”
- “Eliminate men’s preventive services from the women’s preventive services mandate. In December 2021, HRSA [Health Resources and Services Administration] updated its women’s preventive services guidelines to include male condoms.”
- “Prioritize funding for home-based childcare, not universal day care.”
- “ The Office of the Secretary should eliminate the HHS Reproductive Healthcare Access Task Force and install a pro-life task force to ensure that all of the department’s divisions seek to use their authority to promote the life and health of women and their unborn children.”
- “The ASH [Assistant Secretary for Health] and SG [Surgeon General] positions should be combined into one four-star position with the rank, responsibilities, and authority of the ASH retained but with the title of Surgeon General.”
- “OCR [Office for Civil Rights] should withdraw its Health Insurance Portability and Accountability Act (HIPAA) guidance on abortion.”
Dr. Lundberg is Editor in Chief, Cancer Commons, and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Heritage Foundation sponsored and developed Project 2025 for the explicit, stated purpose of building a conservative victory through policy, personnel, and training with a 180-day game plan after a sympathetic new President of the United States takes office. To date, Project 2025 has not been formally endorsed by any presidential campaign.
Chapter 14 of the “Mandate for Leadership” is an exhaustive proposed overhaul of the Department of Health and Human Services (HHS), one of the major existing arms of the executive branch of the US government.
The mandate’s sweeping recommendations, if implemented, would impact the lives of all Americans and all healthcare workers, as outlined in the following excerpts.
Healthcare-Related Excerpts From Project 2025
- “From the moment of conception, every human being possesses inherent dignity and worth, and our humanity does not depend on our age, stage of development, race, or abilities. The Secretary must ensure that all HHS programs and activities are rooted in a deep respect for innocent human life from day one until natural death: Abortion and euthanasia are not health care.”
- “Unfortunately, family policies and programs under President Biden’s HHS are fraught with agenda items focusing on ‘LGBTQ+ equity,’ subsidizing single motherhood, disincentivizing work, and penalizing marriage. These policies should be repealed and replaced by policies that support the formation of stable, married, nuclear families.”
- “The next Administration should guard against the regulatory capture of our public health agencies by pharmaceutical companies, insurers, hospital conglomerates, and related economic interests that these agencies are meant to regulate. We must erect robust firewalls to mitigate these obvious financial conflicts of interest.”
- “All National Institutes of Health, Centers for Disease Control and Prevention, and Food and Drug Administration regulators should be entirely free from private biopharmaceutical funding. In this realm, ‘public–private partnerships’ is a euphemism for agency capture, a thin veneer for corporatism. Funding for agencies and individual government researchers must come directly from the government with robust congressional oversight.”
- “The CDC [Centers for Disease Control and Prevention] operates several programs related to vaccine safety including the Vaccine Adverse Event Reporting System (VAERS); Vaccine Safety Datalink (VSD); and Clinical Immunization Safety Assessment (CISA) Project. Those functions and their associated funding should be transferred to the FDA [Food and Drug Administration], which is responsible for post-market surveillance and evaluation of all other drugs and biological products.”
- “Because liberal states have now become sanctuaries for abortion tourism, HHS should use every available tool, including the cutting of funds, to ensure that every state reports exactly how many abortions take place within its borders, at what gestational age of the child, for what reason, the mother’s state of residence, and by what method. It should also ensure that statistics are separated by category: spontaneous miscarriage; treatments that incidentally result in the death of a child (such as chemotherapy); stillbirths; and induced abortion. In addition, CDC should require monitoring and reporting for complications due to abortion and every instance of children being born alive after an abortion.”
- “The CDC should immediately end its collection of data on gender identity, which legitimizes the unscientific notion that men can become women (and vice versa) and encourages the phenomenon of ever-multiplying subjective identities.”
- “A test developed by a lab in accordance with the protocols developed by another lab (non-commercial sharing) currently constitutes a ‘new’ laboratory-developed test because the lab in which it will be used is different from the initial developing lab. To encourage interlaboratory collaboration and discourage duplicative test creation (and associated regulatory and logistical burdens), the FDA should introduce mechanisms through which laboratory-developed tests can easily be shared with other laboratories without the current regulatory burdens.”
- “[FDA should] Reverse its approval of chemical abortion drugs because the politicized approval process was illegal from the start. The FDA failed to abide by its legal obligations to protect the health, safety, and welfare of girls and women.”
- “[FDA should] Stop promoting or approving mail-order abortions in violation of long-standing federal laws that prohibit the mailing and interstate carriage of abortion drugs.”
- “[HHS should] Promptly restore the ethics advisory committee to oversee abortion-derived fetal tissue research, and Congress should prohibit such research altogether.”
- “[HHS should] End intramural research projects using tissue from aborted children within the NIH, which should end its human embryonic stem cell registry.”
- “Under Francis Collins, NIH became so focused on the #MeToo movement that it refused to sponsor scientific conferences unless there were a certain number of women panelists, which violates federal civil rights law against sex discrimination. This quota practice should be ended, and the NIH Office of Equity, Diversity, and Inclusion, which pushes such unlawful actions, should be abolished.”
- “Make Medicare Advantage [MA] the default enrollment option.”
- “[Legislation reforming legacy (non-MA) Medicare should] Repeal harmful health policies enacted under the Obama and Biden Administrations such as the Medicare Shared Savings Program and Inflation Reduction Act.”
- “…the next Administration should] Add work requirements and match Medicaid benefits to beneficiary needs. Because Medicaid serves a broad and diverse group of individuals, it should be flexible enough to accommodate different designs for different groups.”
- “The No Surprises Act should scrap the dispute resolution process in favor of a truth-in-advertising approach that will protect consumers and free doctors, insurers, and arbiters from confused and conflicting standards for resolving disputes that the disputing parties can best resolve themselves.”
- “Prohibit abortion travel funding. Providing funding for abortions increases the number of abortions and violates the conscience and religious freedom rights of Americans who object to subsidizing the taking of life.”
- “Prohibit Planned Parenthood from receiving Medicaid funds. During the 2020–2021 reporting period, Planned Parenthood performed more than 383,000 abortions.”
- “Protect faith-based grant recipients from religious liberty violations and maintain a biblically based, social science–reinforced definition of marriage and family. Social science reports that assess the objective outcomes for children raised in homes aside from a heterosexual, intact marriage are clear.”
- “Allocate funding to strategy programs promoting father involvement or terminate parental rights quickly.”
- “Eliminate the Head Start program.”
- “Support palliative care. Physician-assisted suicide (PAS) is legal in 10 states and the District of Columbia. Legalizing PAS is a grave mistake that endangers the weak and vulnerable, corrupts the practice of medicine and the doctor–patient relationship, compromises the family and intergenerational commitments, and betrays human dignity and equality before the law.”
- “Eliminate men’s preventive services from the women’s preventive services mandate. In December 2021, HRSA [Health Resources and Services Administration] updated its women’s preventive services guidelines to include male condoms.”
- “Prioritize funding for home-based childcare, not universal day care.”
- “ The Office of the Secretary should eliminate the HHS Reproductive Healthcare Access Task Force and install a pro-life task force to ensure that all of the department’s divisions seek to use their authority to promote the life and health of women and their unborn children.”
- “The ASH [Assistant Secretary for Health] and SG [Surgeon General] positions should be combined into one four-star position with the rank, responsibilities, and authority of the ASH retained but with the title of Surgeon General.”
- “OCR [Office for Civil Rights] should withdraw its Health Insurance Portability and Accountability Act (HIPAA) guidance on abortion.”
Dr. Lundberg is Editor in Chief, Cancer Commons, and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Racing Heart Signals Trouble in Chronic Kidney Disease
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Remission or Not, Biologics May Mitigate Cardiovascular Risks of RA
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
These Four Factors Account for 18 Years of Life Expectancy
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
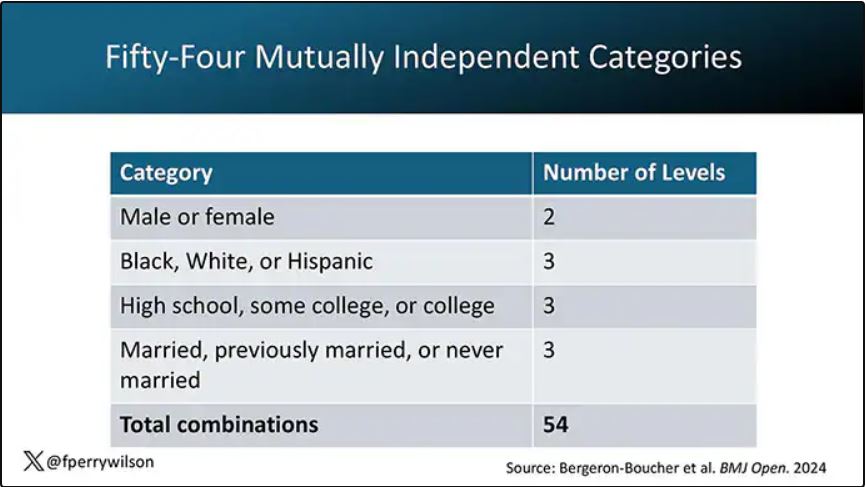
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
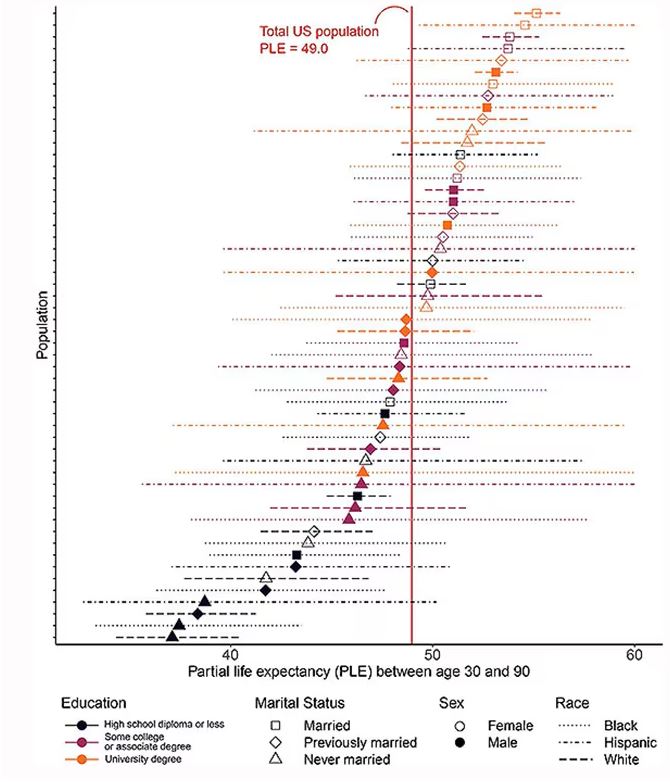
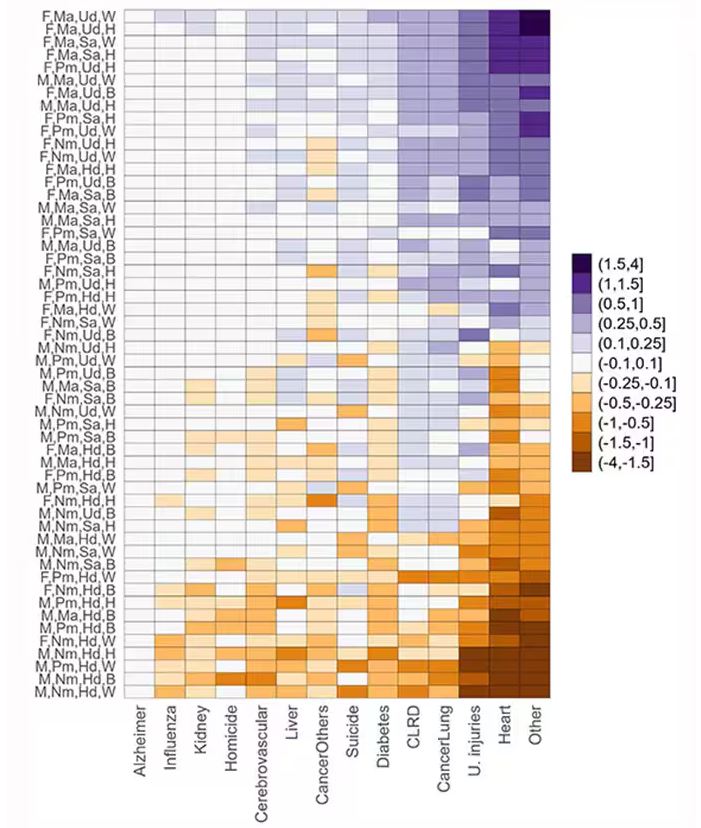
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
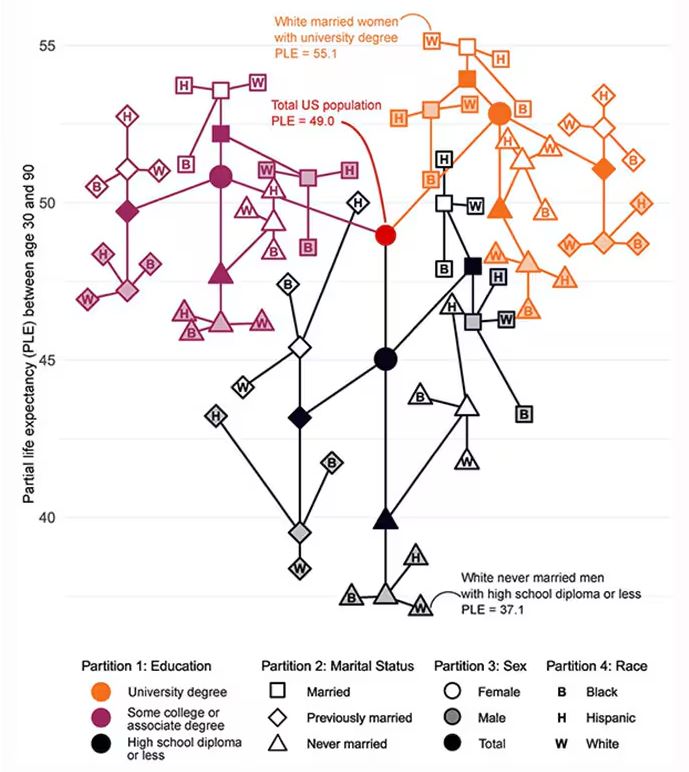
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
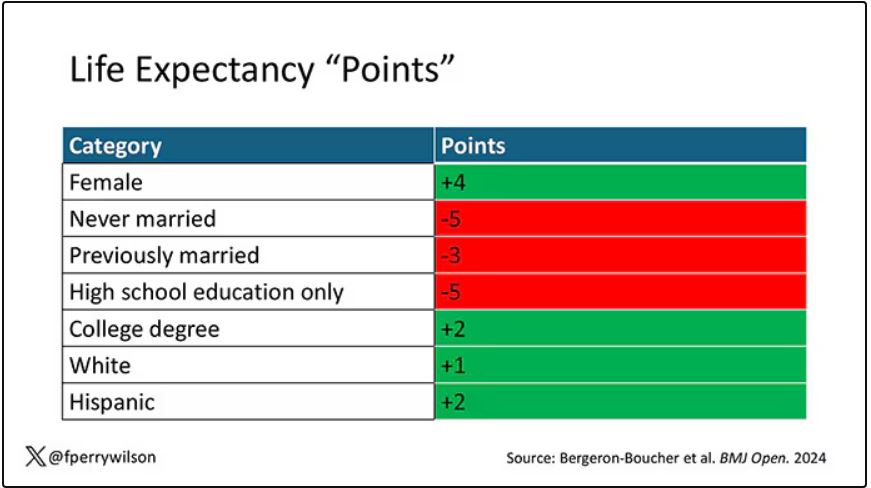
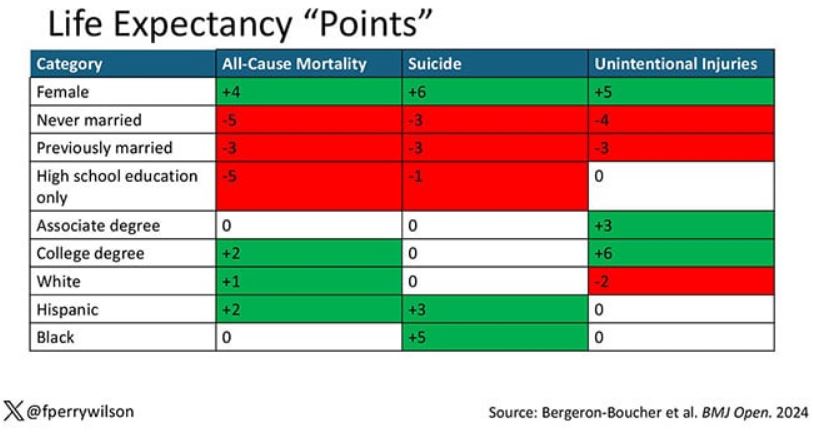
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
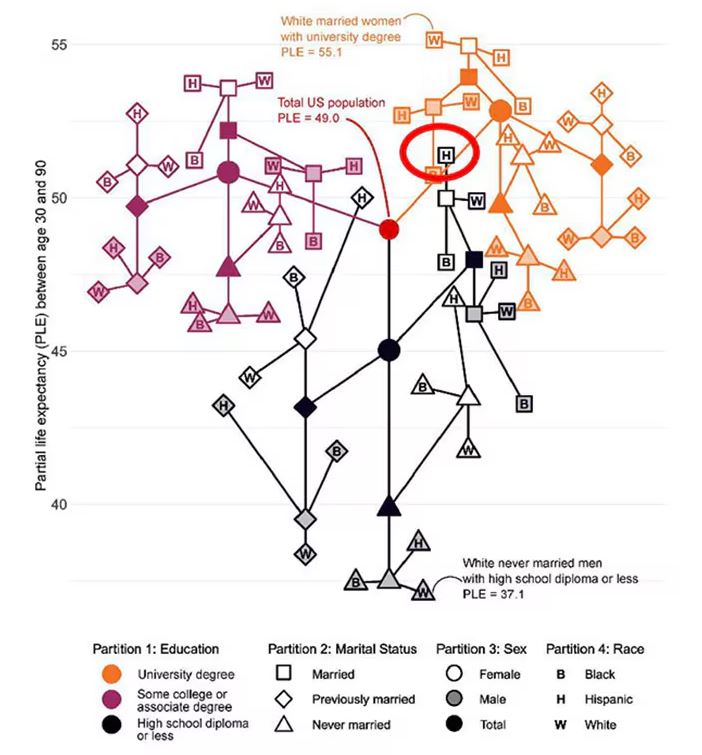
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Xanthelasma Not Linked to Heart Diseases, Study Finds
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New Study Links Sweetener to Heart Risk: What to Know
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Did Statin Decision-Making Just Get Harder?
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On Second Thought: The Truth About Beta-Blockers
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New Guidance on Genetic Testing for Kidney Disease
A new consensus statement recommended genetic testing for all categories of kidney diseases whenever a genetic cause is suspected and offered guidance on who to test, which tests are the most useful, and how to talk to patients about results.
The statement, published online in the American Journal of Kidney Diseases, is the work of four dozen authors — including patients, nephrologists, experts in clinical and laboratory genetics, kidney pathology, genetic counseling, and ethics. The experts were brought together by the National Kidney Foundation (NKF) with the goal of broadening use and understanding of the tests.
About 10% or more of kidney diseases in adults and 70% of selected chronic kidney diseases (CKDs) in children have genetic causes. But nephrologists have reported a lack of education about genetic testing, and other barriers to wider use, including limited access to testing, cost, insurance coverage, and a small number of genetic counselors who are versed in kidney genetics.
Genetic testing “in the kidney field is a little less developed than in other fields,” said co–lead author Nora Franceschini, MD, MPH, a professor of epidemiology at the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and a nephrologist who studies the genetic epidemiology of hypertension and kidney and cardiovascular diseases.
There are already many known variants that play a role in various kidney diseases and more are on the horizon, Dr. Franceschini told this news organization. More genetic tests will be available in the near future. “The workforce needs to be prepared,” she said.
The statement is an initial step that gets clinicians thinking about testing in a more systematic way, said Dr. Franceschini. “Genetic testing is just another test that physicians can use to complete the story when evaluating patients.
“I think clinicians are ready to implement” testing, said Dr. Franceschini. “We just need to have better guidance.”
Who, When, What to Test
The NKF statement is not the first to try to address gaps in use and knowledge. A European Renal Association Working Group published guidelines in 2022.
The NKF Working Group came up with 56 recommendations and separate algorithms to guide testing for adult and pediatric individuals who are considered at-risk (and currently asymptomatic) and for those who already have clinical disease.
Testing can help determine a cause if there’s an atypical clinical presentation, and it can help avoid biopsies, said the group. Tests can also guide choice of therapy.
For at-risk individuals, there are two broad situations in which testing might be considered: In family members of a patient who already has kidney disease and in potential kidney donors. But testing at-risk children younger than 18 years should only be done if there is an intervention available that could prevent, treat, or slow progression of disease, said the authors.
For patients with an established genetic diagnosis, at-risk family members should be tested with the known single-gene variant diagnostic instead of a broad panel, said the group.
Single-gene variant testing is most appropriate in situations when clinical disease is already evident or when there is known genetic disease in the family, according to the NKF panel. A large diagnostic panel that covers the many common genetic causes of kidney disease is recommended for the majority of patients.
The group recommended that apolipoprotein L1 (APOL1) testing should be included in gene panels for CKD, and it should be offered to any patient “with clinical findings suggestive of APOL1-association nephropathy, regardless of race and ethnicity.”
High-risk APOL1 genotypes confer a 5- to 10-fold increased risk for CKD and are found in one out of seven individuals of African ancestry, which means the focus has largely been on testing those with that ancestry.
However, with many unknowns about APOL1, the NKF panel did not want to “profile” individuals and suggest that testing should not be based on skin color or race/ethnicity, said Dr. Franceschini.
In addition, only about 10% of those with the variant develop disease, so testing is not currently warranted for those who do not already have kidney disease, said the group.
They also recommended against the use of polygenic risk scores, saying that there are not enough data from diverse populations in genome-wide association studies for kidney disease or on their clinical utility.
More Education Needed; Many Barriers
The authors acknowledged that nephrologists generally receive little education in genetics and lack support for interpreting and discussing results.
“Nephrologists should be provided with training and best practice resources to interpret genetic testing and discuss the results with individuals and their families,” they wrote, adding that there’s a need for genomic medicine boards at academic centers that would be available to help nephrologists interpret results and plot clinical management.
The group did not, however, cite some of the other barriers to adoption of testing, including a limited number of sites offering testing, cost, and lack of insurance coverage for the diagnostics.
Medicare may cover genetic testing for kidney disease when an individual has symptoms and there is a Food and Drug Administration–approved test. Joseph Vassalotti, MD, chief medical officer for the NKF, said private insurance may cover the testing if the nephrologist deems it medically necessary, but that he usually confirms coverage before initiating testing. The often-used Renasight panel, which tests for 385 genes related to kidney diseases, costs $300-$400 out of pocket, Dr. Vassalotti told this news organization.
In a survey of 149 nephrologists conducted in 2021, both users (46%) and nonusers of the tests (69%) said that high cost was the most significant perceived barrier to implementing widespread testing. A third of users and almost two thirds of nonusers said that poor availability or lack of ease of testing was the second most significant barrier.
Clinics that test for kidney genes “are largely confined to large academic centers and some specialty clinics,” said Dominic Raj, MD, the Bert B. Brooks chair, and Divya Shankaranarayanan, MD, director of the Kidney Precision Medicine Clinic, both at George Washington University School of Medicine & Health Sciences, Washington, DC, in an email.
Testing is also limited by cultural barriers, lack of genetic literacy, and patients’ concerns that a positive result could lead to a loss of health insurance coverage, said Dr. Raj and Dr. Shankaranarayanan.
Paper Will Help Expand Use
A lack of consensus has also held back expansion. The new statement “may lead to increased and possibly judicious utilization of genetic testing in nephrology practices,” said Dr. Raj and Dr. Shankaranarayanan. “Most importantly, the panel has given specific guidance as to what type of genetic test platform is likely to yield the best and most cost-effective yield.”
The most effective use is “in monogenic kidney diseases and to a lesser extent in oligogenic kidney disease,” said Dr. Raj and Dr. Shankaranarayanan, adding that testing is of less-certain utility in polygenic kidney diseases, “where complex genetic and epigenetic factors determine the phenotype.”
Genetic testing might be especially useful “in atypical clinical presentations” and can help clinicians avoid unnecessary expensive and extensive investigations when multiple organ systems are involved, they said.
“Most importantly, [testing] might prevent unnecessary and potentially harmful treatment and enable targeted specific treatment, when available,” said Dr. Raj and Dr. Shankaranarayanan.
Dr. Franceschini and Dr. Shankaranarayanan reported no relevant financial relationships. Dr. Raj disclosed that he received consulting fees and honoraria from Novo Nordisk and is a national leader for the company’s Zeus trial, studying whether ziltivekimab reduces the risk for cardiovascular events in cardiovascular disease, CKD, and inflammation. He also participated in a study of Natera’s Renasight, a 385-gene panel for kidney disease.
A version of this article first appeared on Medscape.com.
A new consensus statement recommended genetic testing for all categories of kidney diseases whenever a genetic cause is suspected and offered guidance on who to test, which tests are the most useful, and how to talk to patients about results.
The statement, published online in the American Journal of Kidney Diseases, is the work of four dozen authors — including patients, nephrologists, experts in clinical and laboratory genetics, kidney pathology, genetic counseling, and ethics. The experts were brought together by the National Kidney Foundation (NKF) with the goal of broadening use and understanding of the tests.
About 10% or more of kidney diseases in adults and 70% of selected chronic kidney diseases (CKDs) in children have genetic causes. But nephrologists have reported a lack of education about genetic testing, and other barriers to wider use, including limited access to testing, cost, insurance coverage, and a small number of genetic counselors who are versed in kidney genetics.
Genetic testing “in the kidney field is a little less developed than in other fields,” said co–lead author Nora Franceschini, MD, MPH, a professor of epidemiology at the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and a nephrologist who studies the genetic epidemiology of hypertension and kidney and cardiovascular diseases.
There are already many known variants that play a role in various kidney diseases and more are on the horizon, Dr. Franceschini told this news organization. More genetic tests will be available in the near future. “The workforce needs to be prepared,” she said.
The statement is an initial step that gets clinicians thinking about testing in a more systematic way, said Dr. Franceschini. “Genetic testing is just another test that physicians can use to complete the story when evaluating patients.
“I think clinicians are ready to implement” testing, said Dr. Franceschini. “We just need to have better guidance.”
Who, When, What to Test
The NKF statement is not the first to try to address gaps in use and knowledge. A European Renal Association Working Group published guidelines in 2022.
The NKF Working Group came up with 56 recommendations and separate algorithms to guide testing for adult and pediatric individuals who are considered at-risk (and currently asymptomatic) and for those who already have clinical disease.
Testing can help determine a cause if there’s an atypical clinical presentation, and it can help avoid biopsies, said the group. Tests can also guide choice of therapy.
For at-risk individuals, there are two broad situations in which testing might be considered: In family members of a patient who already has kidney disease and in potential kidney donors. But testing at-risk children younger than 18 years should only be done if there is an intervention available that could prevent, treat, or slow progression of disease, said the authors.
For patients with an established genetic diagnosis, at-risk family members should be tested with the known single-gene variant diagnostic instead of a broad panel, said the group.
Single-gene variant testing is most appropriate in situations when clinical disease is already evident or when there is known genetic disease in the family, according to the NKF panel. A large diagnostic panel that covers the many common genetic causes of kidney disease is recommended for the majority of patients.
The group recommended that apolipoprotein L1 (APOL1) testing should be included in gene panels for CKD, and it should be offered to any patient “with clinical findings suggestive of APOL1-association nephropathy, regardless of race and ethnicity.”
High-risk APOL1 genotypes confer a 5- to 10-fold increased risk for CKD and are found in one out of seven individuals of African ancestry, which means the focus has largely been on testing those with that ancestry.
However, with many unknowns about APOL1, the NKF panel did not want to “profile” individuals and suggest that testing should not be based on skin color or race/ethnicity, said Dr. Franceschini.
In addition, only about 10% of those with the variant develop disease, so testing is not currently warranted for those who do not already have kidney disease, said the group.
They also recommended against the use of polygenic risk scores, saying that there are not enough data from diverse populations in genome-wide association studies for kidney disease or on their clinical utility.
More Education Needed; Many Barriers
The authors acknowledged that nephrologists generally receive little education in genetics and lack support for interpreting and discussing results.
“Nephrologists should be provided with training and best practice resources to interpret genetic testing and discuss the results with individuals and their families,” they wrote, adding that there’s a need for genomic medicine boards at academic centers that would be available to help nephrologists interpret results and plot clinical management.
The group did not, however, cite some of the other barriers to adoption of testing, including a limited number of sites offering testing, cost, and lack of insurance coverage for the diagnostics.
Medicare may cover genetic testing for kidney disease when an individual has symptoms and there is a Food and Drug Administration–approved test. Joseph Vassalotti, MD, chief medical officer for the NKF, said private insurance may cover the testing if the nephrologist deems it medically necessary, but that he usually confirms coverage before initiating testing. The often-used Renasight panel, which tests for 385 genes related to kidney diseases, costs $300-$400 out of pocket, Dr. Vassalotti told this news organization.
In a survey of 149 nephrologists conducted in 2021, both users (46%) and nonusers of the tests (69%) said that high cost was the most significant perceived barrier to implementing widespread testing. A third of users and almost two thirds of nonusers said that poor availability or lack of ease of testing was the second most significant barrier.
Clinics that test for kidney genes “are largely confined to large academic centers and some specialty clinics,” said Dominic Raj, MD, the Bert B. Brooks chair, and Divya Shankaranarayanan, MD, director of the Kidney Precision Medicine Clinic, both at George Washington University School of Medicine & Health Sciences, Washington, DC, in an email.
Testing is also limited by cultural barriers, lack of genetic literacy, and patients’ concerns that a positive result could lead to a loss of health insurance coverage, said Dr. Raj and Dr. Shankaranarayanan.
Paper Will Help Expand Use
A lack of consensus has also held back expansion. The new statement “may lead to increased and possibly judicious utilization of genetic testing in nephrology practices,” said Dr. Raj and Dr. Shankaranarayanan. “Most importantly, the panel has given specific guidance as to what type of genetic test platform is likely to yield the best and most cost-effective yield.”
The most effective use is “in monogenic kidney diseases and to a lesser extent in oligogenic kidney disease,” said Dr. Raj and Dr. Shankaranarayanan, adding that testing is of less-certain utility in polygenic kidney diseases, “where complex genetic and epigenetic factors determine the phenotype.”
Genetic testing might be especially useful “in atypical clinical presentations” and can help clinicians avoid unnecessary expensive and extensive investigations when multiple organ systems are involved, they said.
“Most importantly, [testing] might prevent unnecessary and potentially harmful treatment and enable targeted specific treatment, when available,” said Dr. Raj and Dr. Shankaranarayanan.
Dr. Franceschini and Dr. Shankaranarayanan reported no relevant financial relationships. Dr. Raj disclosed that he received consulting fees and honoraria from Novo Nordisk and is a national leader for the company’s Zeus trial, studying whether ziltivekimab reduces the risk for cardiovascular events in cardiovascular disease, CKD, and inflammation. He also participated in a study of Natera’s Renasight, a 385-gene panel for kidney disease.
A version of this article first appeared on Medscape.com.
A new consensus statement recommended genetic testing for all categories of kidney diseases whenever a genetic cause is suspected and offered guidance on who to test, which tests are the most useful, and how to talk to patients about results.
The statement, published online in the American Journal of Kidney Diseases, is the work of four dozen authors — including patients, nephrologists, experts in clinical and laboratory genetics, kidney pathology, genetic counseling, and ethics. The experts were brought together by the National Kidney Foundation (NKF) with the goal of broadening use and understanding of the tests.
About 10% or more of kidney diseases in adults and 70% of selected chronic kidney diseases (CKDs) in children have genetic causes. But nephrologists have reported a lack of education about genetic testing, and other barriers to wider use, including limited access to testing, cost, insurance coverage, and a small number of genetic counselors who are versed in kidney genetics.
Genetic testing “in the kidney field is a little less developed than in other fields,” said co–lead author Nora Franceschini, MD, MPH, a professor of epidemiology at the University of North Carolina Gillings School of Global Public Health, Chapel Hill, and a nephrologist who studies the genetic epidemiology of hypertension and kidney and cardiovascular diseases.
There are already many known variants that play a role in various kidney diseases and more are on the horizon, Dr. Franceschini told this news organization. More genetic tests will be available in the near future. “The workforce needs to be prepared,” she said.
The statement is an initial step that gets clinicians thinking about testing in a more systematic way, said Dr. Franceschini. “Genetic testing is just another test that physicians can use to complete the story when evaluating patients.
“I think clinicians are ready to implement” testing, said Dr. Franceschini. “We just need to have better guidance.”
Who, When, What to Test
The NKF statement is not the first to try to address gaps in use and knowledge. A European Renal Association Working Group published guidelines in 2022.
The NKF Working Group came up with 56 recommendations and separate algorithms to guide testing for adult and pediatric individuals who are considered at-risk (and currently asymptomatic) and for those who already have clinical disease.
Testing can help determine a cause if there’s an atypical clinical presentation, and it can help avoid biopsies, said the group. Tests can also guide choice of therapy.
For at-risk individuals, there are two broad situations in which testing might be considered: In family members of a patient who already has kidney disease and in potential kidney donors. But testing at-risk children younger than 18 years should only be done if there is an intervention available that could prevent, treat, or slow progression of disease, said the authors.
For patients with an established genetic diagnosis, at-risk family members should be tested with the known single-gene variant diagnostic instead of a broad panel, said the group.
Single-gene variant testing is most appropriate in situations when clinical disease is already evident or when there is known genetic disease in the family, according to the NKF panel. A large diagnostic panel that covers the many common genetic causes of kidney disease is recommended for the majority of patients.
The group recommended that apolipoprotein L1 (APOL1) testing should be included in gene panels for CKD, and it should be offered to any patient “with clinical findings suggestive of APOL1-association nephropathy, regardless of race and ethnicity.”
High-risk APOL1 genotypes confer a 5- to 10-fold increased risk for CKD and are found in one out of seven individuals of African ancestry, which means the focus has largely been on testing those with that ancestry.
However, with many unknowns about APOL1, the NKF panel did not want to “profile” individuals and suggest that testing should not be based on skin color or race/ethnicity, said Dr. Franceschini.
In addition, only about 10% of those with the variant develop disease, so testing is not currently warranted for those who do not already have kidney disease, said the group.
They also recommended against the use of polygenic risk scores, saying that there are not enough data from diverse populations in genome-wide association studies for kidney disease or on their clinical utility.
More Education Needed; Many Barriers
The authors acknowledged that nephrologists generally receive little education in genetics and lack support for interpreting and discussing results.
“Nephrologists should be provided with training and best practice resources to interpret genetic testing and discuss the results with individuals and their families,” they wrote, adding that there’s a need for genomic medicine boards at academic centers that would be available to help nephrologists interpret results and plot clinical management.
The group did not, however, cite some of the other barriers to adoption of testing, including a limited number of sites offering testing, cost, and lack of insurance coverage for the diagnostics.
Medicare may cover genetic testing for kidney disease when an individual has symptoms and there is a Food and Drug Administration–approved test. Joseph Vassalotti, MD, chief medical officer for the NKF, said private insurance may cover the testing if the nephrologist deems it medically necessary, but that he usually confirms coverage before initiating testing. The often-used Renasight panel, which tests for 385 genes related to kidney diseases, costs $300-$400 out of pocket, Dr. Vassalotti told this news organization.
In a survey of 149 nephrologists conducted in 2021, both users (46%) and nonusers of the tests (69%) said that high cost was the most significant perceived barrier to implementing widespread testing. A third of users and almost two thirds of nonusers said that poor availability or lack of ease of testing was the second most significant barrier.
Clinics that test for kidney genes “are largely confined to large academic centers and some specialty clinics,” said Dominic Raj, MD, the Bert B. Brooks chair, and Divya Shankaranarayanan, MD, director of the Kidney Precision Medicine Clinic, both at George Washington University School of Medicine & Health Sciences, Washington, DC, in an email.
Testing is also limited by cultural barriers, lack of genetic literacy, and patients’ concerns that a positive result could lead to a loss of health insurance coverage, said Dr. Raj and Dr. Shankaranarayanan.
Paper Will Help Expand Use
A lack of consensus has also held back expansion. The new statement “may lead to increased and possibly judicious utilization of genetic testing in nephrology practices,” said Dr. Raj and Dr. Shankaranarayanan. “Most importantly, the panel has given specific guidance as to what type of genetic test platform is likely to yield the best and most cost-effective yield.”
The most effective use is “in monogenic kidney diseases and to a lesser extent in oligogenic kidney disease,” said Dr. Raj and Dr. Shankaranarayanan, adding that testing is of less-certain utility in polygenic kidney diseases, “where complex genetic and epigenetic factors determine the phenotype.”
Genetic testing might be especially useful “in atypical clinical presentations” and can help clinicians avoid unnecessary expensive and extensive investigations when multiple organ systems are involved, they said.
“Most importantly, [testing] might prevent unnecessary and potentially harmful treatment and enable targeted specific treatment, when available,” said Dr. Raj and Dr. Shankaranarayanan.
Dr. Franceschini and Dr. Shankaranarayanan reported no relevant financial relationships. Dr. Raj disclosed that he received consulting fees and honoraria from Novo Nordisk and is a national leader for the company’s Zeus trial, studying whether ziltivekimab reduces the risk for cardiovascular events in cardiovascular disease, CKD, and inflammation. He also participated in a study of Natera’s Renasight, a 385-gene panel for kidney disease.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF KIDNEY DISEASES
Weight Loss in Obesity May Create ‘Positive’ Hormone Changes
TOPLINE:
In middle-aged patients with severe obesity, changes in endogenous sex hormones may be proportional to the amount of weight loss after bariatric surgery and dietary intervention, leading to an improved hormonal balance, with more pronounced androgen changes in women.
METHODOLOGY:
- Obesity-related hormonal imbalances are common among those seeking weight loss treatment.
- This prospective observational study evaluated the incremental effect of weight loss by three bariatric procedures and a dietary intervention on endogenous sex hormones in men and women over 3 years.
- The study included 61 adults (median age, 50.9 years; baseline mean body mass index, 40.2; 72% women) from obesity clinics and private bariatric services in Sydney, Australia, between 2009 and 2012, who underwent bariatric surgery or received dietary interventions based on their probability of diabetes remission.
- The researchers evaluated weight loss and hormone levels at baseline and at 6, 12, 24, and 36 months.
- Changes in hormones were also compared among patients who received dietary intervention and those who underwent bariatric procedures such as Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic gastric banding.
TAKEAWAY:
- In women, testosterone levels decreased and sex hormone–binding globulin (SHBG) levels increased at 6 months; these changes were maintained at 24 and 36 months and remained statistically significant when controlled for age and menopausal status.
- In men, testosterone levels were significantly higher at 12, 24, and 36 months, and SHBG levels increased at 12 and 24 months. There were no differences in the estradiol levels among men and women.
- Women who underwent Roux-en-Y gastric bypass surgery experienced the greatest weight loss and the largest reduction (54%) in testosterone levels (P = .004), and sleeve gastrectomy led to an increase of 51% in SHBG levels (P = .0001), all compared with dietary interventions. In men, there were no differences in testosterone and SHBG levels between the diet and surgical groups.
IN PRACTICE:
“Ongoing monitoring of hormone levels and metabolic parameters is crucial for patients undergoing bariatric procedures to ensure long-term optimal health outcomes,” the authors wrote.
SOURCE:
This study was led by Malgorzata M. Brzozowska, MD, PhD, UNSW Sydney, Sydney, Australia, and was published online in the International Journal of Obesity.
LIMITATIONS:
The main limitations were a small sample size, lack of randomization, and absence of data on clinical outcomes related to hormone changes. Additionally, the researchers did not evaluate women for polycystic ovary syndrome or menstrual irregularities, and the clinical significance of testosterone reductions within the normal range remains unknown.
DISCLOSURES:
The study was funded by the National Health and Medical Research Council. Some authors have received honoraria and consulting and research support from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
In middle-aged patients with severe obesity, changes in endogenous sex hormones may be proportional to the amount of weight loss after bariatric surgery and dietary intervention, leading to an improved hormonal balance, with more pronounced androgen changes in women.
METHODOLOGY:
- Obesity-related hormonal imbalances are common among those seeking weight loss treatment.
- This prospective observational study evaluated the incremental effect of weight loss by three bariatric procedures and a dietary intervention on endogenous sex hormones in men and women over 3 years.
- The study included 61 adults (median age, 50.9 years; baseline mean body mass index, 40.2; 72% women) from obesity clinics and private bariatric services in Sydney, Australia, between 2009 and 2012, who underwent bariatric surgery or received dietary interventions based on their probability of diabetes remission.
- The researchers evaluated weight loss and hormone levels at baseline and at 6, 12, 24, and 36 months.
- Changes in hormones were also compared among patients who received dietary intervention and those who underwent bariatric procedures such as Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic gastric banding.
TAKEAWAY:
- In women, testosterone levels decreased and sex hormone–binding globulin (SHBG) levels increased at 6 months; these changes were maintained at 24 and 36 months and remained statistically significant when controlled for age and menopausal status.
- In men, testosterone levels were significantly higher at 12, 24, and 36 months, and SHBG levels increased at 12 and 24 months. There were no differences in the estradiol levels among men and women.
- Women who underwent Roux-en-Y gastric bypass surgery experienced the greatest weight loss and the largest reduction (54%) in testosterone levels (P = .004), and sleeve gastrectomy led to an increase of 51% in SHBG levels (P = .0001), all compared with dietary interventions. In men, there were no differences in testosterone and SHBG levels between the diet and surgical groups.
IN PRACTICE:
“Ongoing monitoring of hormone levels and metabolic parameters is crucial for patients undergoing bariatric procedures to ensure long-term optimal health outcomes,” the authors wrote.
SOURCE:
This study was led by Malgorzata M. Brzozowska, MD, PhD, UNSW Sydney, Sydney, Australia, and was published online in the International Journal of Obesity.
LIMITATIONS:
The main limitations were a small sample size, lack of randomization, and absence of data on clinical outcomes related to hormone changes. Additionally, the researchers did not evaluate women for polycystic ovary syndrome or menstrual irregularities, and the clinical significance of testosterone reductions within the normal range remains unknown.
DISCLOSURES:
The study was funded by the National Health and Medical Research Council. Some authors have received honoraria and consulting and research support from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
In middle-aged patients with severe obesity, changes in endogenous sex hormones may be proportional to the amount of weight loss after bariatric surgery and dietary intervention, leading to an improved hormonal balance, with more pronounced androgen changes in women.
METHODOLOGY:
- Obesity-related hormonal imbalances are common among those seeking weight loss treatment.
- This prospective observational study evaluated the incremental effect of weight loss by three bariatric procedures and a dietary intervention on endogenous sex hormones in men and women over 3 years.
- The study included 61 adults (median age, 50.9 years; baseline mean body mass index, 40.2; 72% women) from obesity clinics and private bariatric services in Sydney, Australia, between 2009 and 2012, who underwent bariatric surgery or received dietary interventions based on their probability of diabetes remission.
- The researchers evaluated weight loss and hormone levels at baseline and at 6, 12, 24, and 36 months.
- Changes in hormones were also compared among patients who received dietary intervention and those who underwent bariatric procedures such as Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic gastric banding.
TAKEAWAY:
- In women, testosterone levels decreased and sex hormone–binding globulin (SHBG) levels increased at 6 months; these changes were maintained at 24 and 36 months and remained statistically significant when controlled for age and menopausal status.
- In men, testosterone levels were significantly higher at 12, 24, and 36 months, and SHBG levels increased at 12 and 24 months. There were no differences in the estradiol levels among men and women.
- Women who underwent Roux-en-Y gastric bypass surgery experienced the greatest weight loss and the largest reduction (54%) in testosterone levels (P = .004), and sleeve gastrectomy led to an increase of 51% in SHBG levels (P = .0001), all compared with dietary interventions. In men, there were no differences in testosterone and SHBG levels between the diet and surgical groups.
IN PRACTICE:
“Ongoing monitoring of hormone levels and metabolic parameters is crucial for patients undergoing bariatric procedures to ensure long-term optimal health outcomes,” the authors wrote.
SOURCE:
This study was led by Malgorzata M. Brzozowska, MD, PhD, UNSW Sydney, Sydney, Australia, and was published online in the International Journal of Obesity.
LIMITATIONS:
The main limitations were a small sample size, lack of randomization, and absence of data on clinical outcomes related to hormone changes. Additionally, the researchers did not evaluate women for polycystic ovary syndrome or menstrual irregularities, and the clinical significance of testosterone reductions within the normal range remains unknown.
DISCLOSURES:
The study was funded by the National Health and Medical Research Council. Some authors have received honoraria and consulting and research support from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.