User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Recent Developments in Psychodermatology and Psychopharmacology for Delusional Patients
The management of delusional infestation (DI), also known as Morgellons disease or delusional parasitosis, can lead to some of the most difficult and stressful patient encounters in dermatology. As a specialty, dermatology providers are trained to respect scientific objectivity and pride themselves on their visual diagnostic acumen. Therefore, having to accommodate a patient’s erroneous ideations and potentially treat a psychiatric pathology poses a challenge for many dermatology providers because it requires shifting their mindset to where the subjective reality becomes the primary issue during the visit. This disconnect may lead to strife between the patient and the provider. All of these issues may make it difficult for dermatologists to connect with DI patients with the usual courtesy and consideration given to other patients. Moreover, some dermatologists find it difficult to respect the chief concern, which often is seen as purely psychological because there may be some lingering bias where psychological concerns perhaps are not seen as bona fide or legitimate disorders.
Is There a Biologic Basis for DI? A New Theory on the Etiology of Delusional Parasitosis
It is important to distinguish DI phenomenology into primary and secondary causes. Primary DI refers to cases where the delusion and formication occur spontaneously. In contrast, in secondary DI the delusion and other manifestations (eg, formication) happen secondarily to underlying broader diagnoses such as illicit substance abuse, primary psychiatric conditions including schizophrenia, organic brain syndrome, and vitamin B12 deficiency.
It is well known that primary DI overwhelmingly occurs in older women, whereas secondary DI does not show this same predilection. It has been a big unanswered question as to why primary DI so often occurs not only in women but specifically in older women. The latest theory that has been advancing in Europe and is supported by some data, including magnetic resonance imaging of the brain, involves the dopamine transporter (DAT) system, which is important in making sure the dopamine level in the intersynaptic space is not excessive.1 The DAT system is much more prominent in woman vs men and deteriorates with age due to declining estrogen levels. This age-related loss of striatal DAT is thought to be one possible etiology of DI. It has been hypothesized that decreased DAT functioning may cause an increase in extracellular striatal dopamine levels in the synapse that can lead to tactile hallucinations and delusions, which are hallmark symptoms seen in DI. Given that women experience a greater age-related DAT decline in striatal subregions than men, it is thought that primary DI mainly affects older women due to the decline of neuroprotective effects of estrogen on DAT activity with age.2 Further studies should evaluate the possibility of estrogen replacement therapy for treatment of DI.
Improving Care of Psychodermatology Patients in Clinic
There are several medications that are known to be effective for the treatment of DI, including pimozide, risperidone, aripiprazole, and olanzapine, among others. Pimozide is uniquely accepted by DI patients because it has no official psychiatric indication from the US Food and Drug Administration (FDA); it is only indicated in the United States for Tourette syndrome, which is a neurologic disorder. Therefore, pimozide arguably can be disregarded as a true antipsychotic agent. The fact that its chemical structure is similar to those of bona fide antipsychotic medications does not necessarily put it in this same category, as there also are antiemetic and antitussive medications (eg, prochlorperazine, promethazine) with chemical structures similar to antipsychotics, but clinicians generally do not think of these drugs as antipsychotics despite the similarities. This nuanced and admittedly somewhat arbitrary categorization is critical to patient care; in our clinic, we have found that patients who categorically refuse to consider all psychiatric medications are much more willing to try pimozide for this very reason, that this medication can uniquely be presented to the DI patient as an agent not used in psychiatry. We have found great success in treatment with pimozide, even with relatively low doses.3,4
One of the main reasons dermatologists are reluctant to prescribe antipsychotic medications or even pimozide is the concern for side effects, especially tardive dyskinesia (TD), which is thought to be irreversible and untreatable. However, after a half century of worldwide use of pimozide in dermatology, a PubMed search of English-language articles indexed for MEDLINE using the terms pimozide and tardive dyskinesia, tardive dyskinesia and delusions of parasitosis, tardive dyskinesia and dermatology, and tardive dyskinesia and delusional infestation/Morgellons disease yielded only 1 known case of TD reported in dermatologic use for DI.5 In this particular case, TD-like symptoms did not appear until after pimozide had been discontinued for 1 month. Therefore, it is not clear if this case was true TD or a condition known as withdrawal dyskinesia, which mimics TD and usually is self-limiting.5
The senior author (J.K.) has been using pimozide for treatment of DI for more than 30 years and has not encountered TD or any other notable side effects. The reason for this extremely low incidence of side effects may be due to its high efficacy in treating DI; hence, only a low dose of pimozide usually is needed. At the University of California, San Francisco, Psychodermatology Clinic, pimozide typically is used to treat DI at a low dose of 3 mg or less daily, starting with 0.5 or 1 mg and slowly titrating upward until a clinically effective dose is reached. Pimozide rarely is used long-term; after the resolution of symptoms, the dose usually is continued at the clinically effective dose for a few months and then is slowly tapered off. In contrast, for a condition such as schizophrenia, an antipsychotic medication often is needed at high doses for life, resulting in higher TD occurrences being reported. Therefore, even though the newer antipsychotic agents are preferable to pimozide because of their somewhat lower risk for TD, in actual clinical practice many, if not most, DI patients detest any suggestion of taking a medication for “crazy people.” Thus, we find that pimozide’s inherent superior acceptability among DI patients often is critical to enabling any effective treatment to occur at all due to the fact that the provider can honestly say that pimozide has no FDA psychiatric indication.
Still, one of the biggest apprehensions with initiating and continuing these medications in dermatology is fear of TD. Now, dermatologists can be made aware that if this very rare side effect occurs, there are medications approved to treat TD, even if the anti-TD therapy is administered by a neurologist. For the first time, 2 medications were approved by the FDA for treatment of TD in 2017, namely valbenazine and deutetrabenazine. These medications represent a class known as vesicular monoamine transporter type 2 inhibitors and function by ultimately reducing the amount of dopamine released from the presynaptic dopaminergic neurons. In phase 3 trials for valbenazine and deutetrabenazine, 40% (N=234) and 34% (N=222) of patients, respectively, achieved a response, which was defined as at least a 50% decrease from baseline on the abnormal involuntary movement scale dyskinesia score in 6 to 12 weeks compared to 9% and 12%, respectively, with placebo.Discontinuation because of an adverse event was seldom encountered with both medications.6
Conclusion
The recent developments in psychodermatology with regard to DI are encouraging. The advent of new evidence and theories suggestive of an organic basis for DI could help this condition become more respected in the eyes of the dermatologist as a bona fide disorder. Moreover, the new developments and availability of medications that can treat TD can further make it easier for dermatologists to consider offering DI patients truly meaningful treatment that they desperately need. Therefore, both of these developments are welcomed for our specialty.
- Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. a new insight of etiology? Med Hypotheses. 2007;68:1351-1358.
- Chan SY, Koo J. Sex differences in primary delusional infestation: an insight into etiology and potential novel therapy. Int J Women Dermatol. 2020;6:226.
- Lorenzo CR, Koo J. Pimozide in dermatologic practice: a comprehensive review. Am J Clin Dermatol. 2004;5:339-349.
- Brownstone ND, Beck K, Sekhon S, et al. Morgellons Disease. 2nd ed. Kindle Direct Publishing; 2020.
- Thomson AM, Wallace J, Kobylecki C. Tardive dyskinesia after drug withdrawal in two older adults: clinical features, complications and management. Geriatr Gerontol Int. 2019;19:563-564.
- Citrome L. Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective. Expert Rev Neurother. 2018;18:323-332.
The management of delusional infestation (DI), also known as Morgellons disease or delusional parasitosis, can lead to some of the most difficult and stressful patient encounters in dermatology. As a specialty, dermatology providers are trained to respect scientific objectivity and pride themselves on their visual diagnostic acumen. Therefore, having to accommodate a patient’s erroneous ideations and potentially treat a psychiatric pathology poses a challenge for many dermatology providers because it requires shifting their mindset to where the subjective reality becomes the primary issue during the visit. This disconnect may lead to strife between the patient and the provider. All of these issues may make it difficult for dermatologists to connect with DI patients with the usual courtesy and consideration given to other patients. Moreover, some dermatologists find it difficult to respect the chief concern, which often is seen as purely psychological because there may be some lingering bias where psychological concerns perhaps are not seen as bona fide or legitimate disorders.
Is There a Biologic Basis for DI? A New Theory on the Etiology of Delusional Parasitosis
It is important to distinguish DI phenomenology into primary and secondary causes. Primary DI refers to cases where the delusion and formication occur spontaneously. In contrast, in secondary DI the delusion and other manifestations (eg, formication) happen secondarily to underlying broader diagnoses such as illicit substance abuse, primary psychiatric conditions including schizophrenia, organic brain syndrome, and vitamin B12 deficiency.
It is well known that primary DI overwhelmingly occurs in older women, whereas secondary DI does not show this same predilection. It has been a big unanswered question as to why primary DI so often occurs not only in women but specifically in older women. The latest theory that has been advancing in Europe and is supported by some data, including magnetic resonance imaging of the brain, involves the dopamine transporter (DAT) system, which is important in making sure the dopamine level in the intersynaptic space is not excessive.1 The DAT system is much more prominent in woman vs men and deteriorates with age due to declining estrogen levels. This age-related loss of striatal DAT is thought to be one possible etiology of DI. It has been hypothesized that decreased DAT functioning may cause an increase in extracellular striatal dopamine levels in the synapse that can lead to tactile hallucinations and delusions, which are hallmark symptoms seen in DI. Given that women experience a greater age-related DAT decline in striatal subregions than men, it is thought that primary DI mainly affects older women due to the decline of neuroprotective effects of estrogen on DAT activity with age.2 Further studies should evaluate the possibility of estrogen replacement therapy for treatment of DI.
Improving Care of Psychodermatology Patients in Clinic
There are several medications that are known to be effective for the treatment of DI, including pimozide, risperidone, aripiprazole, and olanzapine, among others. Pimozide is uniquely accepted by DI patients because it has no official psychiatric indication from the US Food and Drug Administration (FDA); it is only indicated in the United States for Tourette syndrome, which is a neurologic disorder. Therefore, pimozide arguably can be disregarded as a true antipsychotic agent. The fact that its chemical structure is similar to those of bona fide antipsychotic medications does not necessarily put it in this same category, as there also are antiemetic and antitussive medications (eg, prochlorperazine, promethazine) with chemical structures similar to antipsychotics, but clinicians generally do not think of these drugs as antipsychotics despite the similarities. This nuanced and admittedly somewhat arbitrary categorization is critical to patient care; in our clinic, we have found that patients who categorically refuse to consider all psychiatric medications are much more willing to try pimozide for this very reason, that this medication can uniquely be presented to the DI patient as an agent not used in psychiatry. We have found great success in treatment with pimozide, even with relatively low doses.3,4
One of the main reasons dermatologists are reluctant to prescribe antipsychotic medications or even pimozide is the concern for side effects, especially tardive dyskinesia (TD), which is thought to be irreversible and untreatable. However, after a half century of worldwide use of pimozide in dermatology, a PubMed search of English-language articles indexed for MEDLINE using the terms pimozide and tardive dyskinesia, tardive dyskinesia and delusions of parasitosis, tardive dyskinesia and dermatology, and tardive dyskinesia and delusional infestation/Morgellons disease yielded only 1 known case of TD reported in dermatologic use for DI.5 In this particular case, TD-like symptoms did not appear until after pimozide had been discontinued for 1 month. Therefore, it is not clear if this case was true TD or a condition known as withdrawal dyskinesia, which mimics TD and usually is self-limiting.5
The senior author (J.K.) has been using pimozide for treatment of DI for more than 30 years and has not encountered TD or any other notable side effects. The reason for this extremely low incidence of side effects may be due to its high efficacy in treating DI; hence, only a low dose of pimozide usually is needed. At the University of California, San Francisco, Psychodermatology Clinic, pimozide typically is used to treat DI at a low dose of 3 mg or less daily, starting with 0.5 or 1 mg and slowly titrating upward until a clinically effective dose is reached. Pimozide rarely is used long-term; after the resolution of symptoms, the dose usually is continued at the clinically effective dose for a few months and then is slowly tapered off. In contrast, for a condition such as schizophrenia, an antipsychotic medication often is needed at high doses for life, resulting in higher TD occurrences being reported. Therefore, even though the newer antipsychotic agents are preferable to pimozide because of their somewhat lower risk for TD, in actual clinical practice many, if not most, DI patients detest any suggestion of taking a medication for “crazy people.” Thus, we find that pimozide’s inherent superior acceptability among DI patients often is critical to enabling any effective treatment to occur at all due to the fact that the provider can honestly say that pimozide has no FDA psychiatric indication.
Still, one of the biggest apprehensions with initiating and continuing these medications in dermatology is fear of TD. Now, dermatologists can be made aware that if this very rare side effect occurs, there are medications approved to treat TD, even if the anti-TD therapy is administered by a neurologist. For the first time, 2 medications were approved by the FDA for treatment of TD in 2017, namely valbenazine and deutetrabenazine. These medications represent a class known as vesicular monoamine transporter type 2 inhibitors and function by ultimately reducing the amount of dopamine released from the presynaptic dopaminergic neurons. In phase 3 trials for valbenazine and deutetrabenazine, 40% (N=234) and 34% (N=222) of patients, respectively, achieved a response, which was defined as at least a 50% decrease from baseline on the abnormal involuntary movement scale dyskinesia score in 6 to 12 weeks compared to 9% and 12%, respectively, with placebo.Discontinuation because of an adverse event was seldom encountered with both medications.6
Conclusion
The recent developments in psychodermatology with regard to DI are encouraging. The advent of new evidence and theories suggestive of an organic basis for DI could help this condition become more respected in the eyes of the dermatologist as a bona fide disorder. Moreover, the new developments and availability of medications that can treat TD can further make it easier for dermatologists to consider offering DI patients truly meaningful treatment that they desperately need. Therefore, both of these developments are welcomed for our specialty.
The management of delusional infestation (DI), also known as Morgellons disease or delusional parasitosis, can lead to some of the most difficult and stressful patient encounters in dermatology. As a specialty, dermatology providers are trained to respect scientific objectivity and pride themselves on their visual diagnostic acumen. Therefore, having to accommodate a patient’s erroneous ideations and potentially treat a psychiatric pathology poses a challenge for many dermatology providers because it requires shifting their mindset to where the subjective reality becomes the primary issue during the visit. This disconnect may lead to strife between the patient and the provider. All of these issues may make it difficult for dermatologists to connect with DI patients with the usual courtesy and consideration given to other patients. Moreover, some dermatologists find it difficult to respect the chief concern, which often is seen as purely psychological because there may be some lingering bias where psychological concerns perhaps are not seen as bona fide or legitimate disorders.
Is There a Biologic Basis for DI? A New Theory on the Etiology of Delusional Parasitosis
It is important to distinguish DI phenomenology into primary and secondary causes. Primary DI refers to cases where the delusion and formication occur spontaneously. In contrast, in secondary DI the delusion and other manifestations (eg, formication) happen secondarily to underlying broader diagnoses such as illicit substance abuse, primary psychiatric conditions including schizophrenia, organic brain syndrome, and vitamin B12 deficiency.
It is well known that primary DI overwhelmingly occurs in older women, whereas secondary DI does not show this same predilection. It has been a big unanswered question as to why primary DI so often occurs not only in women but specifically in older women. The latest theory that has been advancing in Europe and is supported by some data, including magnetic resonance imaging of the brain, involves the dopamine transporter (DAT) system, which is important in making sure the dopamine level in the intersynaptic space is not excessive.1 The DAT system is much more prominent in woman vs men and deteriorates with age due to declining estrogen levels. This age-related loss of striatal DAT is thought to be one possible etiology of DI. It has been hypothesized that decreased DAT functioning may cause an increase in extracellular striatal dopamine levels in the synapse that can lead to tactile hallucinations and delusions, which are hallmark symptoms seen in DI. Given that women experience a greater age-related DAT decline in striatal subregions than men, it is thought that primary DI mainly affects older women due to the decline of neuroprotective effects of estrogen on DAT activity with age.2 Further studies should evaluate the possibility of estrogen replacement therapy for treatment of DI.
Improving Care of Psychodermatology Patients in Clinic
There are several medications that are known to be effective for the treatment of DI, including pimozide, risperidone, aripiprazole, and olanzapine, among others. Pimozide is uniquely accepted by DI patients because it has no official psychiatric indication from the US Food and Drug Administration (FDA); it is only indicated in the United States for Tourette syndrome, which is a neurologic disorder. Therefore, pimozide arguably can be disregarded as a true antipsychotic agent. The fact that its chemical structure is similar to those of bona fide antipsychotic medications does not necessarily put it in this same category, as there also are antiemetic and antitussive medications (eg, prochlorperazine, promethazine) with chemical structures similar to antipsychotics, but clinicians generally do not think of these drugs as antipsychotics despite the similarities. This nuanced and admittedly somewhat arbitrary categorization is critical to patient care; in our clinic, we have found that patients who categorically refuse to consider all psychiatric medications are much more willing to try pimozide for this very reason, that this medication can uniquely be presented to the DI patient as an agent not used in psychiatry. We have found great success in treatment with pimozide, even with relatively low doses.3,4
One of the main reasons dermatologists are reluctant to prescribe antipsychotic medications or even pimozide is the concern for side effects, especially tardive dyskinesia (TD), which is thought to be irreversible and untreatable. However, after a half century of worldwide use of pimozide in dermatology, a PubMed search of English-language articles indexed for MEDLINE using the terms pimozide and tardive dyskinesia, tardive dyskinesia and delusions of parasitosis, tardive dyskinesia and dermatology, and tardive dyskinesia and delusional infestation/Morgellons disease yielded only 1 known case of TD reported in dermatologic use for DI.5 In this particular case, TD-like symptoms did not appear until after pimozide had been discontinued for 1 month. Therefore, it is not clear if this case was true TD or a condition known as withdrawal dyskinesia, which mimics TD and usually is self-limiting.5
The senior author (J.K.) has been using pimozide for treatment of DI for more than 30 years and has not encountered TD or any other notable side effects. The reason for this extremely low incidence of side effects may be due to its high efficacy in treating DI; hence, only a low dose of pimozide usually is needed. At the University of California, San Francisco, Psychodermatology Clinic, pimozide typically is used to treat DI at a low dose of 3 mg or less daily, starting with 0.5 or 1 mg and slowly titrating upward until a clinically effective dose is reached. Pimozide rarely is used long-term; after the resolution of symptoms, the dose usually is continued at the clinically effective dose for a few months and then is slowly tapered off. In contrast, for a condition such as schizophrenia, an antipsychotic medication often is needed at high doses for life, resulting in higher TD occurrences being reported. Therefore, even though the newer antipsychotic agents are preferable to pimozide because of their somewhat lower risk for TD, in actual clinical practice many, if not most, DI patients detest any suggestion of taking a medication for “crazy people.” Thus, we find that pimozide’s inherent superior acceptability among DI patients often is critical to enabling any effective treatment to occur at all due to the fact that the provider can honestly say that pimozide has no FDA psychiatric indication.
Still, one of the biggest apprehensions with initiating and continuing these medications in dermatology is fear of TD. Now, dermatologists can be made aware that if this very rare side effect occurs, there are medications approved to treat TD, even if the anti-TD therapy is administered by a neurologist. For the first time, 2 medications were approved by the FDA for treatment of TD in 2017, namely valbenazine and deutetrabenazine. These medications represent a class known as vesicular monoamine transporter type 2 inhibitors and function by ultimately reducing the amount of dopamine released from the presynaptic dopaminergic neurons. In phase 3 trials for valbenazine and deutetrabenazine, 40% (N=234) and 34% (N=222) of patients, respectively, achieved a response, which was defined as at least a 50% decrease from baseline on the abnormal involuntary movement scale dyskinesia score in 6 to 12 weeks compared to 9% and 12%, respectively, with placebo.Discontinuation because of an adverse event was seldom encountered with both medications.6
Conclusion
The recent developments in psychodermatology with regard to DI are encouraging. The advent of new evidence and theories suggestive of an organic basis for DI could help this condition become more respected in the eyes of the dermatologist as a bona fide disorder. Moreover, the new developments and availability of medications that can treat TD can further make it easier for dermatologists to consider offering DI patients truly meaningful treatment that they desperately need. Therefore, both of these developments are welcomed for our specialty.
- Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. a new insight of etiology? Med Hypotheses. 2007;68:1351-1358.
- Chan SY, Koo J. Sex differences in primary delusional infestation: an insight into etiology and potential novel therapy. Int J Women Dermatol. 2020;6:226.
- Lorenzo CR, Koo J. Pimozide in dermatologic practice: a comprehensive review. Am J Clin Dermatol. 2004;5:339-349.
- Brownstone ND, Beck K, Sekhon S, et al. Morgellons Disease. 2nd ed. Kindle Direct Publishing; 2020.
- Thomson AM, Wallace J, Kobylecki C. Tardive dyskinesia after drug withdrawal in two older adults: clinical features, complications and management. Geriatr Gerontol Int. 2019;19:563-564.
- Citrome L. Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective. Expert Rev Neurother. 2018;18:323-332.
- Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. a new insight of etiology? Med Hypotheses. 2007;68:1351-1358.
- Chan SY, Koo J. Sex differences in primary delusional infestation: an insight into etiology and potential novel therapy. Int J Women Dermatol. 2020;6:226.
- Lorenzo CR, Koo J. Pimozide in dermatologic practice: a comprehensive review. Am J Clin Dermatol. 2004;5:339-349.
- Brownstone ND, Beck K, Sekhon S, et al. Morgellons Disease. 2nd ed. Kindle Direct Publishing; 2020.
- Thomson AM, Wallace J, Kobylecki C. Tardive dyskinesia after drug withdrawal in two older adults: clinical features, complications and management. Geriatr Gerontol Int. 2019;19:563-564.
- Citrome L. Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective. Expert Rev Neurother. 2018;18:323-332.
Erythema Ab Igne and Malignant Transformation to Squamous Cell Carcinoma
Case Report
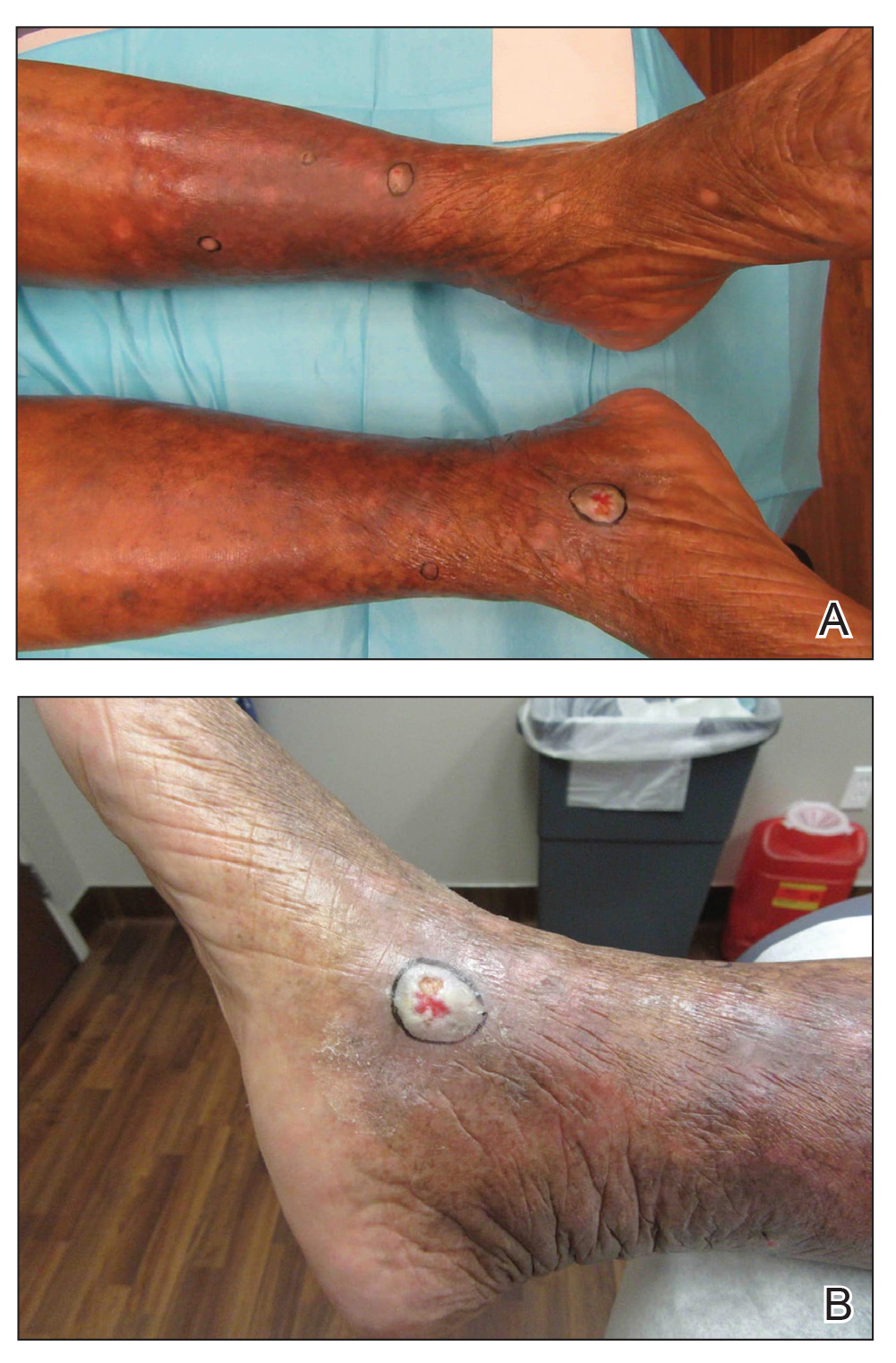
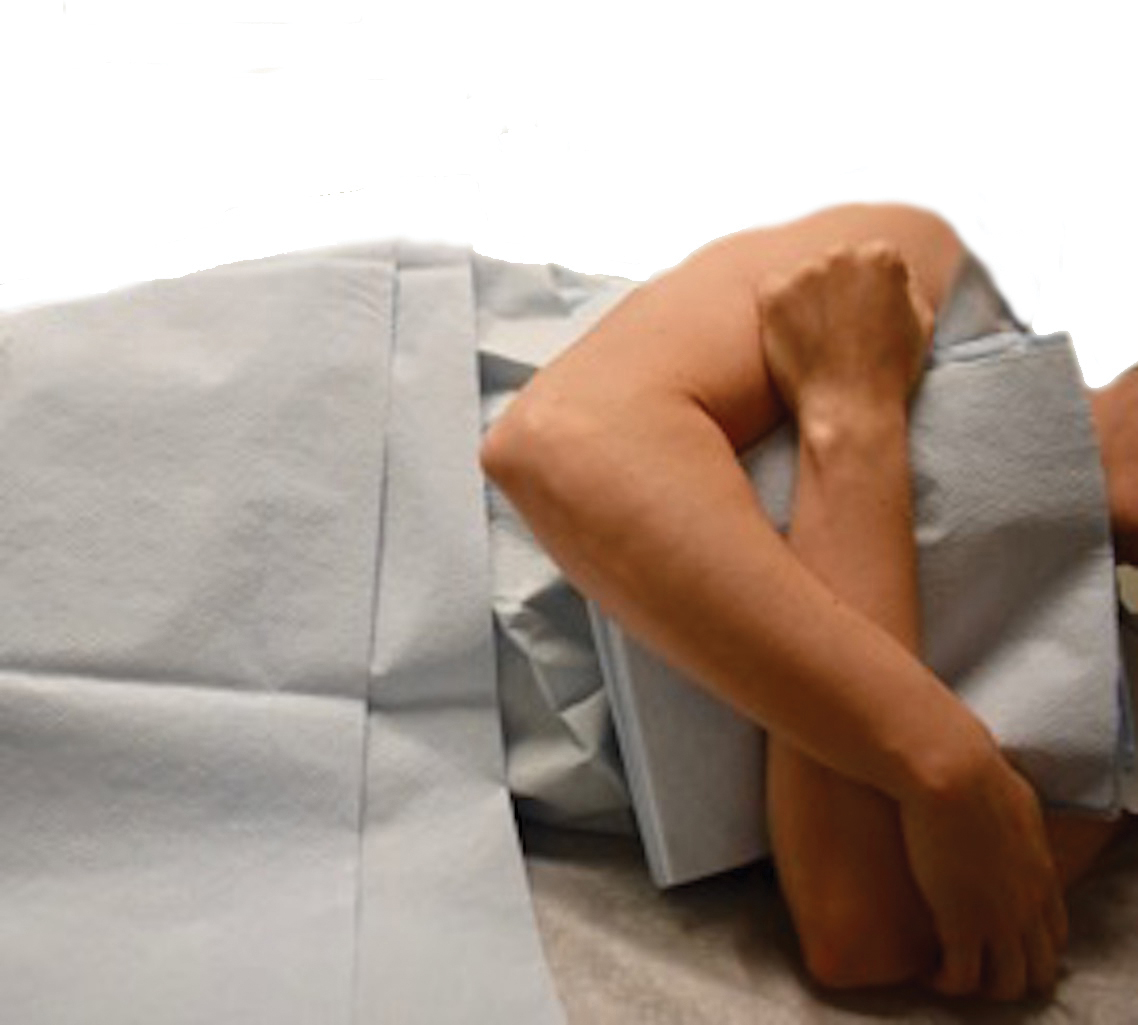
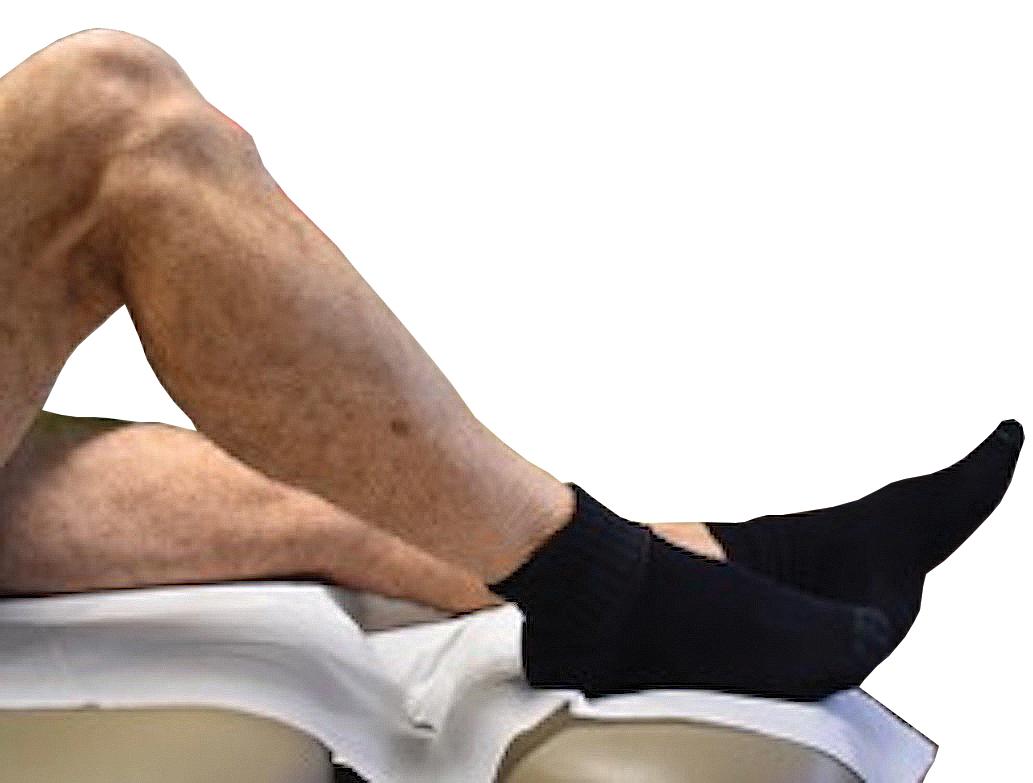
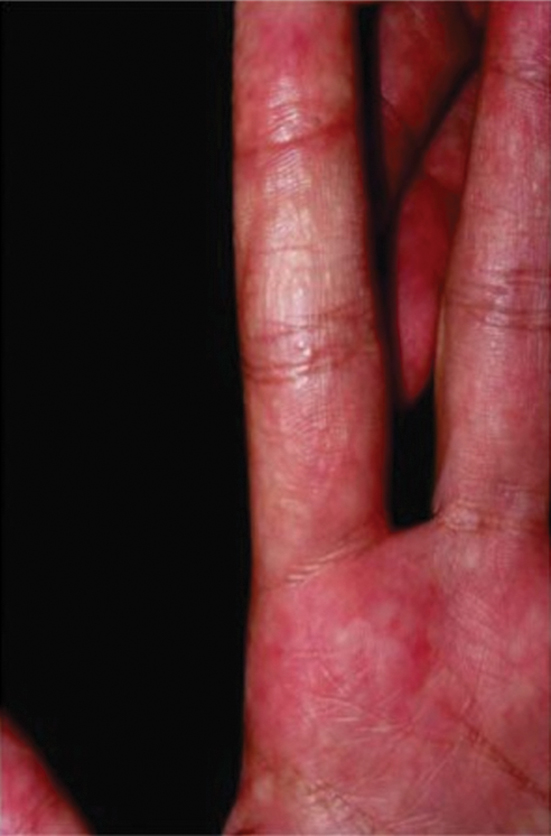
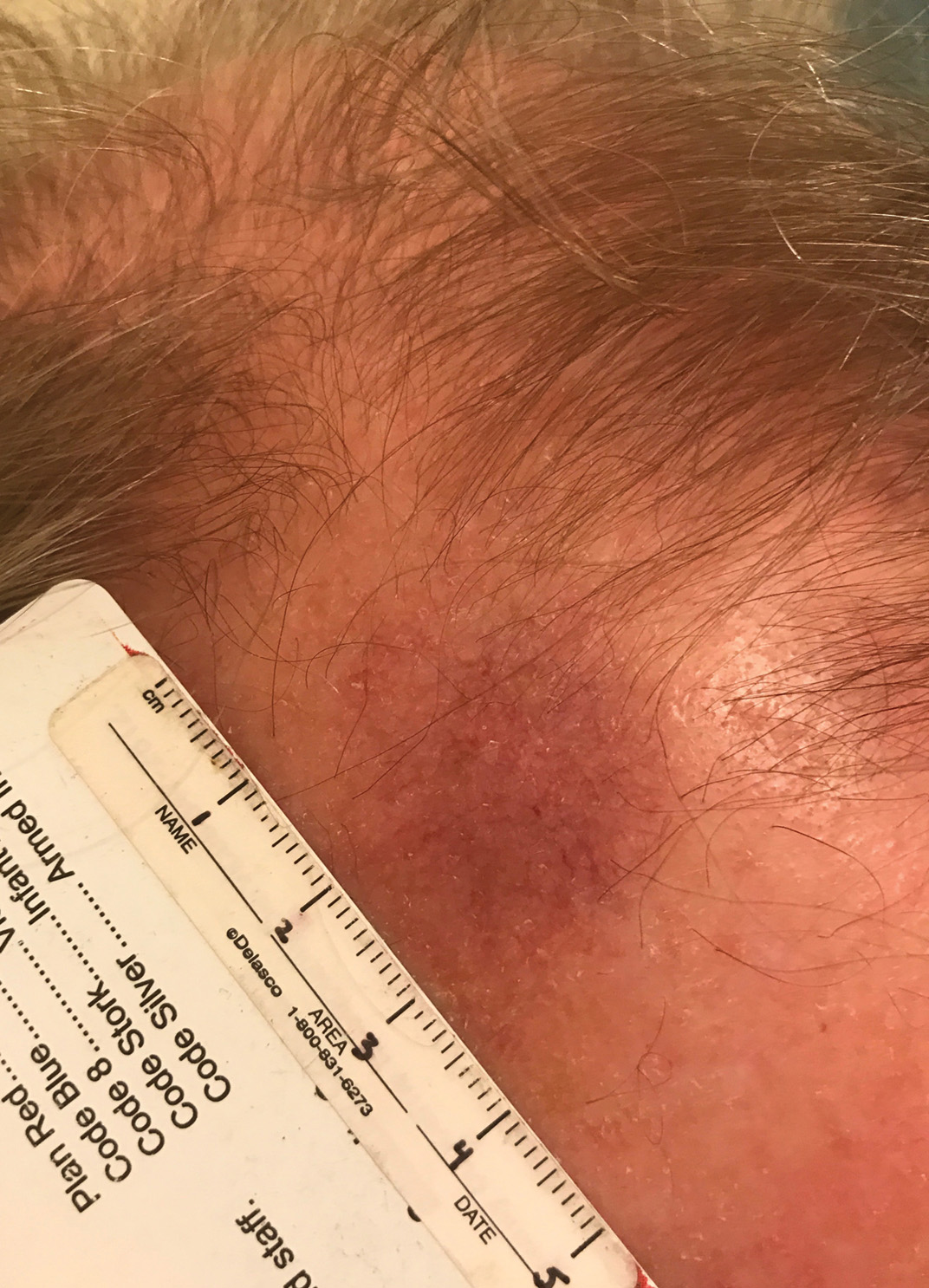
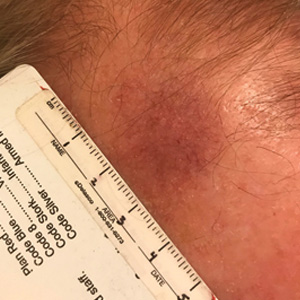
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).
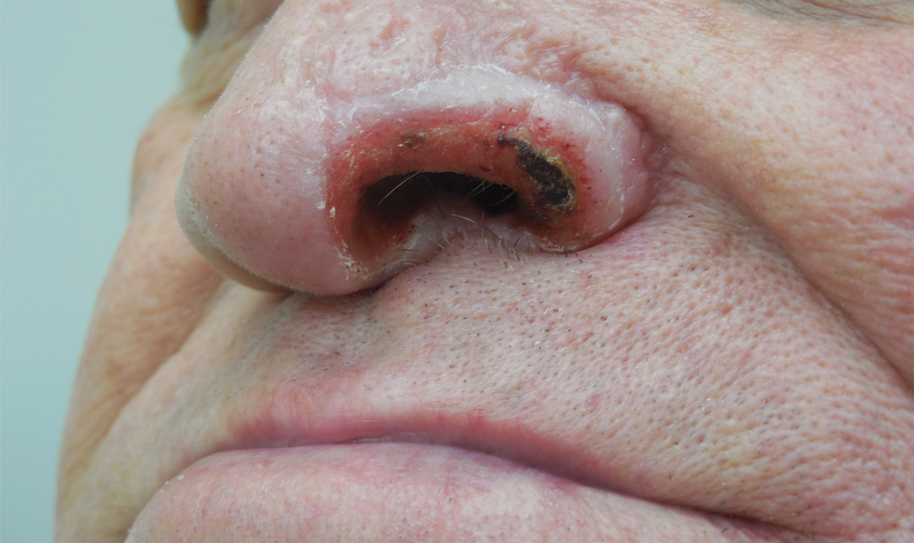
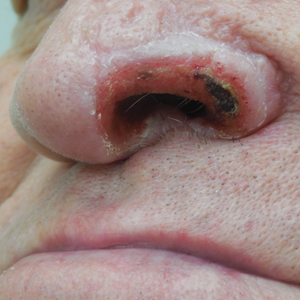
Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.
Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
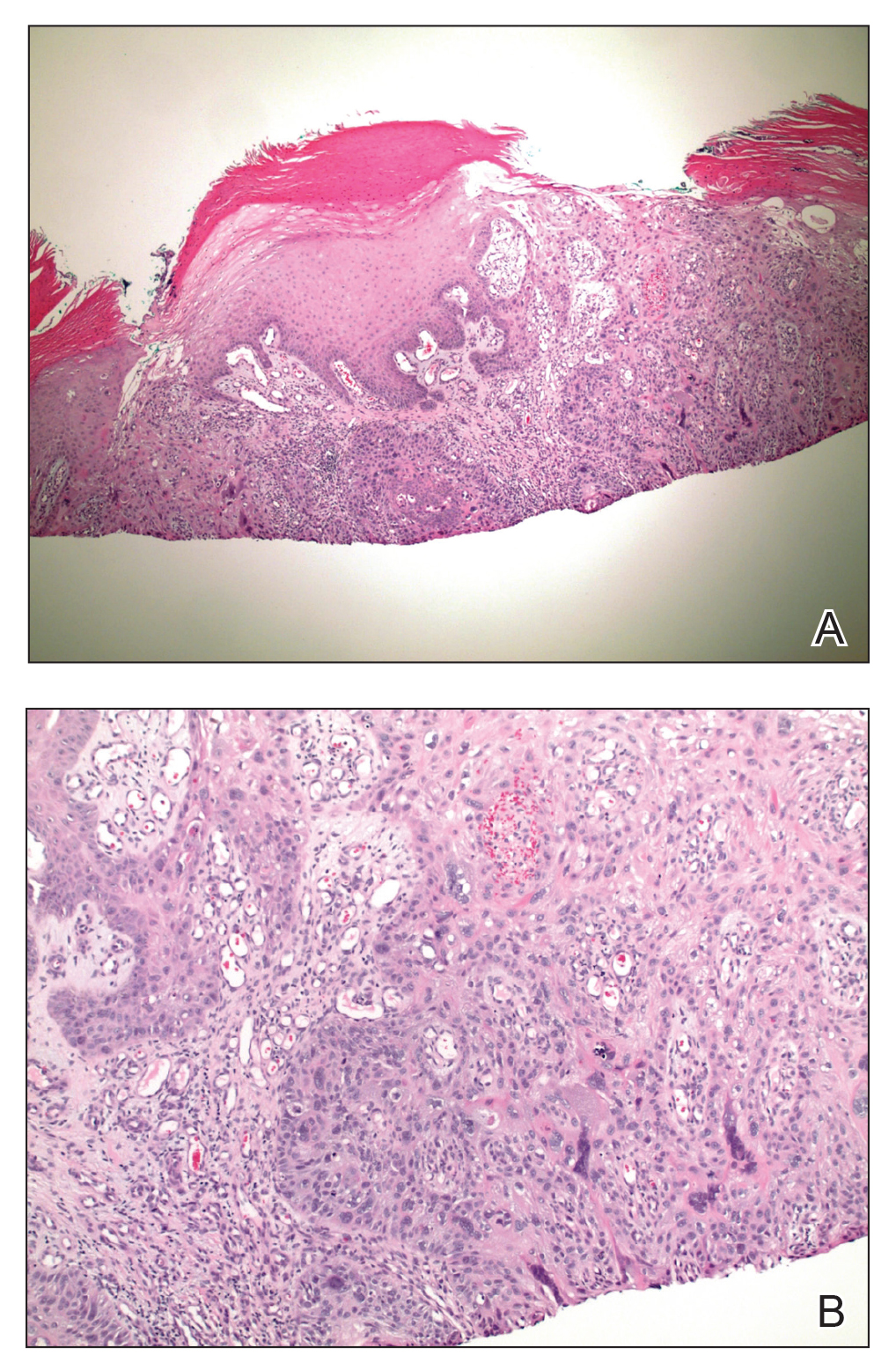
Histopathology of EAI
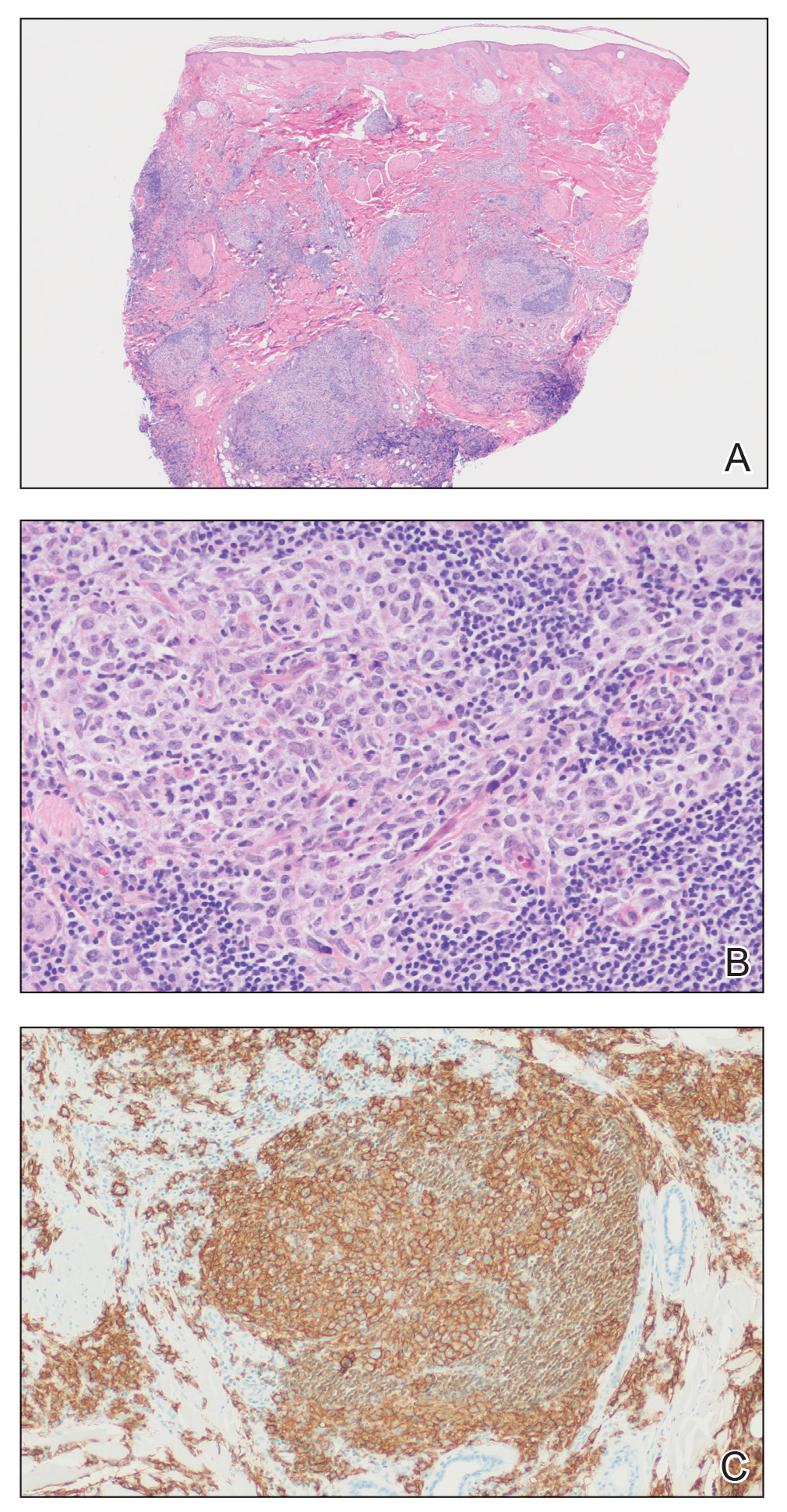
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
Case Report
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).
Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.
Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
Histopathology of EAI
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
Case Report
A 67-year-old Black woman presented with a long-standing history of pruritus and “scaly thick bumps” on the lower extremities. Upon further questioning, she reported a 30-year history of placing her feet by an electric space heater and daily baths in “very hot” water. A review of systems and medical history were unremarkable, and the patient was not on any medications. Initial physical examination of the lower extremities demonstrated lichenified plaques and scattered, firm, ulcerated nodules surrounded by mottled postinflammatory hyperpigmentation with sharp demarcation at the midcalf bilaterally (Figure 1).
Subsequently, the patient was shown to have multiple actinic keratoses and SCCs, both in situ and invasive, within the areas of EAI (Figure 2). The patient had no actinic keratoses or other cutaneous malignant neoplasms elsewhere on the skin. Management of actinic keratoses, SCC in situ, and invasive SCC on the lower extremities included numerous excisions, treatment with liquid nitrogen, and topical 5-fluorouracil under occlusion. The patient continues to be monitored frequently.
Comment
Presentation of EAI
Erythema ab igne is a cutaneous reaction resulting from prolonged exposure to an infrared heat source at temperatures insufficient to cause a burn (37 °F to 11
Histopathology of EAI
Histologically, later stages of EAI can demonstrate focal hyperkeratosis with dyskeratosis and increased dermal elastosis, similar to actinic damage, with a predisposition to develop SCC.2 Notably, early reports document various heat-induced carcinomas, including kangri-burn cancers among Kashmiris, kang thermal cancers in China, and kairo cancers in Japan.2,4,5 More recent reports identify cutaneous carcinomas arising specifically in the setting of EAI, most commonly SCC3; Merkel cell carcinoma and cutaneous marginal zone lymphoma are less commonly reported malignancies.6,7 Given the frequency of malignant transformation within sites of thermal exposure, chronic heat exposure may share a common pathophysiology with SCC and other neoplasms, including Merkel cell carcinoma and cutaneous marginal zone lymphoma.
SCC in Black Individuals
Squamous cell carcinoma is the most common skin cancer in Black individuals, with a notably higher incidence in high-risk subpopulations (immunosuppressed patients). Unlike White individuals, SCCs frequently occur in non–sun-exposed areas in Black individuals and are associated with unique risk factors, such as human papillomavirus, as demonstrated in Black transplant patients.8 A retrospective study examining the characteristics of SCC on the legs of Black individuals documented atypical hyperkeratotic neoplasms surrounded by abnormal pigmentation and mottling of surrounding skin.9 Morphologic skin changes could be the result of chronic thermal damage: Numerous patients reported a history of leg warming from an open heat source. Other patients had an actual diagnosis of EAI. The predilection for less-exposed skin suggests UV radiation (UVR) might be a less important predisposing risk factor for this racial group, and the increased mortality associated with SCC in Black individuals might represent a more aggressive nature to this subset of SCCs.9 Furthermore, infrared radiation (IRR), such as fires and coal stoves, might have the potential to stimulate skin changes similar to those associated with UVR and ultimately malignant changes.
Infrared Radiation
Compared to UVR, little is known about the biological effects of IRR (wavelength, 760 nm to 1 mm), to which human skin is constantly exposed from natural and artificial light sources. Early studies have demonstrated the carcinogenic potential of IRR, observing an augmentation of UVR-induced tumorigenesis in the presence of heat. More recently, IRR was observed to stimulate increased collagenase production from dermal fibroblasts and influence pathways (extracellular signal-related kinases 1/2 and p38 mitogen-activated protein kinases) in a similar fashion to UVB and UVA.10,11 Therefore, IRR might be capable of eliciting molecular responses comparable to those caused by UVR.
Conclusion
Although SCC in association with EAI is uncommon, historical reports of thermal cancers and scientific observations of IRR-induced biological and molecular effects support EAI as a predisposing risk factor for SCC and the important need for close monitoring by physicians. Studies are needed to further elucidate the pathologic effects of IRR, with more promotion of caution relating to thermal exposure.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;2016:1862480.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28. Accessed December 10, 2020. https://escholarship.org/uc/item/47z4v01z
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Neve EF. Kangri-burn cancer. Br Med J. 1923;2:1255-1256.
- Laycock HT. The kang cancer of North-West China. Br Med J. 1948;1:982.
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081.
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Freeman RG, Knox JM. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89:858-864.
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed. 2003;19:228-234.
Practice Points
- Erythema ab igne (EAI) is a cutaneous reaction in response to prolonged exposure to infrared heat sources at temperatures insufficient to induce a burn.
- Common infrared heat sources include open fires, coal stoves, heating pads, laptop computers, and electric space heaters.
- Although considered a chronic pigmentary disorder, EAI rarely can progress to malignant transformation, including squamous cell carcinoma. Patients with EAI should be monitored long-term for malignant transformation.
Perception of Executive Order on Medicare Pay for Advanced Practice Providers: A Study of Comments From Medical Professionals
The ability of advanced practice providers (APPs) to practice independently has been a recent topic of discussion among both the medical community and legislatures. Advanced practice provider is an umbrella term that includes physician assistants (PAs) and advanced practice registered nurses, including nurse practitioners (NPs), clinical nurse specialists, certified nurse-midwives, and certified registered nurse anesthetists. Since Congress passed the Balanced Budget Act of 1997, APPs can bill and be paid independently if they are not practicing incident to a physician or in a facility.1 Currently, NPs can practice independently in 27 states and Washington, DC. Physician assistants are required to practice under the supervision of a physician; however, the extent of supervision varies by state.2 Advocates for broadening the scope of practice for APPs argue that NPs and PAs will help to fill the physician deficit, particularly in primary care and rural regions. It has been projected that by 2025, the United States will require an additional 46,000 primary care providers to meet growing medical needs.3
On October 3, 2019, President Donald Trump issued the Executive Order on Protecting and Improving Medicare for Our Nation’s Seniors, in which he proposed an alternative to “Medicare for all.”4 This order instructed the Secretary of Health and Human Services to prepare a regulation that would “eliminate burdensome regulatory billing requirements, conditions of participation, supervision requirements, benefit definitions and all other licensure requirements . . . that are more stringent than applicable Federal or State laws require and that limit professionals from practicing at the top of their field.” Furthermore, President Trump proposed that “services provided by clinicians, including physicians, physician assistants, and nurse practitioners, are appropriately reimbursed in accordance with the work performed rather than the clinician’s occupation.”4
In response to the executive order, members of the medical community utilized Reddit, an online public forum, and Medscape, a medical news website, to vocalize opinions on the executive order.5,6 Our goal was to analyze the characteristics of those who participated in the discussion and their points of view on the plan to broaden the scope of practice and change the Medicare reimbursement plans for APPs.
Methods
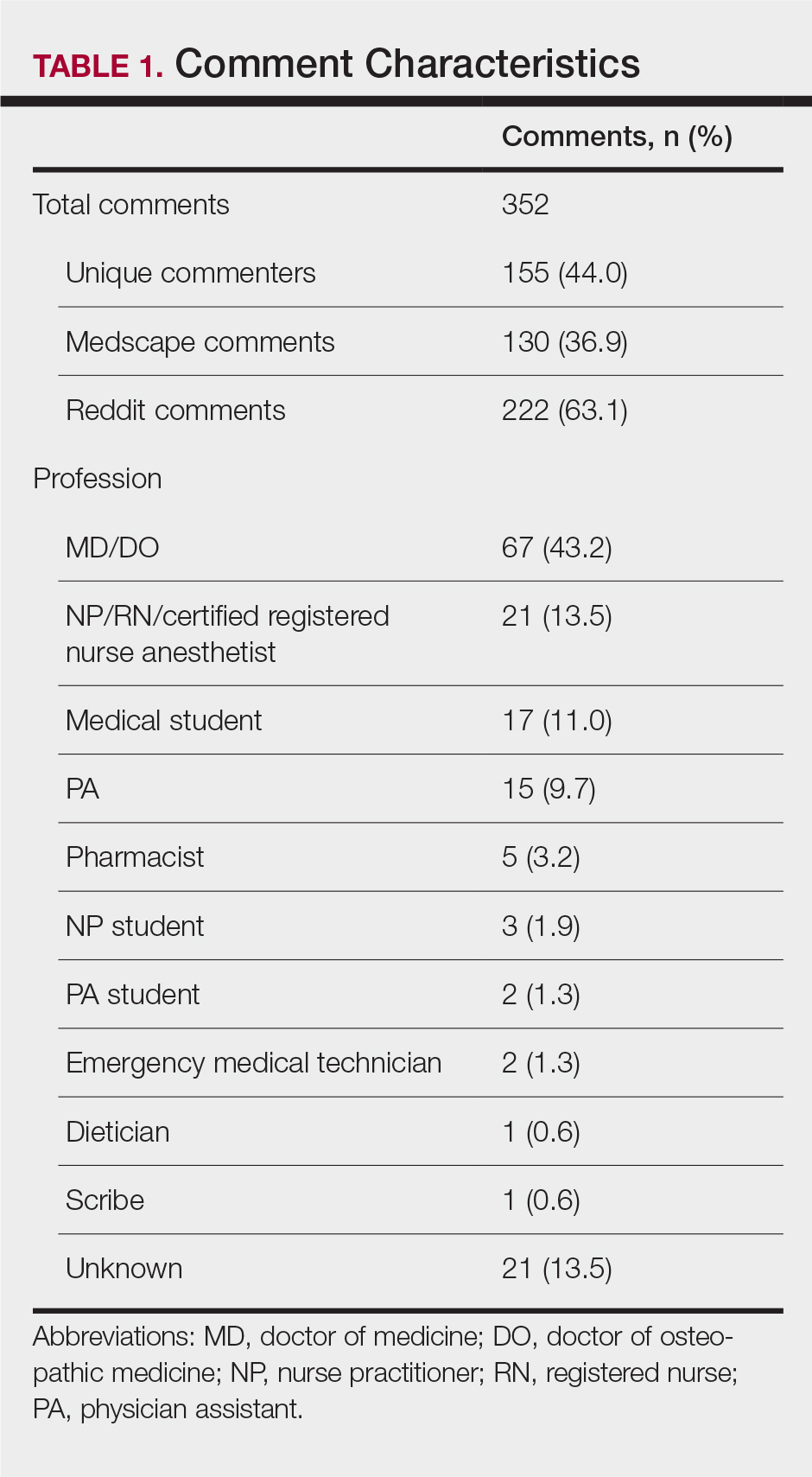
All comments on the October 3, 2019, Medscape article, “Trump Executive Order Seeks Proposals on Medicare Pay for NPs, PAs,”5 and the corresponding Reddit discussion on this article6 were reviewed and characterized by the type of commenter—doctor of medicine (MD)/doctor of osteopathic medicine (DO), NP/RN/certified registered nurse anesthetist, PA, medical student, PA student, NP student, pharmacist, dietician, emergency medical technician, scribe, or unknown—as identified in their username, title, or in the text of the comment. Gender of the commenter was recorded when provided. Commenters were further grouped by their support or lack of support for the executive order based on their comments. Patients’ comments underwent further qualitative analysis to identify general themes.
All analyses were conducted with RStudio statistical software. Analyses were reported as proportions. Variables were compared by χ2 and Fisher exact tests. Odds ratios with 95% CIs were calculated. P<.05 was considered statistically significant.
Results
A total of 352 comments (130 on Medscape and 222 on Reddit) posted by 155 unique users (57 on Medscape and 98 on Reddit) were included in the analysis (Table 1). Of the 51 Medscape commenters who identified a gender, 60.7% were male and 39.2% were female. Reddit commenters did not identify a gender. Commenters included MD and DO physicians (43.2%), NPs/RNs/certified registered nurse anesthetists (13.5%), medical students (11.0%), PAs (9.7%), pharmacists (3.2%), NP students (1.9%), PA students (1.3%), emergency medical technicians (1.3%), dieticians (0.6%), and scribes (0.6%). Physicians (54.5% vs 36.73%; P=.032) and NPs (22.8% vs 8.2%; P=.009) made up a larger percentage of all comments on Medscape compared to Reddit, where medical students were more prevalent (16.3% vs 1.8%; P=.005). Nursing students and PA students more commonly posted on Reddit (4.08% of Reddit commenters vs 1.75% of Medscape commenters), though this difference did not achieve statistical significance.
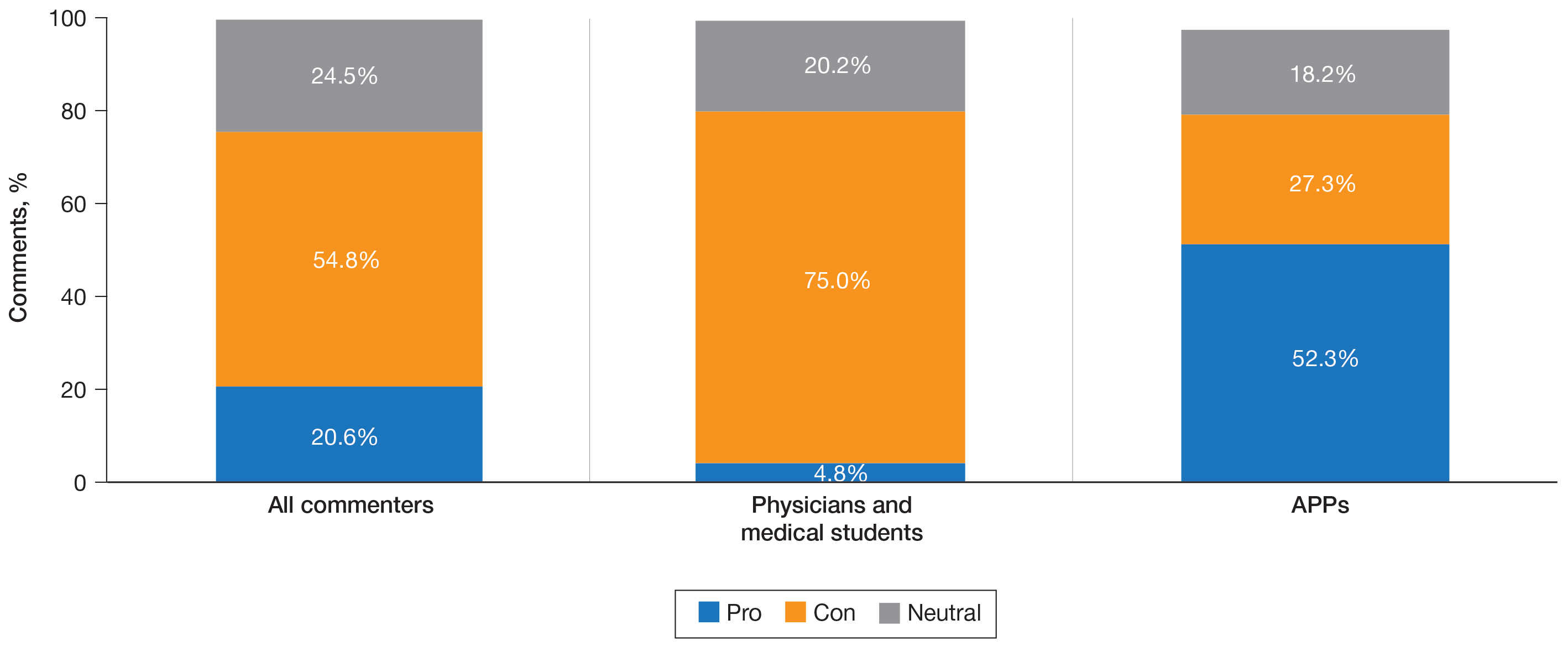
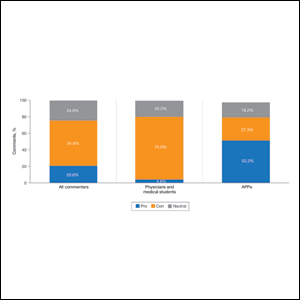
A majority of commenters did not support the executive order, with only 20.6% approving of the plan, 54.8% disapproving, and 24.5% remaining neutral (Figure). Advanced practice providers—NPs, PAs, NP/PA students, and APPs not otherwise specified—were more likely to support the executive order, with 52.3% voicing their support compared to only 4.8% of physicians and medical students expressing support (P<.0001). Similarly, physicians and medical students were more likely to disapprove of the order, with 75.0% voicing concerns compared to only 27.3% of APPs dissenting (P<.0001). A similar percentage of both physicians/medical students and APPs remained neutral (20.2% vs 18.2%). Commenters on Medscape were more likely to voice support for the executive order than those on Reddit (36.8% vs 11.2%; P=.0002), likely due to the higher percentage of NP and PA comments on the former.
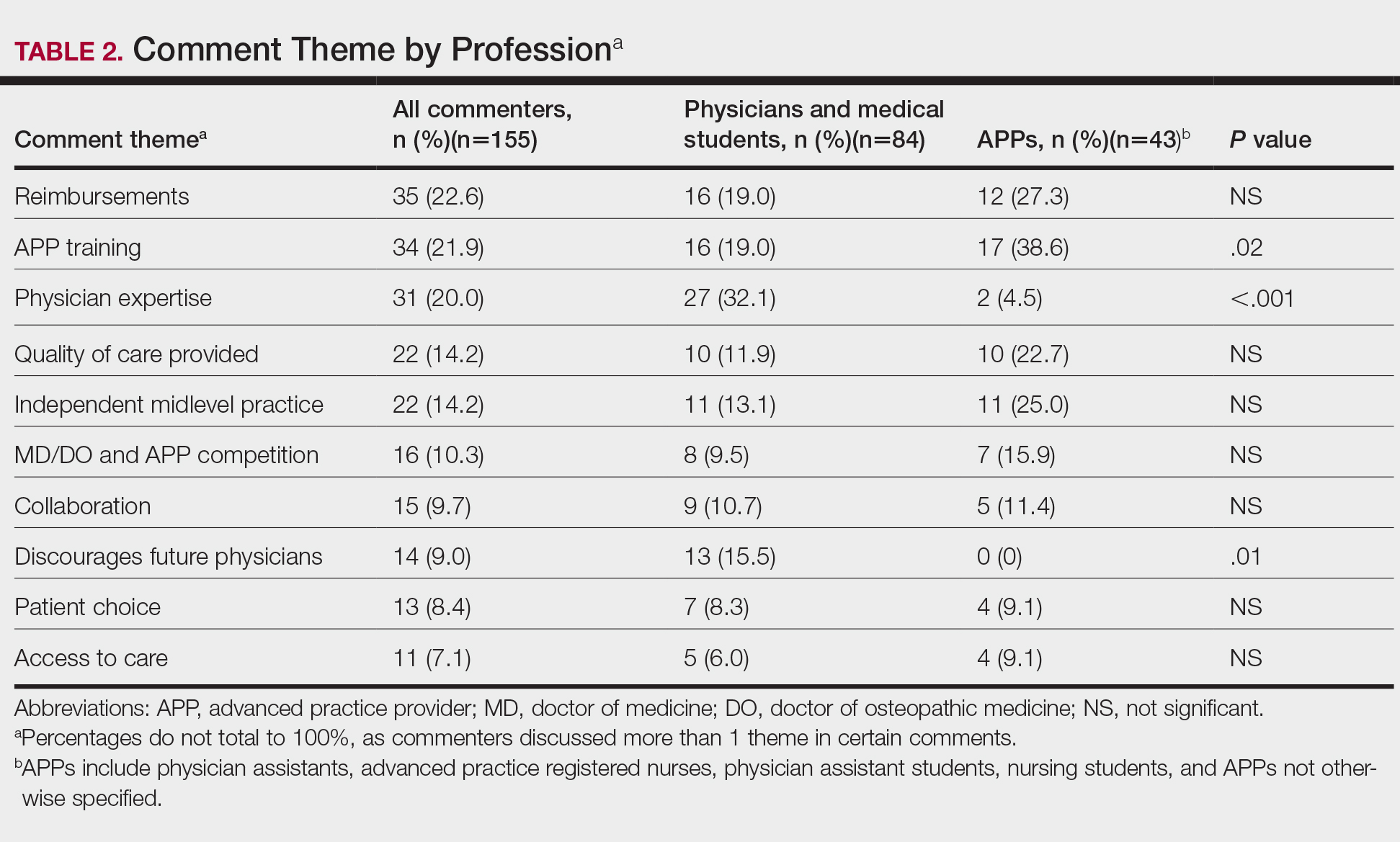
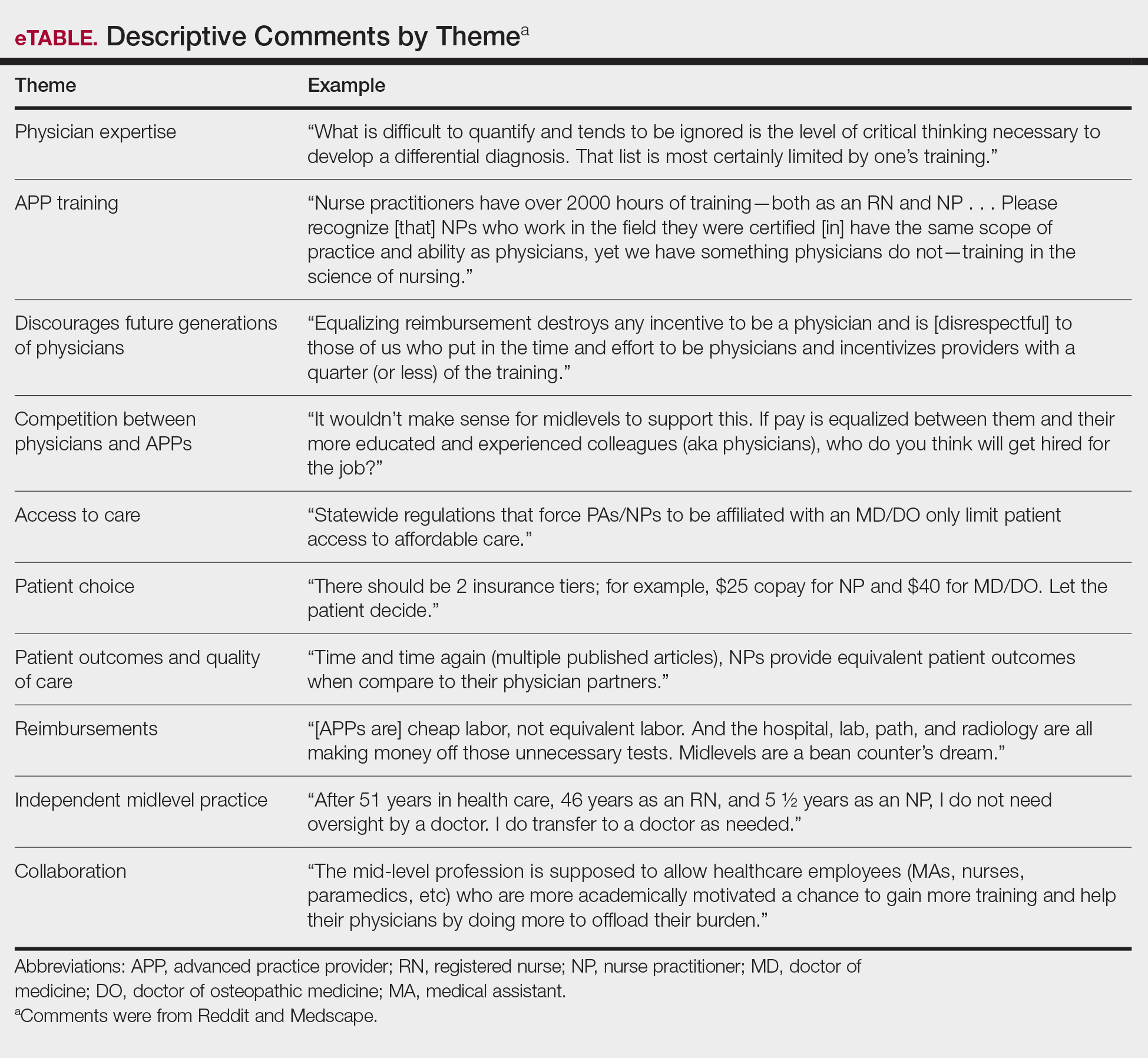
Overall, the most commonly discussed topic was provider reimbursement (22.6% of all comments)(Table 2). Physicians and medical students were more likely to discuss physician expertise compared to APPs (32.1% vs 4.5%; P<.001). They also were more likely to raise concerns that the executive order would discourage future generations of physicians from pursuing medicine (15.5% vs 0%; P=.01). Advanced practice providers were more likely than physicians/medical students to comment on the breadth of NP and/or PA training (38.6% vs 19.0%; P=.02). The eTable shows representative comments for each theme encountered.
A subgroup analysis of the comments written by physicians supporting the executive order (n=4) and APPs disapproving of the order (n=12) was performed to identify the dissenting opinions. Physicians who supported the order discussed the need for improved pay for equal work (n=3), the competency of NP and PA training (n=2), the ability of a practice to generate more profit from APPs (n=1), and possible benefits of APPs providing primary care while MDs perform more specialized care (n=1). Of the APPs who did not support the order, there were 4 PAs, 2 registered nurses, 2 NPs, 2 NP students, and 2 PA students. The most common themes discussed were the differences in APP education and training (n=6), lack of desire for further responsibilities (n=4), and the adequacy of the current scope of practice (n=3).
Comment
President Trump’s executive order follows a trend of decreasing required oversight of APPs; however, this study indicates that these policies would face pushback from many physicians. These results are consistent with a prior study that analyzed 309 comments on an article in The New York Times made by physicians, APPs, patients, and laypeople, in which 24.7% had mistrust of APPs and 14.9% had concerns over APP supervision compared to 9% who supported APP independent practice.7 It is clear that there is a serious divide in opinion that threatens to harm the existing collaborations between physicians and APPs.
Primary Care Coverage With APPs
In the comments analyzed in our study, supporters of the executive order argued that an increase in APPs practicing independently would provide much-needed primary care coverage to patients in underserved regions. However, APPs are instead well represented across most specialties, with a majority in dermatology. Of the 4 million procedures billed independently by APPs in 2012, 54.8% were in the field of dermatology.8 The employment of APPs by dermatologists has grown from 28% of practices in 2005 to 46% in 2014, making this issue of particular importance to our field.9,10
Education and Training of APPs
In our analysis, many physicians cited concerns about the education and training of APPs. Dermatologists receive approximately 10,000 hours of training over the course of residency. Per the American Academy of Physician Assistants, PAs spend more than 2000 hours over a 26-month period on various clinical rotations, “with an emphasis on primary care.”11 There are multiple routes to become an advanced practice RN with varying classroom and clinical requirements, with one pathway requiring a bachelor of science in nursing, followed by a master’s degree requiring 500 to 700 hours of supervised clinical work. Although the Dermatology Nurses’ Association and Society of Dermatology Physician Assistants (http://www.dermpa.org) provide online modules, annual conventions with training workshops, and short fellowship programs, neither have formal guidelines on minimum requirements to diagnose and treat dermatologic conditions.2 Despite the lack of formalized dermatologic training, APPs billed for 13.4% of all dermatology procedures submitted to Medicare in 2015.12
Quality of Patient Care
In our study, physicians also voiced concern over reduced quality of patient care. In a review of 33,647 skin cancer screening examinations, PAs biopsied an average of 39.4 skin lesions, while dermatologists biopsied an average of 25.4 skin lesions to diagnose 1 case of melanoma.13 In addition, nonphysician providers accounted for 37.9% of defendants in 174 legal cases related to injury from cutaneous laser surgery.14 Before further laws are enacted regarding the independent practice and billing by NPs and PAs in the field of dermatology, further research is needed to address patient outcomes and safety.
Limitations
This study was subject to several limitations. Because of a lack of other sources offering discussions on the topic, our sample size was limited. Self-identification of users presents a challenge, as an individual can pose as a physician or APP without validation of credentials. Although great care was taken to minimize bias, grouping comments into broad categories may misinterpret a poster’s intentions. Furthermore, the data collected represent only a small proportion of the medical community—readers of Medscape and Reddit who have the motivation to create a user profile and post a comment rather than put their efforts into lobbying or contacting legislators. Those posting may have stronger political opinions or more poignant experiences than the general public. Although selection bias impacts the generalizability of our findings, this analysis allows for deeper insight into the beliefs of a vocal subset of the medical community who may not have the opportunity to present their opinions elsewhere.
Conclusion
Our analysis of the response to President Trump’s executive order reveals that a rollout of these regulations would be met with strong opposition. On October 29, 2019, more than 100 professional organizations, including the American Medical Association and the American Academy of Dermatology, wrote a letter to the Secretary of Health and Human Services that eloquently echoed the sentiments of the physician commenters in this study: “Scope of practice of health care professionals should be based on standardized, adequate training and demonstrated competence in patient care, not politics. While all health care professionals share an important role in providing care to patients, their skillset is not interchangeable with that of a fully trained physician.”15 The executive order would lead to a major shift in the current medical landscape, and as such, it is prudent that these concerns are addressed.
- Balanced Budget Act of 1997, 42 USC §1395x (1997). Accessed December 15, 2020. https://www.govinfo.gov/content/pkg/PLAW-105publ33/html/PLAW-105publ33.htm
- State practice environment. American Association of Nurse Practitioners. Updated October 20, 2020. Accessed December 8, 2020. https://www.aanp.org/advocacy/state/state-practice-environment
- Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2015. Ann Fam Med. 2012;10:503-509.
- United States, Executive Office of the President [Donald Trump]. Executive Order 13890: Protecting and Improving Medicare for Our Nation’s Seniors. October 3, 2019. Fed Regist. 2019;84:53573-53576.
- Young KD. Trump executive order seeks proposals on Medicare pay for NPs, PAs. Medscape. Published October 3, 2019. Accessed December 8, 2020. https://www.medscape.com/viewarticle/919415
- Trump seeks proposals on Medicare pay for NPs, PAs. Reddit. Accessed December 8, 2020. https://www.reddit.com/r/medicine/comments/ddy03w/trump_seeks_proposals_on_medicare_pay_for_nps_pas/
- Martin E, Huang WW, Strowd LC, et al. Public perception of ethical issues in dermatology: evidenced by New York Times commenters. Dermatol Surg. 2018;44:1571-1577.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14.
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752.
- Become a PA. American Academy of Physician Assistants. Accessed December 8, 2020. https://www.aapa.org/career-central/become-a-pa/.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis of physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- American Medical Association. Open letter to the Honorable Alex M. Azar II. Published October 29, 2019. Accessed December 11, 2020. https://searchlf.ama-assn.org/undefined/documentDownload?uri=%2Funstructured%2Fbinary%2Fletter%2FLETTERS%2F2019-10-29-Final-Sign-on-re-10-3-Executive-Order.pdf
The ability of advanced practice providers (APPs) to practice independently has been a recent topic of discussion among both the medical community and legislatures. Advanced practice provider is an umbrella term that includes physician assistants (PAs) and advanced practice registered nurses, including nurse practitioners (NPs), clinical nurse specialists, certified nurse-midwives, and certified registered nurse anesthetists. Since Congress passed the Balanced Budget Act of 1997, APPs can bill and be paid independently if they are not practicing incident to a physician or in a facility.1 Currently, NPs can practice independently in 27 states and Washington, DC. Physician assistants are required to practice under the supervision of a physician; however, the extent of supervision varies by state.2 Advocates for broadening the scope of practice for APPs argue that NPs and PAs will help to fill the physician deficit, particularly in primary care and rural regions. It has been projected that by 2025, the United States will require an additional 46,000 primary care providers to meet growing medical needs.3
On October 3, 2019, President Donald Trump issued the Executive Order on Protecting and Improving Medicare for Our Nation’s Seniors, in which he proposed an alternative to “Medicare for all.”4 This order instructed the Secretary of Health and Human Services to prepare a regulation that would “eliminate burdensome regulatory billing requirements, conditions of participation, supervision requirements, benefit definitions and all other licensure requirements . . . that are more stringent than applicable Federal or State laws require and that limit professionals from practicing at the top of their field.” Furthermore, President Trump proposed that “services provided by clinicians, including physicians, physician assistants, and nurse practitioners, are appropriately reimbursed in accordance with the work performed rather than the clinician’s occupation.”4
In response to the executive order, members of the medical community utilized Reddit, an online public forum, and Medscape, a medical news website, to vocalize opinions on the executive order.5,6 Our goal was to analyze the characteristics of those who participated in the discussion and their points of view on the plan to broaden the scope of practice and change the Medicare reimbursement plans for APPs.
Methods
All comments on the October 3, 2019, Medscape article, “Trump Executive Order Seeks Proposals on Medicare Pay for NPs, PAs,”5 and the corresponding Reddit discussion on this article6 were reviewed and characterized by the type of commenter—doctor of medicine (MD)/doctor of osteopathic medicine (DO), NP/RN/certified registered nurse anesthetist, PA, medical student, PA student, NP student, pharmacist, dietician, emergency medical technician, scribe, or unknown—as identified in their username, title, or in the text of the comment. Gender of the commenter was recorded when provided. Commenters were further grouped by their support or lack of support for the executive order based on their comments. Patients’ comments underwent further qualitative analysis to identify general themes.
All analyses were conducted with RStudio statistical software. Analyses were reported as proportions. Variables were compared by χ2 and Fisher exact tests. Odds ratios with 95% CIs were calculated. P<.05 was considered statistically significant.
Results
A total of 352 comments (130 on Medscape and 222 on Reddit) posted by 155 unique users (57 on Medscape and 98 on Reddit) were included in the analysis (Table 1). Of the 51 Medscape commenters who identified a gender, 60.7% were male and 39.2% were female. Reddit commenters did not identify a gender. Commenters included MD and DO physicians (43.2%), NPs/RNs/certified registered nurse anesthetists (13.5%), medical students (11.0%), PAs (9.7%), pharmacists (3.2%), NP students (1.9%), PA students (1.3%), emergency medical technicians (1.3%), dieticians (0.6%), and scribes (0.6%). Physicians (54.5% vs 36.73%; P=.032) and NPs (22.8% vs 8.2%; P=.009) made up a larger percentage of all comments on Medscape compared to Reddit, where medical students were more prevalent (16.3% vs 1.8%; P=.005). Nursing students and PA students more commonly posted on Reddit (4.08% of Reddit commenters vs 1.75% of Medscape commenters), though this difference did not achieve statistical significance.
A majority of commenters did not support the executive order, with only 20.6% approving of the plan, 54.8% disapproving, and 24.5% remaining neutral (Figure). Advanced practice providers—NPs, PAs, NP/PA students, and APPs not otherwise specified—were more likely to support the executive order, with 52.3% voicing their support compared to only 4.8% of physicians and medical students expressing support (P<.0001). Similarly, physicians and medical students were more likely to disapprove of the order, with 75.0% voicing concerns compared to only 27.3% of APPs dissenting (P<.0001). A similar percentage of both physicians/medical students and APPs remained neutral (20.2% vs 18.2%). Commenters on Medscape were more likely to voice support for the executive order than those on Reddit (36.8% vs 11.2%; P=.0002), likely due to the higher percentage of NP and PA comments on the former.
Overall, the most commonly discussed topic was provider reimbursement (22.6% of all comments)(Table 2). Physicians and medical students were more likely to discuss physician expertise compared to APPs (32.1% vs 4.5%; P<.001). They also were more likely to raise concerns that the executive order would discourage future generations of physicians from pursuing medicine (15.5% vs 0%; P=.01). Advanced practice providers were more likely than physicians/medical students to comment on the breadth of NP and/or PA training (38.6% vs 19.0%; P=.02). The eTable shows representative comments for each theme encountered.
A subgroup analysis of the comments written by physicians supporting the executive order (n=4) and APPs disapproving of the order (n=12) was performed to identify the dissenting opinions. Physicians who supported the order discussed the need for improved pay for equal work (n=3), the competency of NP and PA training (n=2), the ability of a practice to generate more profit from APPs (n=1), and possible benefits of APPs providing primary care while MDs perform more specialized care (n=1). Of the APPs who did not support the order, there were 4 PAs, 2 registered nurses, 2 NPs, 2 NP students, and 2 PA students. The most common themes discussed were the differences in APP education and training (n=6), lack of desire for further responsibilities (n=4), and the adequacy of the current scope of practice (n=3).
Comment
President Trump’s executive order follows a trend of decreasing required oversight of APPs; however, this study indicates that these policies would face pushback from many physicians. These results are consistent with a prior study that analyzed 309 comments on an article in The New York Times made by physicians, APPs, patients, and laypeople, in which 24.7% had mistrust of APPs and 14.9% had concerns over APP supervision compared to 9% who supported APP independent practice.7 It is clear that there is a serious divide in opinion that threatens to harm the existing collaborations between physicians and APPs.
Primary Care Coverage With APPs
In the comments analyzed in our study, supporters of the executive order argued that an increase in APPs practicing independently would provide much-needed primary care coverage to patients in underserved regions. However, APPs are instead well represented across most specialties, with a majority in dermatology. Of the 4 million procedures billed independently by APPs in 2012, 54.8% were in the field of dermatology.8 The employment of APPs by dermatologists has grown from 28% of practices in 2005 to 46% in 2014, making this issue of particular importance to our field.9,10
Education and Training of APPs
In our analysis, many physicians cited concerns about the education and training of APPs. Dermatologists receive approximately 10,000 hours of training over the course of residency. Per the American Academy of Physician Assistants, PAs spend more than 2000 hours over a 26-month period on various clinical rotations, “with an emphasis on primary care.”11 There are multiple routes to become an advanced practice RN with varying classroom and clinical requirements, with one pathway requiring a bachelor of science in nursing, followed by a master’s degree requiring 500 to 700 hours of supervised clinical work. Although the Dermatology Nurses’ Association and Society of Dermatology Physician Assistants (http://www.dermpa.org) provide online modules, annual conventions with training workshops, and short fellowship programs, neither have formal guidelines on minimum requirements to diagnose and treat dermatologic conditions.2 Despite the lack of formalized dermatologic training, APPs billed for 13.4% of all dermatology procedures submitted to Medicare in 2015.12
Quality of Patient Care
In our study, physicians also voiced concern over reduced quality of patient care. In a review of 33,647 skin cancer screening examinations, PAs biopsied an average of 39.4 skin lesions, while dermatologists biopsied an average of 25.4 skin lesions to diagnose 1 case of melanoma.13 In addition, nonphysician providers accounted for 37.9% of defendants in 174 legal cases related to injury from cutaneous laser surgery.14 Before further laws are enacted regarding the independent practice and billing by NPs and PAs in the field of dermatology, further research is needed to address patient outcomes and safety.
Limitations
This study was subject to several limitations. Because of a lack of other sources offering discussions on the topic, our sample size was limited. Self-identification of users presents a challenge, as an individual can pose as a physician or APP without validation of credentials. Although great care was taken to minimize bias, grouping comments into broad categories may misinterpret a poster’s intentions. Furthermore, the data collected represent only a small proportion of the medical community—readers of Medscape and Reddit who have the motivation to create a user profile and post a comment rather than put their efforts into lobbying or contacting legislators. Those posting may have stronger political opinions or more poignant experiences than the general public. Although selection bias impacts the generalizability of our findings, this analysis allows for deeper insight into the beliefs of a vocal subset of the medical community who may not have the opportunity to present their opinions elsewhere.
Conclusion
Our analysis of the response to President Trump’s executive order reveals that a rollout of these regulations would be met with strong opposition. On October 29, 2019, more than 100 professional organizations, including the American Medical Association and the American Academy of Dermatology, wrote a letter to the Secretary of Health and Human Services that eloquently echoed the sentiments of the physician commenters in this study: “Scope of practice of health care professionals should be based on standardized, adequate training and demonstrated competence in patient care, not politics. While all health care professionals share an important role in providing care to patients, their skillset is not interchangeable with that of a fully trained physician.”15 The executive order would lead to a major shift in the current medical landscape, and as such, it is prudent that these concerns are addressed.
The ability of advanced practice providers (APPs) to practice independently has been a recent topic of discussion among both the medical community and legislatures. Advanced practice provider is an umbrella term that includes physician assistants (PAs) and advanced practice registered nurses, including nurse practitioners (NPs), clinical nurse specialists, certified nurse-midwives, and certified registered nurse anesthetists. Since Congress passed the Balanced Budget Act of 1997, APPs can bill and be paid independently if they are not practicing incident to a physician or in a facility.1 Currently, NPs can practice independently in 27 states and Washington, DC. Physician assistants are required to practice under the supervision of a physician; however, the extent of supervision varies by state.2 Advocates for broadening the scope of practice for APPs argue that NPs and PAs will help to fill the physician deficit, particularly in primary care and rural regions. It has been projected that by 2025, the United States will require an additional 46,000 primary care providers to meet growing medical needs.3
On October 3, 2019, President Donald Trump issued the Executive Order on Protecting and Improving Medicare for Our Nation’s Seniors, in which he proposed an alternative to “Medicare for all.”4 This order instructed the Secretary of Health and Human Services to prepare a regulation that would “eliminate burdensome regulatory billing requirements, conditions of participation, supervision requirements, benefit definitions and all other licensure requirements . . . that are more stringent than applicable Federal or State laws require and that limit professionals from practicing at the top of their field.” Furthermore, President Trump proposed that “services provided by clinicians, including physicians, physician assistants, and nurse practitioners, are appropriately reimbursed in accordance with the work performed rather than the clinician’s occupation.”4
In response to the executive order, members of the medical community utilized Reddit, an online public forum, and Medscape, a medical news website, to vocalize opinions on the executive order.5,6 Our goal was to analyze the characteristics of those who participated in the discussion and their points of view on the plan to broaden the scope of practice and change the Medicare reimbursement plans for APPs.
Methods
All comments on the October 3, 2019, Medscape article, “Trump Executive Order Seeks Proposals on Medicare Pay for NPs, PAs,”5 and the corresponding Reddit discussion on this article6 were reviewed and characterized by the type of commenter—doctor of medicine (MD)/doctor of osteopathic medicine (DO), NP/RN/certified registered nurse anesthetist, PA, medical student, PA student, NP student, pharmacist, dietician, emergency medical technician, scribe, or unknown—as identified in their username, title, or in the text of the comment. Gender of the commenter was recorded when provided. Commenters were further grouped by their support or lack of support for the executive order based on their comments. Patients’ comments underwent further qualitative analysis to identify general themes.
All analyses were conducted with RStudio statistical software. Analyses were reported as proportions. Variables were compared by χ2 and Fisher exact tests. Odds ratios with 95% CIs were calculated. P<.05 was considered statistically significant.
Results
A total of 352 comments (130 on Medscape and 222 on Reddit) posted by 155 unique users (57 on Medscape and 98 on Reddit) were included in the analysis (Table 1). Of the 51 Medscape commenters who identified a gender, 60.7% were male and 39.2% were female. Reddit commenters did not identify a gender. Commenters included MD and DO physicians (43.2%), NPs/RNs/certified registered nurse anesthetists (13.5%), medical students (11.0%), PAs (9.7%), pharmacists (3.2%), NP students (1.9%), PA students (1.3%), emergency medical technicians (1.3%), dieticians (0.6%), and scribes (0.6%). Physicians (54.5% vs 36.73%; P=.032) and NPs (22.8% vs 8.2%; P=.009) made up a larger percentage of all comments on Medscape compared to Reddit, where medical students were more prevalent (16.3% vs 1.8%; P=.005). Nursing students and PA students more commonly posted on Reddit (4.08% of Reddit commenters vs 1.75% of Medscape commenters), though this difference did not achieve statistical significance.
A majority of commenters did not support the executive order, with only 20.6% approving of the plan, 54.8% disapproving, and 24.5% remaining neutral (Figure). Advanced practice providers—NPs, PAs, NP/PA students, and APPs not otherwise specified—were more likely to support the executive order, with 52.3% voicing their support compared to only 4.8% of physicians and medical students expressing support (P<.0001). Similarly, physicians and medical students were more likely to disapprove of the order, with 75.0% voicing concerns compared to only 27.3% of APPs dissenting (P<.0001). A similar percentage of both physicians/medical students and APPs remained neutral (20.2% vs 18.2%). Commenters on Medscape were more likely to voice support for the executive order than those on Reddit (36.8% vs 11.2%; P=.0002), likely due to the higher percentage of NP and PA comments on the former.
Overall, the most commonly discussed topic was provider reimbursement (22.6% of all comments)(Table 2). Physicians and medical students were more likely to discuss physician expertise compared to APPs (32.1% vs 4.5%; P<.001). They also were more likely to raise concerns that the executive order would discourage future generations of physicians from pursuing medicine (15.5% vs 0%; P=.01). Advanced practice providers were more likely than physicians/medical students to comment on the breadth of NP and/or PA training (38.6% vs 19.0%; P=.02). The eTable shows representative comments for each theme encountered.
A subgroup analysis of the comments written by physicians supporting the executive order (n=4) and APPs disapproving of the order (n=12) was performed to identify the dissenting opinions. Physicians who supported the order discussed the need for improved pay for equal work (n=3), the competency of NP and PA training (n=2), the ability of a practice to generate more profit from APPs (n=1), and possible benefits of APPs providing primary care while MDs perform more specialized care (n=1). Of the APPs who did not support the order, there were 4 PAs, 2 registered nurses, 2 NPs, 2 NP students, and 2 PA students. The most common themes discussed were the differences in APP education and training (n=6), lack of desire for further responsibilities (n=4), and the adequacy of the current scope of practice (n=3).
Comment
President Trump’s executive order follows a trend of decreasing required oversight of APPs; however, this study indicates that these policies would face pushback from many physicians. These results are consistent with a prior study that analyzed 309 comments on an article in The New York Times made by physicians, APPs, patients, and laypeople, in which 24.7% had mistrust of APPs and 14.9% had concerns over APP supervision compared to 9% who supported APP independent practice.7 It is clear that there is a serious divide in opinion that threatens to harm the existing collaborations between physicians and APPs.
Primary Care Coverage With APPs
In the comments analyzed in our study, supporters of the executive order argued that an increase in APPs practicing independently would provide much-needed primary care coverage to patients in underserved regions. However, APPs are instead well represented across most specialties, with a majority in dermatology. Of the 4 million procedures billed independently by APPs in 2012, 54.8% were in the field of dermatology.8 The employment of APPs by dermatologists has grown from 28% of practices in 2005 to 46% in 2014, making this issue of particular importance to our field.9,10
Education and Training of APPs
In our analysis, many physicians cited concerns about the education and training of APPs. Dermatologists receive approximately 10,000 hours of training over the course of residency. Per the American Academy of Physician Assistants, PAs spend more than 2000 hours over a 26-month period on various clinical rotations, “with an emphasis on primary care.”11 There are multiple routes to become an advanced practice RN with varying classroom and clinical requirements, with one pathway requiring a bachelor of science in nursing, followed by a master’s degree requiring 500 to 700 hours of supervised clinical work. Although the Dermatology Nurses’ Association and Society of Dermatology Physician Assistants (http://www.dermpa.org) provide online modules, annual conventions with training workshops, and short fellowship programs, neither have formal guidelines on minimum requirements to diagnose and treat dermatologic conditions.2 Despite the lack of formalized dermatologic training, APPs billed for 13.4% of all dermatology procedures submitted to Medicare in 2015.12
Quality of Patient Care
In our study, physicians also voiced concern over reduced quality of patient care. In a review of 33,647 skin cancer screening examinations, PAs biopsied an average of 39.4 skin lesions, while dermatologists biopsied an average of 25.4 skin lesions to diagnose 1 case of melanoma.13 In addition, nonphysician providers accounted for 37.9% of defendants in 174 legal cases related to injury from cutaneous laser surgery.14 Before further laws are enacted regarding the independent practice and billing by NPs and PAs in the field of dermatology, further research is needed to address patient outcomes and safety.
Limitations
This study was subject to several limitations. Because of a lack of other sources offering discussions on the topic, our sample size was limited. Self-identification of users presents a challenge, as an individual can pose as a physician or APP without validation of credentials. Although great care was taken to minimize bias, grouping comments into broad categories may misinterpret a poster’s intentions. Furthermore, the data collected represent only a small proportion of the medical community—readers of Medscape and Reddit who have the motivation to create a user profile and post a comment rather than put their efforts into lobbying or contacting legislators. Those posting may have stronger political opinions or more poignant experiences than the general public. Although selection bias impacts the generalizability of our findings, this analysis allows for deeper insight into the beliefs of a vocal subset of the medical community who may not have the opportunity to present their opinions elsewhere.
Conclusion
Our analysis of the response to President Trump’s executive order reveals that a rollout of these regulations would be met with strong opposition. On October 29, 2019, more than 100 professional organizations, including the American Medical Association and the American Academy of Dermatology, wrote a letter to the Secretary of Health and Human Services that eloquently echoed the sentiments of the physician commenters in this study: “Scope of practice of health care professionals should be based on standardized, adequate training and demonstrated competence in patient care, not politics. While all health care professionals share an important role in providing care to patients, their skillset is not interchangeable with that of a fully trained physician.”15 The executive order would lead to a major shift in the current medical landscape, and as such, it is prudent that these concerns are addressed.
- Balanced Budget Act of 1997, 42 USC §1395x (1997). Accessed December 15, 2020. https://www.govinfo.gov/content/pkg/PLAW-105publ33/html/PLAW-105publ33.htm
- State practice environment. American Association of Nurse Practitioners. Updated October 20, 2020. Accessed December 8, 2020. https://www.aanp.org/advocacy/state/state-practice-environment
- Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2015. Ann Fam Med. 2012;10:503-509.
- United States, Executive Office of the President [Donald Trump]. Executive Order 13890: Protecting and Improving Medicare for Our Nation’s Seniors. October 3, 2019. Fed Regist. 2019;84:53573-53576.
- Young KD. Trump executive order seeks proposals on Medicare pay for NPs, PAs. Medscape. Published October 3, 2019. Accessed December 8, 2020. https://www.medscape.com/viewarticle/919415
- Trump seeks proposals on Medicare pay for NPs, PAs. Reddit. Accessed December 8, 2020. https://www.reddit.com/r/medicine/comments/ddy03w/trump_seeks_proposals_on_medicare_pay_for_nps_pas/
- Martin E, Huang WW, Strowd LC, et al. Public perception of ethical issues in dermatology: evidenced by New York Times commenters. Dermatol Surg. 2018;44:1571-1577.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14.
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752.
- Become a PA. American Academy of Physician Assistants. Accessed December 8, 2020. https://www.aapa.org/career-central/become-a-pa/.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis of physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- American Medical Association. Open letter to the Honorable Alex M. Azar II. Published October 29, 2019. Accessed December 11, 2020. https://searchlf.ama-assn.org/undefined/documentDownload?uri=%2Funstructured%2Fbinary%2Fletter%2FLETTERS%2F2019-10-29-Final-Sign-on-re-10-3-Executive-Order.pdf
- Balanced Budget Act of 1997, 42 USC §1395x (1997). Accessed December 15, 2020. https://www.govinfo.gov/content/pkg/PLAW-105publ33/html/PLAW-105publ33.htm
- State practice environment. American Association of Nurse Practitioners. Updated October 20, 2020. Accessed December 8, 2020. https://www.aanp.org/advocacy/state/state-practice-environment
- Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2015. Ann Fam Med. 2012;10:503-509.
- United States, Executive Office of the President [Donald Trump]. Executive Order 13890: Protecting and Improving Medicare for Our Nation’s Seniors. October 3, 2019. Fed Regist. 2019;84:53573-53576.
- Young KD. Trump executive order seeks proposals on Medicare pay for NPs, PAs. Medscape. Published October 3, 2019. Accessed December 8, 2020. https://www.medscape.com/viewarticle/919415
- Trump seeks proposals on Medicare pay for NPs, PAs. Reddit. Accessed December 8, 2020. https://www.reddit.com/r/medicine/comments/ddy03w/trump_seeks_proposals_on_medicare_pay_for_nps_pas/
- Martin E, Huang WW, Strowd LC, et al. Public perception of ethical issues in dermatology: evidenced by New York Times commenters. Dermatol Surg. 2018;44:1571-1577.
- Coldiron B, Ratnarathorn M. Scope of physician procedures independently billed by mid-level providers in the office setting. JAMA Dermatol. 2014;150:1153-1159.
- Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14.
- Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol. 2017;77:746-752.
- Become a PA. American Academy of Physician Assistants. Accessed December 8, 2020. https://www.aapa.org/career-central/become-a-pa/.
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Anderson AM, Matsumoto M, Saul MI, et al. Accuracy of skin cancer diagnosis of physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154:569-573.
- Jalian HR, Jalian CA, Avram MM. Common causes of injury and legal action in laser surgery. JAMA Dermatol. 2013;149:188-193.
- American Medical Association. Open letter to the Honorable Alex M. Azar II. Published October 29, 2019. Accessed December 11, 2020. https://searchlf.ama-assn.org/undefined/documentDownload?uri=%2Funstructured%2Fbinary%2Fletter%2FLETTERS%2F2019-10-29-Final-Sign-on-re-10-3-Executive-Order.pdf
Practice Points
- On October 3, 2019, President Donald Trump issued the Executive Order on Protecting and Improving Medicare for Our Nation’s Seniors, in which he proposed eliminating supervision requirements for advanced practice providers (APPs) and equalizing Medicare reimbursements among APPs and physicians.
- In a review of comments posted on online forums for medical professionals, a majority of medical professionals disapproved of the executive order.
- Advanced practice providers were more likely to support the plan, citing the breadth of their experience, whereas physicians were more likely to disapprove based on their extensive training within their specialty.
Optimizing Patient Positioning During Dermatologic Surgery
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Rural Residency Curricula: Potential Target for Improved Access to Care?
To the Editor:
There is an irrefutable trend toward urban dermatology practice in the United States, leading to growing problems with rural access to care. The provision of rural clinical experiences and telehealth in dermatology residency training might increase the likelihood of trainees establishing a rural practice.
In 2017, the American Academy of Dermatology released an updated statement supporting direct patient access to board-certified dermatologists in an effort to reduce morbidity and mortality associated with skin disease.1 Twenty percent of the US population lives in a rural and medically underserved location, yet these areas remain largely underserved, in part because of an irrefutable trend toward urban dermatology practice.2-4 Successful approaches to improving rural access to dermatology care are poorly defined in the literature.
Several variables have been shown to influence a young physician’s decision to establish a clinical practice in geographically isolated areas, including rural upbringing, longitudinal rural clinical experiences during medical training, and family influences.5 Location of residency training is an additional variable that impacts practice location, though migration following dermatology residency is a complex phenomenon. However, training location does not guarantee retention of dermatology graduates in any particular geographic area.6 Practice incentives and stipends might encourage rural dermatology practice, yet these programs are underfunded. Last, telemedicine in dermatology (including teledermatology and teledermoscopy), though not always an ideal substitute for a live visit, can improve access to care in geographically isolated or underserved areas in general.7-9
Focused recruitment of medical students interested in rural dermatology practice to accredited dermatology residency programs aligned with this goal represents another approach to improve geographic diversity in the field of dermatology. Online access to this information would be useful for both applicants and their mentors.
We assessed viewable online curricula related to rural dermatology and telemedicine experiences at all Accreditation Council for Graduate Medical Education (ACGME)–accredited residency programs. Telemedicine experiences at Veterans Health Administration (VHA) health systems also were assessed.
Methods
This study was exempt from review by the institutional review board at the University of Minnesota (Minneapolis, Minnesota)(IRB #STUDY00004915) because no human subjects were involved. Online curricula of all ACGME-accredited dermatology residency programs in the United States and Puerto Rico were reviewed from November to December 2018. The following information was recorded: specialized “rural-track” training; optional elective time in rural settings; teledermatology training; and teledermoscopy training.
Additionally, population density at each program’s primary location was determined using US Census Bureau data and with consideration to communities contained within particular Metropolitan Statistical Areas (MSAs)(eTable). Data were obtained from the VHA system to assess teledermatology services at VHA locations affiliated with residency programs.
Results
Of 154 dermatology residency programs identified in the United States and Puerto Rico, 142 were accredited at the time of data collection. Fifteen (10%) were based in communities of 50,000 individuals or fewer that were not near a large metropolitan area. One program (<1%) offered a specific rural track. Fifty-six programs (39%) cited optional rotations or clinical electives, or both, that could be utilized for a rural experience. Eighteen (12%) offered teledermatology experiences and 1 (<1%) offered teledermoscopy during training. Fifty-three programs (37%) offered a rotation at a VHA hospital that had an active teledermatology service.
Comment
Program websites are a free and easily accessible means of acquiring relevant information. The paucity of readily available data on rural dermatology and teledermatology opportunities is unfortunate and a detriment to dermatology residency applicants interested in rural practice, which may result in a missed opportunity to foster a true passion for rural medicine. A brief comment on a website can be impactful, leading to a postgraduate year 4 dermatology elective rotation at a prospective fellowship training site or a rural dermatology experience.
The paucity of dermatologists working directly in rural areas has led to development of teledermatology initiatives to reach deeply into underserved regions. One of the largest providers of teledermatology is the VHA, which standardized its teledermatology efforts in 2012 and provides remarkable educational opportunities for dermatology residents. However, many residency program and VHA websites provide no information about the participation of dermatology residents in the provision of teledermatology services.
A limitation of this study is that it is based on online published curricula. Dermatology residency programs with excellent rural curricula that are not published online might exist.
Residency program directors with an interest in geographic diversity are encouraged to provide rural and teledermatology opportunities and to update these offerings on their websites, which is a simple modifiable strategy that can impact the rural dermatology care gap by recruiting students interested in filling this role. These efforts should be studied to determine whether this strategy impacts resident selection as well as whether focused rural and telemedicine exposure during training increases the likelihood of establishing a rural dermatology practice in the future.
- American Academy of Dermatology. Position statement on access to specialty care and direct access to dermatologic care. Revised May 20, 2017. Accessed December 13, 2020. https://server.aad.org/forms/Policies/Uploads/PS/PS-Access%20to%20Specialty%20Care%20and%20Direct%20Access%20to%20Dermatologic%20Care.pdf
- Dill MJ, Salsberg ES. The Complexities of Physician Supply and Demand: Projections Through 2025. Center for Workforce Studies, Association of American Medical Colleges (AAMC); November 2008. Accessed December 13, 2020. http://innovationlabs.com/pa_future/1/background_docs/AAMC%20Complexities%20of%20physician%20demand,%202008.pdf
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002-2014. Telemed J E Health. 2015;21:769-773.
- Armstrong AW, Kwong MW, Ledo L, et al. Practice models and challenges in teledermatology: a study of collective experiences from teledermatologists. PloS One. 2011;6:e28687.
- Lewis H, Becevic M, Myers D, et al. Dermatology ECHO—an innovative solution to address limited access to dermatology expertise. Rural Remote Health. 2018;18:4415.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. JAMA Dermatol. 2012:148:650-651.
To the Editor:
There is an irrefutable trend toward urban dermatology practice in the United States, leading to growing problems with rural access to care. The provision of rural clinical experiences and telehealth in dermatology residency training might increase the likelihood of trainees establishing a rural practice.
In 2017, the American Academy of Dermatology released an updated statement supporting direct patient access to board-certified dermatologists in an effort to reduce morbidity and mortality associated with skin disease.1 Twenty percent of the US population lives in a rural and medically underserved location, yet these areas remain largely underserved, in part because of an irrefutable trend toward urban dermatology practice.2-4 Successful approaches to improving rural access to dermatology care are poorly defined in the literature.
Several variables have been shown to influence a young physician’s decision to establish a clinical practice in geographically isolated areas, including rural upbringing, longitudinal rural clinical experiences during medical training, and family influences.5 Location of residency training is an additional variable that impacts practice location, though migration following dermatology residency is a complex phenomenon. However, training location does not guarantee retention of dermatology graduates in any particular geographic area.6 Practice incentives and stipends might encourage rural dermatology practice, yet these programs are underfunded. Last, telemedicine in dermatology (including teledermatology and teledermoscopy), though not always an ideal substitute for a live visit, can improve access to care in geographically isolated or underserved areas in general.7-9
Focused recruitment of medical students interested in rural dermatology practice to accredited dermatology residency programs aligned with this goal represents another approach to improve geographic diversity in the field of dermatology. Online access to this information would be useful for both applicants and their mentors.
We assessed viewable online curricula related to rural dermatology and telemedicine experiences at all Accreditation Council for Graduate Medical Education (ACGME)–accredited residency programs. Telemedicine experiences at Veterans Health Administration (VHA) health systems also were assessed.
Methods
This study was exempt from review by the institutional review board at the University of Minnesota (Minneapolis, Minnesota)(IRB #STUDY00004915) because no human subjects were involved. Online curricula of all ACGME-accredited dermatology residency programs in the United States and Puerto Rico were reviewed from November to December 2018. The following information was recorded: specialized “rural-track” training; optional elective time in rural settings; teledermatology training; and teledermoscopy training.
Additionally, population density at each program’s primary location was determined using US Census Bureau data and with consideration to communities contained within particular Metropolitan Statistical Areas (MSAs)(eTable). Data were obtained from the VHA system to assess teledermatology services at VHA locations affiliated with residency programs.
Results
Of 154 dermatology residency programs identified in the United States and Puerto Rico, 142 were accredited at the time of data collection. Fifteen (10%) were based in communities of 50,000 individuals or fewer that were not near a large metropolitan area. One program (<1%) offered a specific rural track. Fifty-six programs (39%) cited optional rotations or clinical electives, or both, that could be utilized for a rural experience. Eighteen (12%) offered teledermatology experiences and 1 (<1%) offered teledermoscopy during training. Fifty-three programs (37%) offered a rotation at a VHA hospital that had an active teledermatology service.
Comment
Program websites are a free and easily accessible means of acquiring relevant information. The paucity of readily available data on rural dermatology and teledermatology opportunities is unfortunate and a detriment to dermatology residency applicants interested in rural practice, which may result in a missed opportunity to foster a true passion for rural medicine. A brief comment on a website can be impactful, leading to a postgraduate year 4 dermatology elective rotation at a prospective fellowship training site or a rural dermatology experience.
The paucity of dermatologists working directly in rural areas has led to development of teledermatology initiatives to reach deeply into underserved regions. One of the largest providers of teledermatology is the VHA, which standardized its teledermatology efforts in 2012 and provides remarkable educational opportunities for dermatology residents. However, many residency program and VHA websites provide no information about the participation of dermatology residents in the provision of teledermatology services.
A limitation of this study is that it is based on online published curricula. Dermatology residency programs with excellent rural curricula that are not published online might exist.
Residency program directors with an interest in geographic diversity are encouraged to provide rural and teledermatology opportunities and to update these offerings on their websites, which is a simple modifiable strategy that can impact the rural dermatology care gap by recruiting students interested in filling this role. These efforts should be studied to determine whether this strategy impacts resident selection as well as whether focused rural and telemedicine exposure during training increases the likelihood of establishing a rural dermatology practice in the future.
To the Editor:
There is an irrefutable trend toward urban dermatology practice in the United States, leading to growing problems with rural access to care. The provision of rural clinical experiences and telehealth in dermatology residency training might increase the likelihood of trainees establishing a rural practice.
In 2017, the American Academy of Dermatology released an updated statement supporting direct patient access to board-certified dermatologists in an effort to reduce morbidity and mortality associated with skin disease.1 Twenty percent of the US population lives in a rural and medically underserved location, yet these areas remain largely underserved, in part because of an irrefutable trend toward urban dermatology practice.2-4 Successful approaches to improving rural access to dermatology care are poorly defined in the literature.
Several variables have been shown to influence a young physician’s decision to establish a clinical practice in geographically isolated areas, including rural upbringing, longitudinal rural clinical experiences during medical training, and family influences.5 Location of residency training is an additional variable that impacts practice location, though migration following dermatology residency is a complex phenomenon. However, training location does not guarantee retention of dermatology graduates in any particular geographic area.6 Practice incentives and stipends might encourage rural dermatology practice, yet these programs are underfunded. Last, telemedicine in dermatology (including teledermatology and teledermoscopy), though not always an ideal substitute for a live visit, can improve access to care in geographically isolated or underserved areas in general.7-9
Focused recruitment of medical students interested in rural dermatology practice to accredited dermatology residency programs aligned with this goal represents another approach to improve geographic diversity in the field of dermatology. Online access to this information would be useful for both applicants and their mentors.
We assessed viewable online curricula related to rural dermatology and telemedicine experiences at all Accreditation Council for Graduate Medical Education (ACGME)–accredited residency programs. Telemedicine experiences at Veterans Health Administration (VHA) health systems also were assessed.
Methods
This study was exempt from review by the institutional review board at the University of Minnesota (Minneapolis, Minnesota)(IRB #STUDY00004915) because no human subjects were involved. Online curricula of all ACGME-accredited dermatology residency programs in the United States and Puerto Rico were reviewed from November to December 2018. The following information was recorded: specialized “rural-track” training; optional elective time in rural settings; teledermatology training; and teledermoscopy training.
Additionally, population density at each program’s primary location was determined using US Census Bureau data and with consideration to communities contained within particular Metropolitan Statistical Areas (MSAs)(eTable). Data were obtained from the VHA system to assess teledermatology services at VHA locations affiliated with residency programs.
Results
Of 154 dermatology residency programs identified in the United States and Puerto Rico, 142 were accredited at the time of data collection. Fifteen (10%) were based in communities of 50,000 individuals or fewer that were not near a large metropolitan area. One program (<1%) offered a specific rural track. Fifty-six programs (39%) cited optional rotations or clinical electives, or both, that could be utilized for a rural experience. Eighteen (12%) offered teledermatology experiences and 1 (<1%) offered teledermoscopy during training. Fifty-three programs (37%) offered a rotation at a VHA hospital that had an active teledermatology service.
Comment
Program websites are a free and easily accessible means of acquiring relevant information. The paucity of readily available data on rural dermatology and teledermatology opportunities is unfortunate and a detriment to dermatology residency applicants interested in rural practice, which may result in a missed opportunity to foster a true passion for rural medicine. A brief comment on a website can be impactful, leading to a postgraduate year 4 dermatology elective rotation at a prospective fellowship training site or a rural dermatology experience.
The paucity of dermatologists working directly in rural areas has led to development of teledermatology initiatives to reach deeply into underserved regions. One of the largest providers of teledermatology is the VHA, which standardized its teledermatology efforts in 2012 and provides remarkable educational opportunities for dermatology residents. However, many residency program and VHA websites provide no information about the participation of dermatology residents in the provision of teledermatology services.
A limitation of this study is that it is based on online published curricula. Dermatology residency programs with excellent rural curricula that are not published online might exist.
Residency program directors with an interest in geographic diversity are encouraged to provide rural and teledermatology opportunities and to update these offerings on their websites, which is a simple modifiable strategy that can impact the rural dermatology care gap by recruiting students interested in filling this role. These efforts should be studied to determine whether this strategy impacts resident selection as well as whether focused rural and telemedicine exposure during training increases the likelihood of establishing a rural dermatology practice in the future.
- American Academy of Dermatology. Position statement on access to specialty care and direct access to dermatologic care. Revised May 20, 2017. Accessed December 13, 2020. https://server.aad.org/forms/Policies/Uploads/PS/PS-Access%20to%20Specialty%20Care%20and%20Direct%20Access%20to%20Dermatologic%20Care.pdf
- Dill MJ, Salsberg ES. The Complexities of Physician Supply and Demand: Projections Through 2025. Center for Workforce Studies, Association of American Medical Colleges (AAMC); November 2008. Accessed December 13, 2020. http://innovationlabs.com/pa_future/1/background_docs/AAMC%20Complexities%20of%20physician%20demand,%202008.pdf
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002-2014. Telemed J E Health. 2015;21:769-773.
- Armstrong AW, Kwong MW, Ledo L, et al. Practice models and challenges in teledermatology: a study of collective experiences from teledermatologists. PloS One. 2011;6:e28687.
- Lewis H, Becevic M, Myers D, et al. Dermatology ECHO—an innovative solution to address limited access to dermatology expertise. Rural Remote Health. 2018;18:4415.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. JAMA Dermatol. 2012:148:650-651.
- American Academy of Dermatology. Position statement on access to specialty care and direct access to dermatologic care. Revised May 20, 2017. Accessed December 13, 2020. https://server.aad.org/forms/Policies/Uploads/PS/PS-Access%20to%20Specialty%20Care%20and%20Direct%20Access%20to%20Dermatologic%20Care.pdf
- Dill MJ, Salsberg ES. The Complexities of Physician Supply and Demand: Projections Through 2025. Center for Workforce Studies, Association of American Medical Colleges (AAMC); November 2008. Accessed December 13, 2020. http://innovationlabs.com/pa_future/1/background_docs/AAMC%20Complexities%20of%20physician%20demand,%202008.pdf
- Glazer AM, Rigel DS. Analysis of trends in geographic distribution of US dermatology workforce density. JAMA Dermatol. 2017;153:472-473.
- Yoo JY, Rigel DS. Trends in dermatology: geographic density of US dermatologists. Arch Dermatol. 2010;146:779.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002-2014. Telemed J E Health. 2015;21:769-773.
- Armstrong AW, Kwong MW, Ledo L, et al. Practice models and challenges in teledermatology: a study of collective experiences from teledermatologists. PloS One. 2011;6:e28687.
- Lewis H, Becevic M, Myers D, et al. Dermatology ECHO—an innovative solution to address limited access to dermatology expertise. Rural Remote Health. 2018;18:4415.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. JAMA Dermatol. 2012:148:650-651.
Practice Points
- Access to dermatologic care in rural areas is a growing problem.
- Dermatology residency programs can influence medical students and resident dermatologists to provide care in rural and geographically isolated areas.
- Presenting detailed curricula that impact access to care on residency program websites could attract applicants with these career goals.
Skin Cancer Screening and Prevention During the COVID-19 Pandemic
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
Practice Points
- It is important for dermatologists to maintain skin cancer screening and prevention efforts during the coronavirus disease 2019 pandemic.
- Patient populations at increased risk for skin cancer should be prioritized for in-person evaluations, but teledermatology should be considered for initial examination in new patients and patients at average risk for skin cancer.
- Teledermatology presents a learning curve for dermatologists and patients, but the confidence level will increase, and evidence-based data will pave the way to enhance this experience.
Aquatic Antagonists: Sponge Dermatitis
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.
Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Practice Points
- Sponges exist in both marine and freshwater environments throughout the world.
- Immediate management of sponge dermatitis should include decontamination by removing the sponge spicules with tape or rubber cement followed by dilute vinegar soaks.
- Topical steroids may be used only after initial decontamination. Use of oral steroids may be needed for more severe reactions.
Skin Cancer in the US Military
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
Practice Points
- An increased risk for melanoma and keratinocyte carcinomas has been identified in those who have served in the US Military.
- UV radiation exposure, low utilization of sun-protective strategies, and low overall education regarding the risks of UV exposure appear to be the primary contributors to increased risks of skin cancer in this population.
- Improving education for military servicemembers on the risks of UV exposure, increasing utilization of sun-protective clothing, and improving access and utilization of sunscreen are viable options to decrease the risk for cutaneous malignancies in US Military servicemembers.
Unilateral Alar Ulceration
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
A 68-year-old man presented with a new left nasal alar ulcer following a recent episode of primary herpes zoster. Physical examination revealed erythema, erosion, and necrosis of the left naris with partial loss of the alar rim. Additional erythema was present without vesicles around the left eye and on the forehead.
Telangiectatic Patch on the Forehead
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.