User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
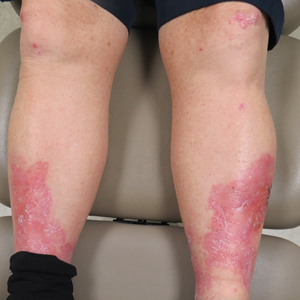
Erythema, Blisters, and Scars on the Elbows, Knees, and Legs
The Diagnosis: Epidermolysis Bullosa Acquisita
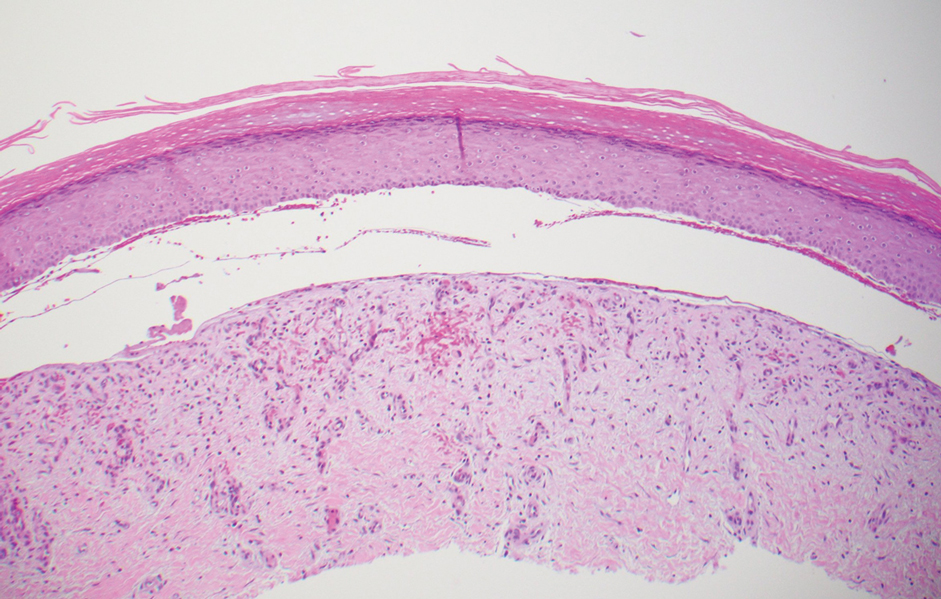
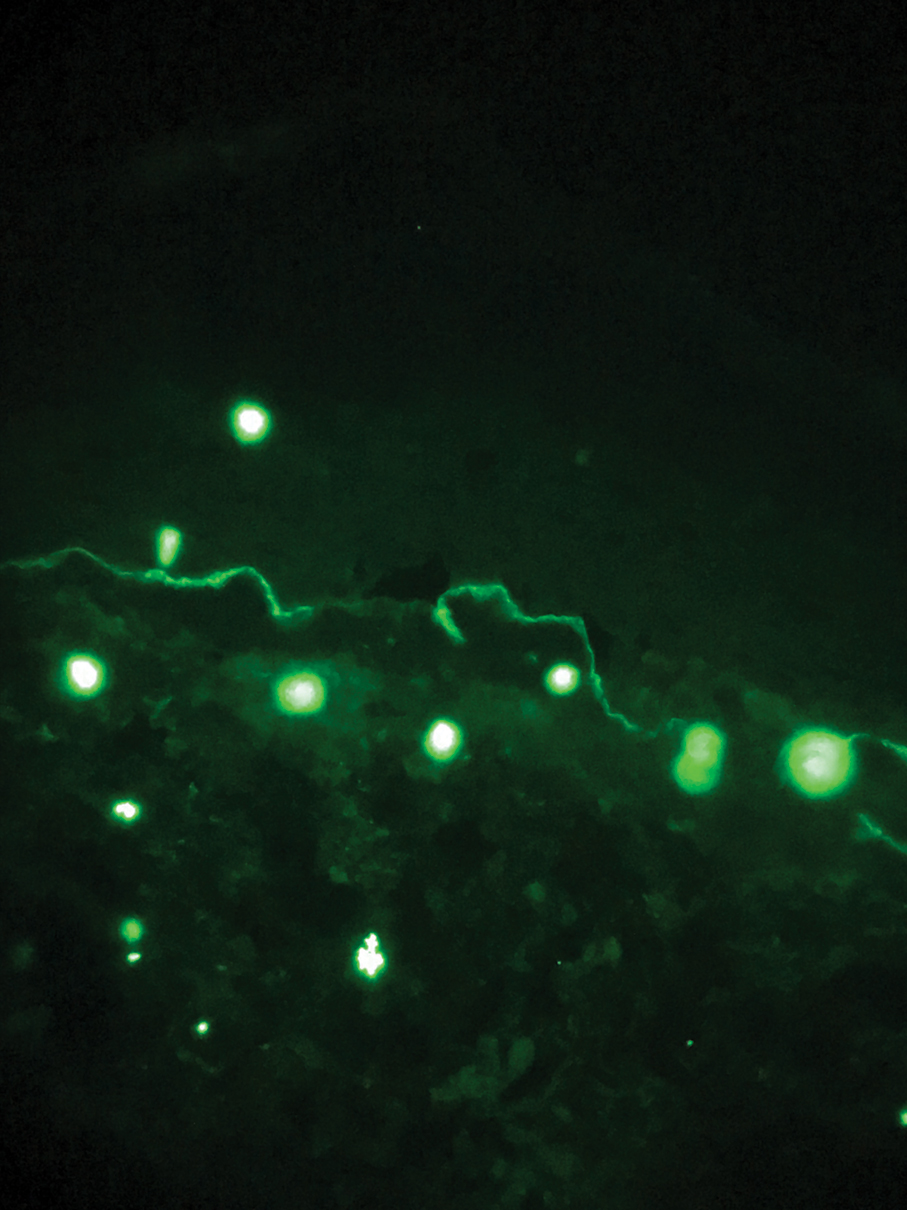
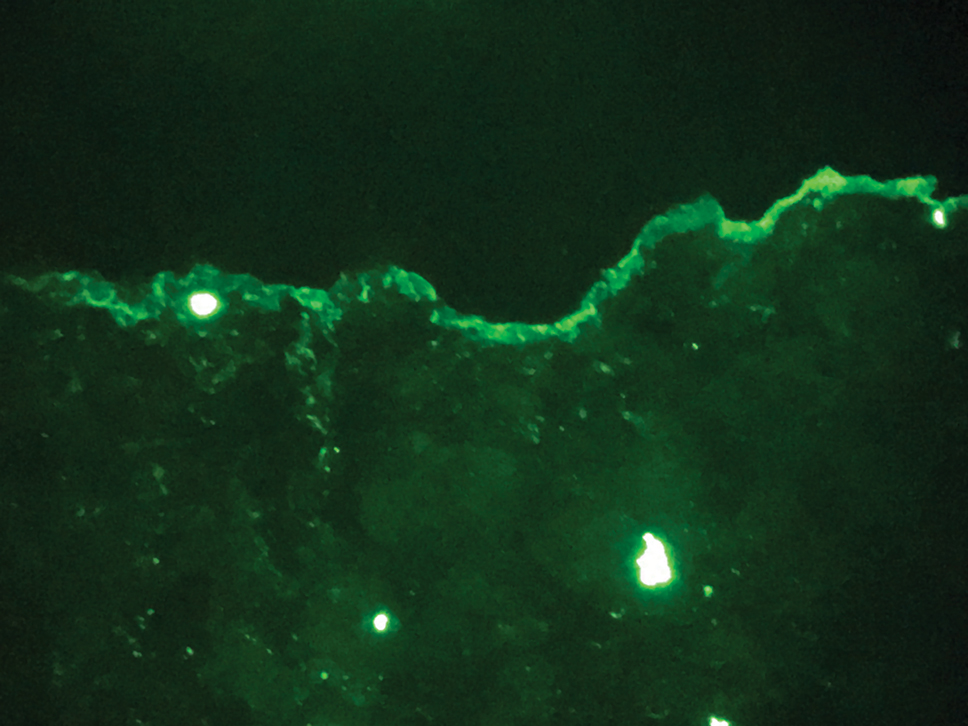
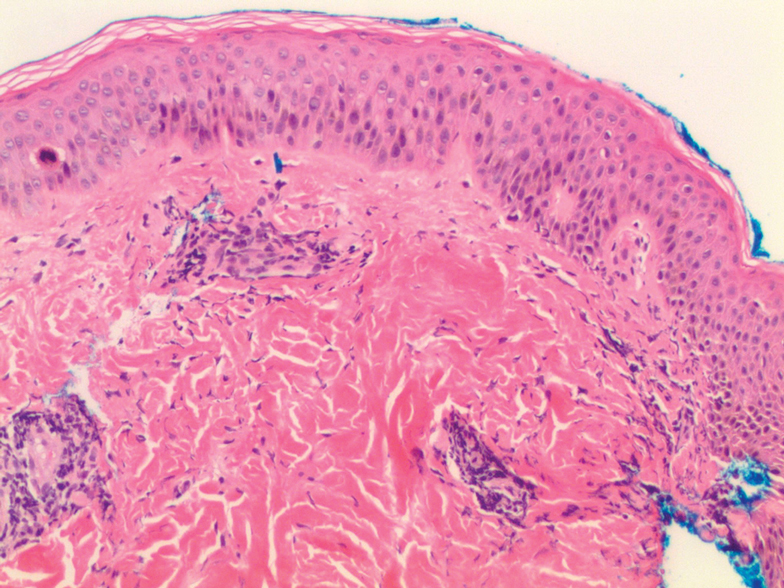
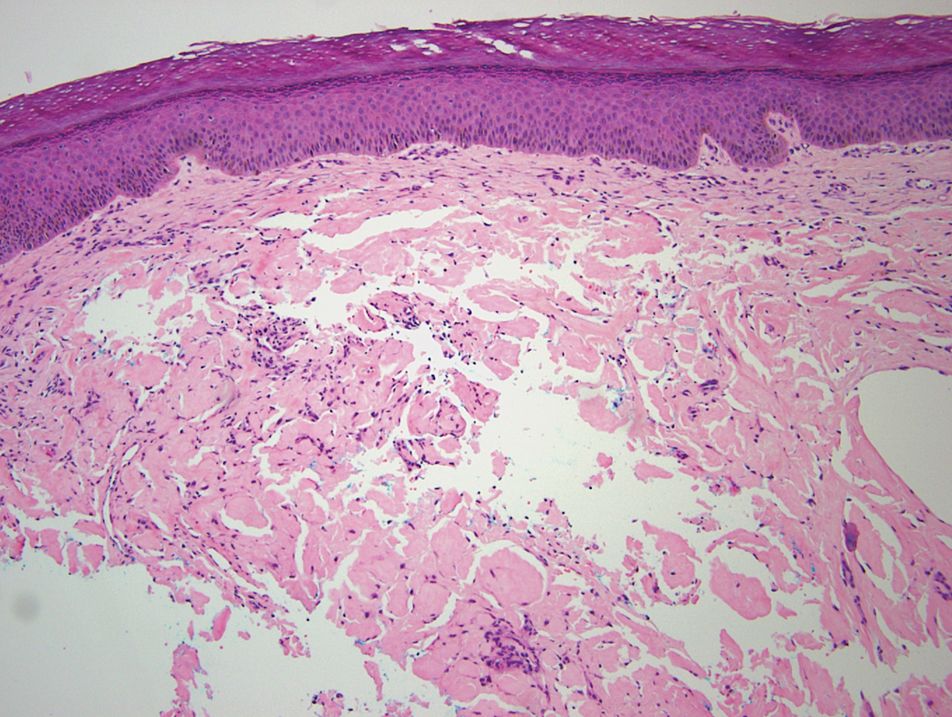
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
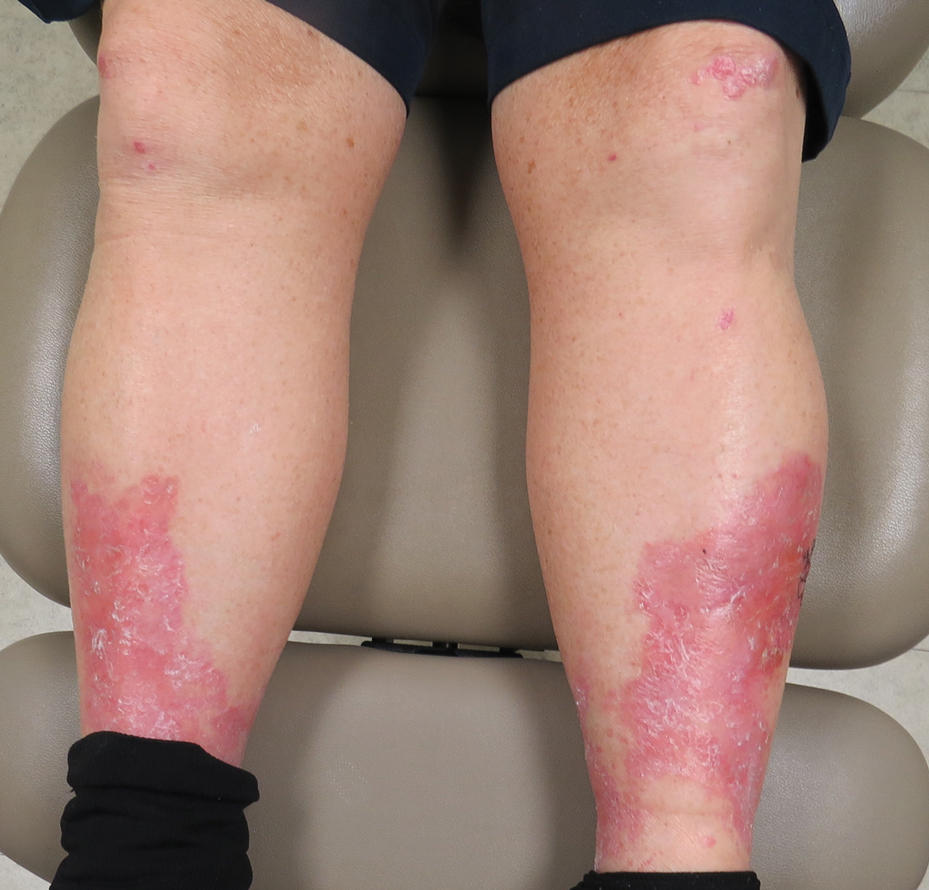
A 69-year-old man presented with an asymptomatic rash on the extensor surfaces of 2 years' duration. He reported recurrent blisters that would then scar over. The lesions did not occur in relation to any known trauma. The patient's medical history revealed dialysis-dependent end-stage renal disease secondary to type 2 diabetes mellitus. His medications were noncontributory, and there was no family history of blistering disorders. He had tried triamcinolone cream without any improvement. Physical examination was remarkable for erythematous blisters and bullae with scales and milia on the elbows, knees, and lower legs. The oral mucosa was unremarkable. Shave biopsies of the skin for direct immunofluorescence and salt-split skin studies were obtained.
Cutaneous Insulin-Derived Amyloidosis Presenting as Hyperkeratotic Nodules
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
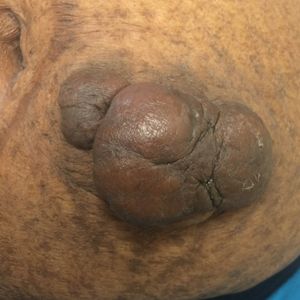
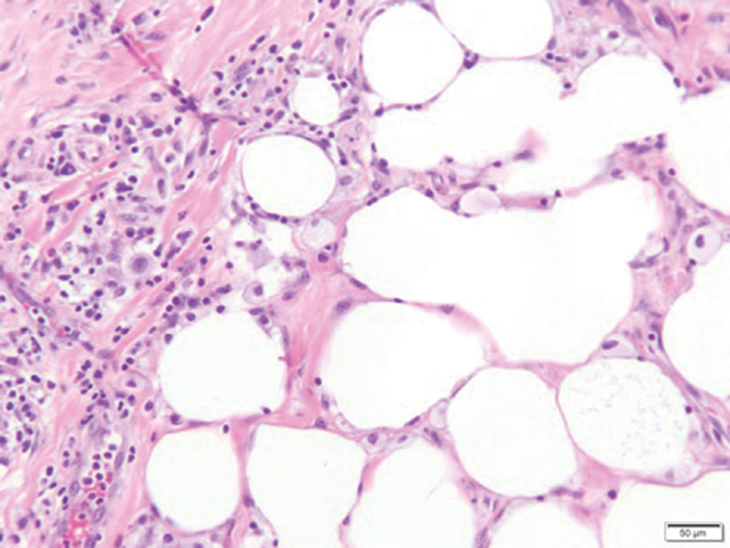
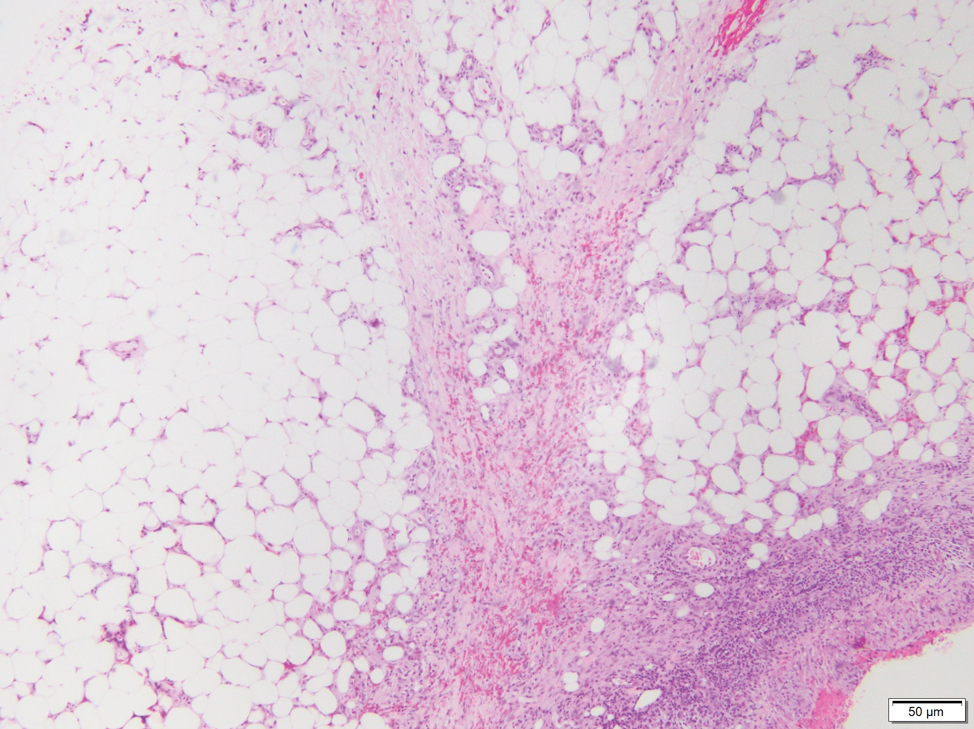
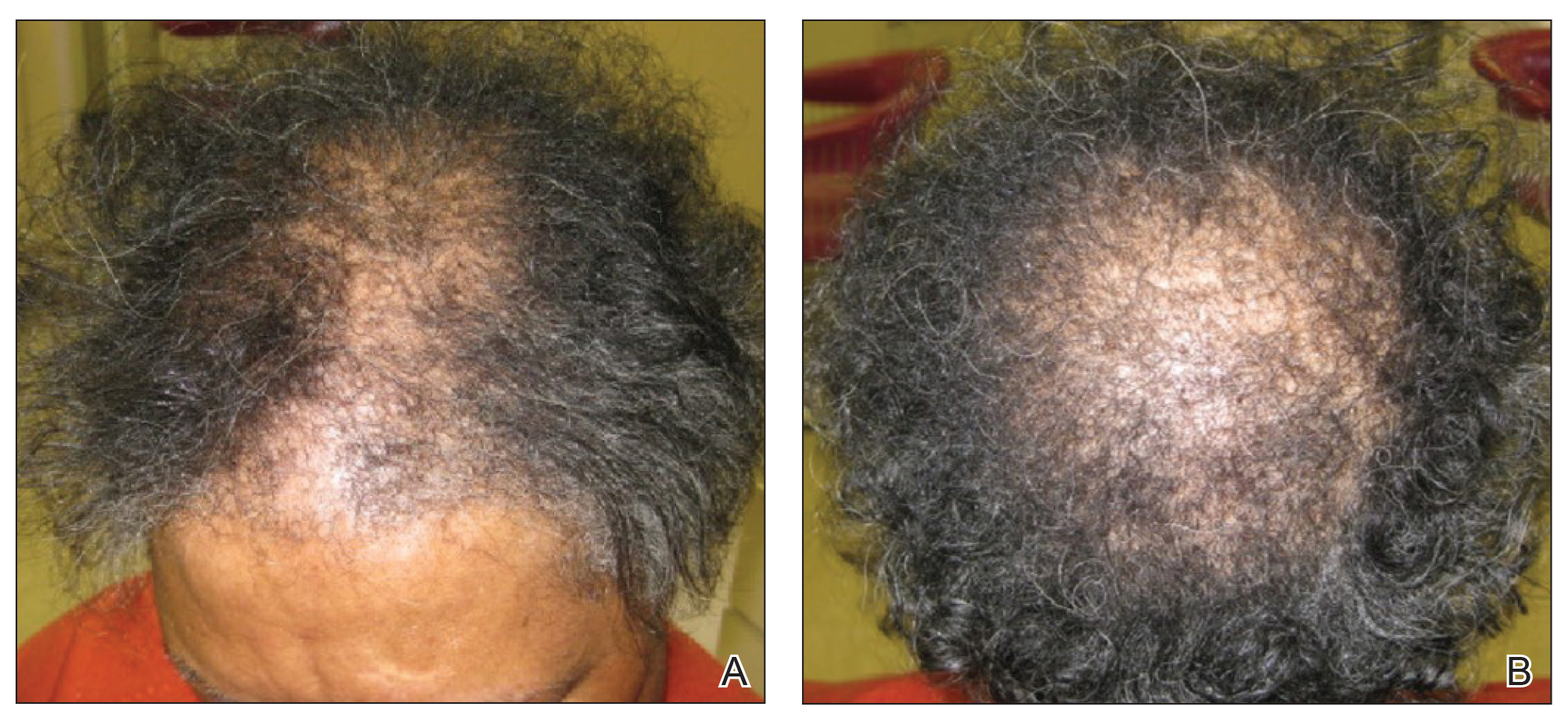
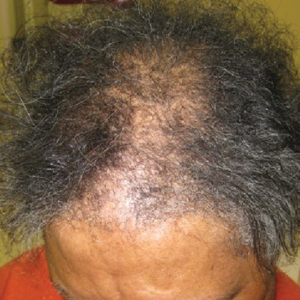
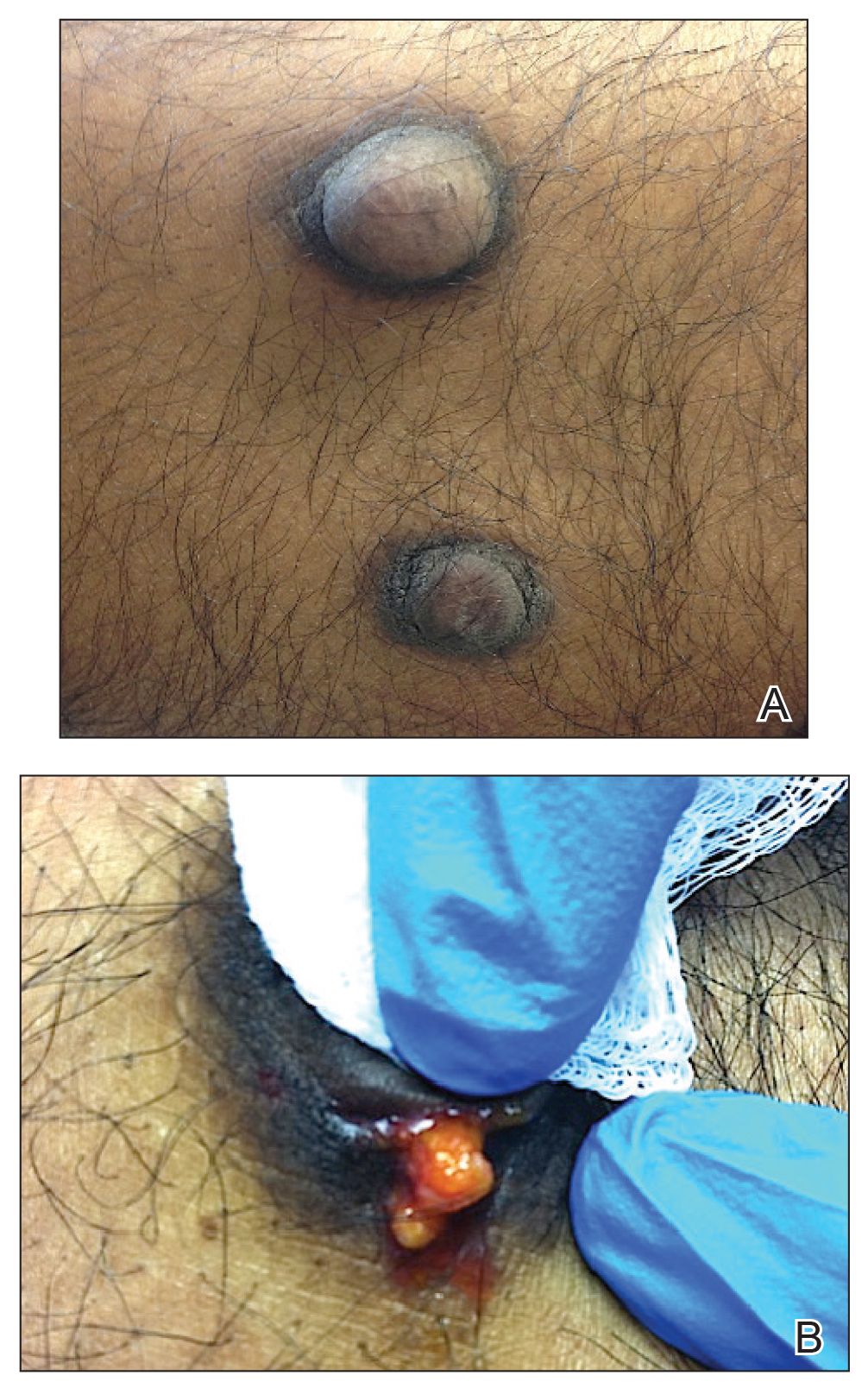
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.
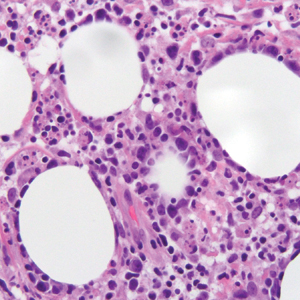
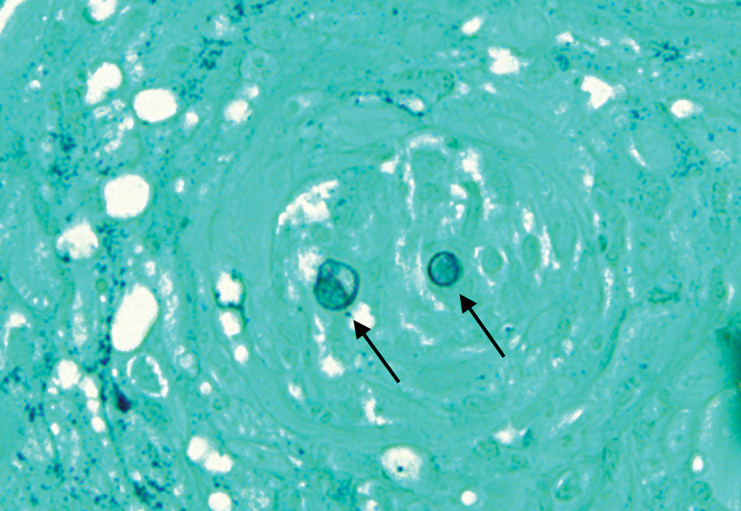
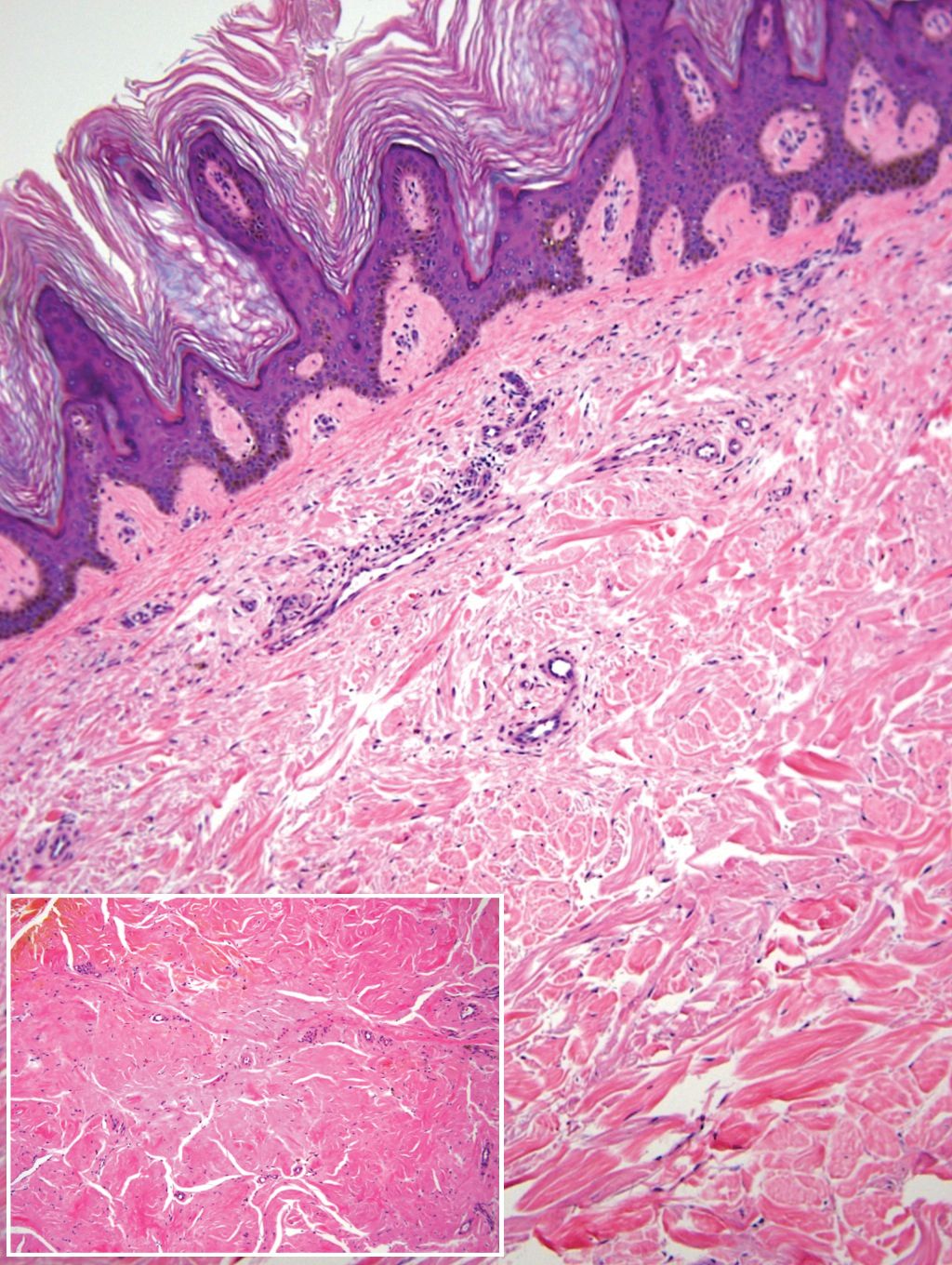
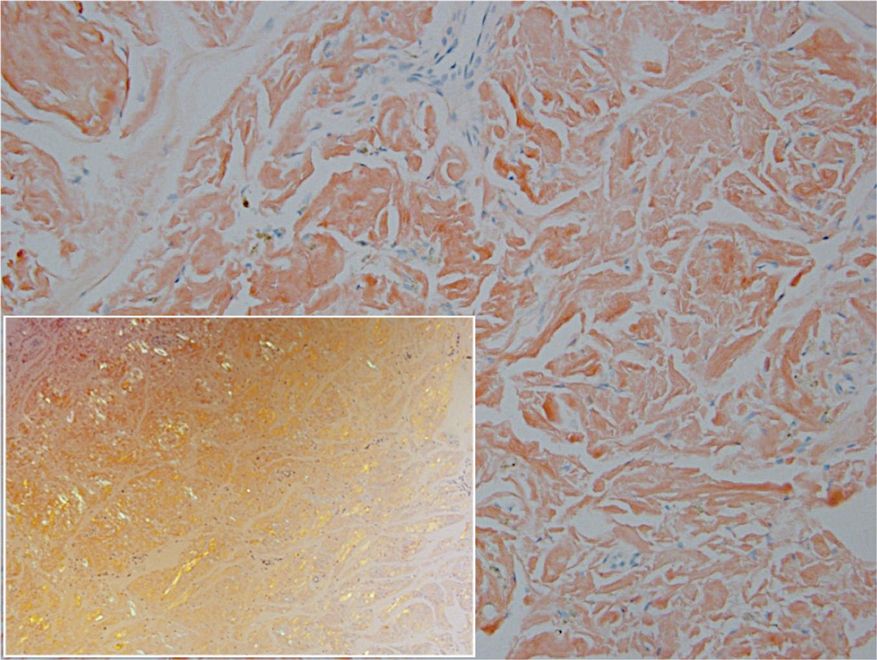
Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.
Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
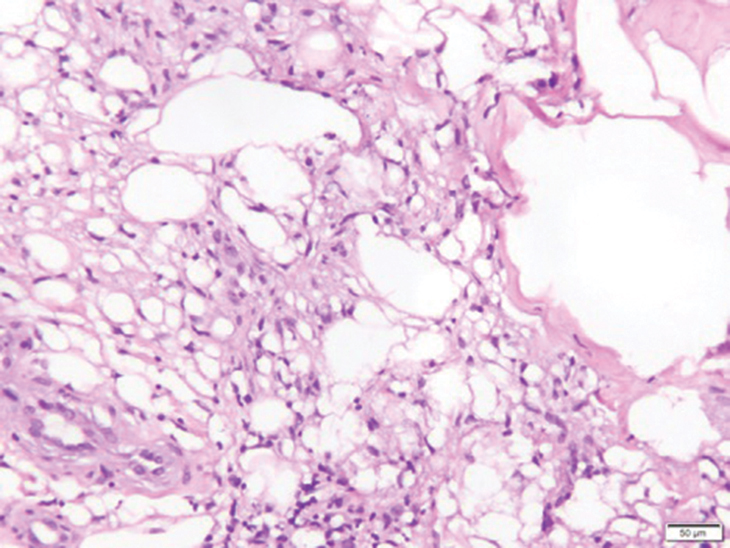
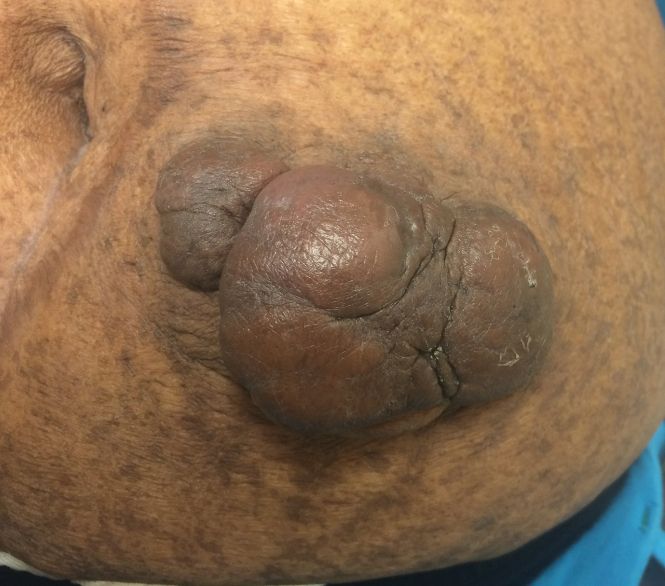
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).
Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.
Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Practice Points
- Deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.
- Patients with insulin-derived (AIns) amyloidosis may initially present after noticing nodular deposits.
- Insulin injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis.
Testosterone Pellet–Induced Generalized Drug Eruption
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
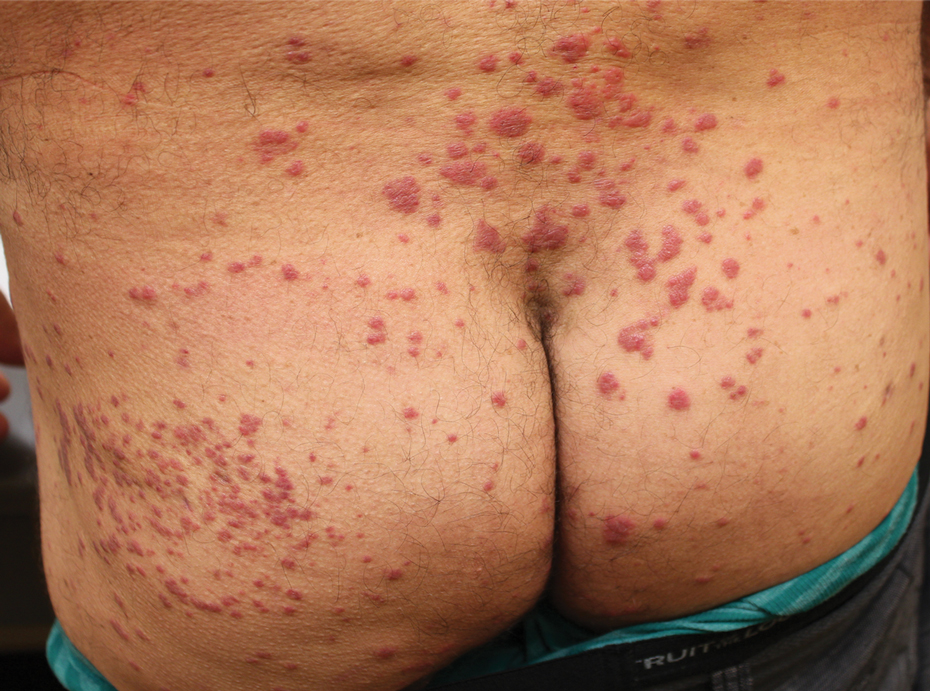
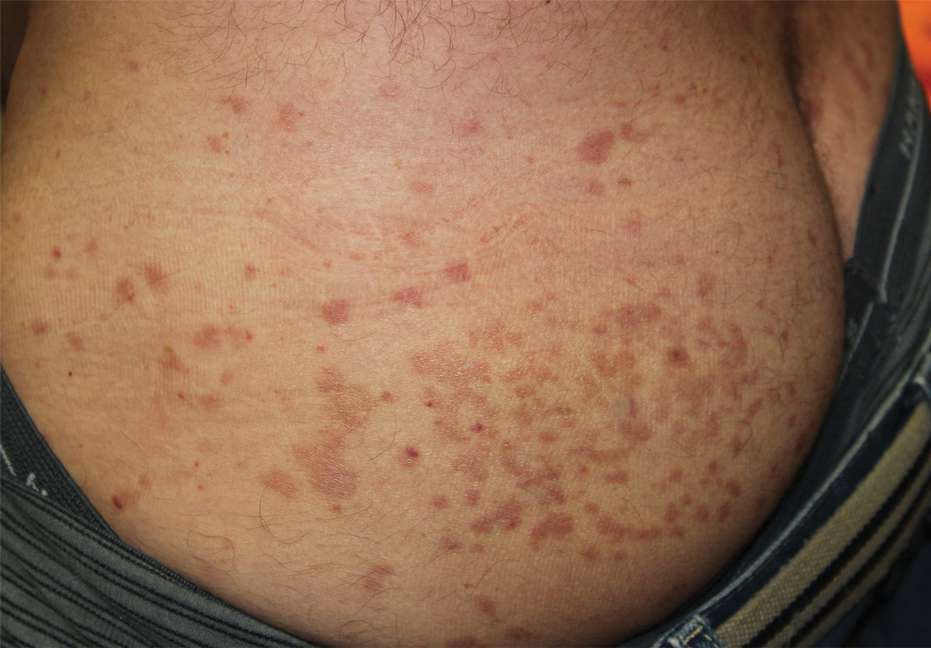
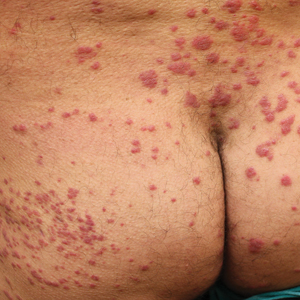
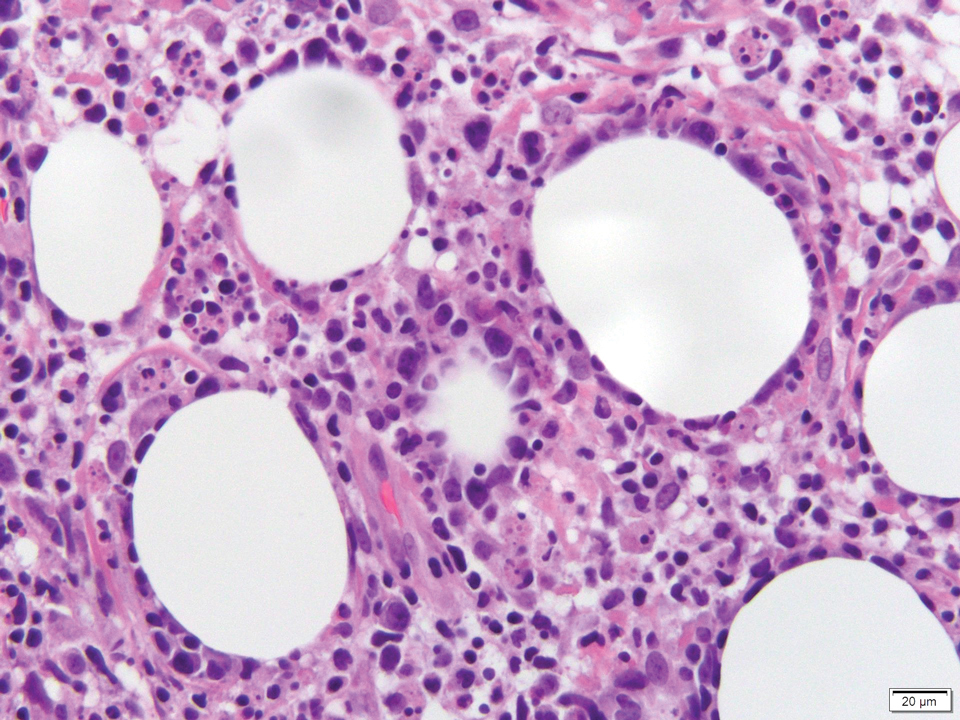
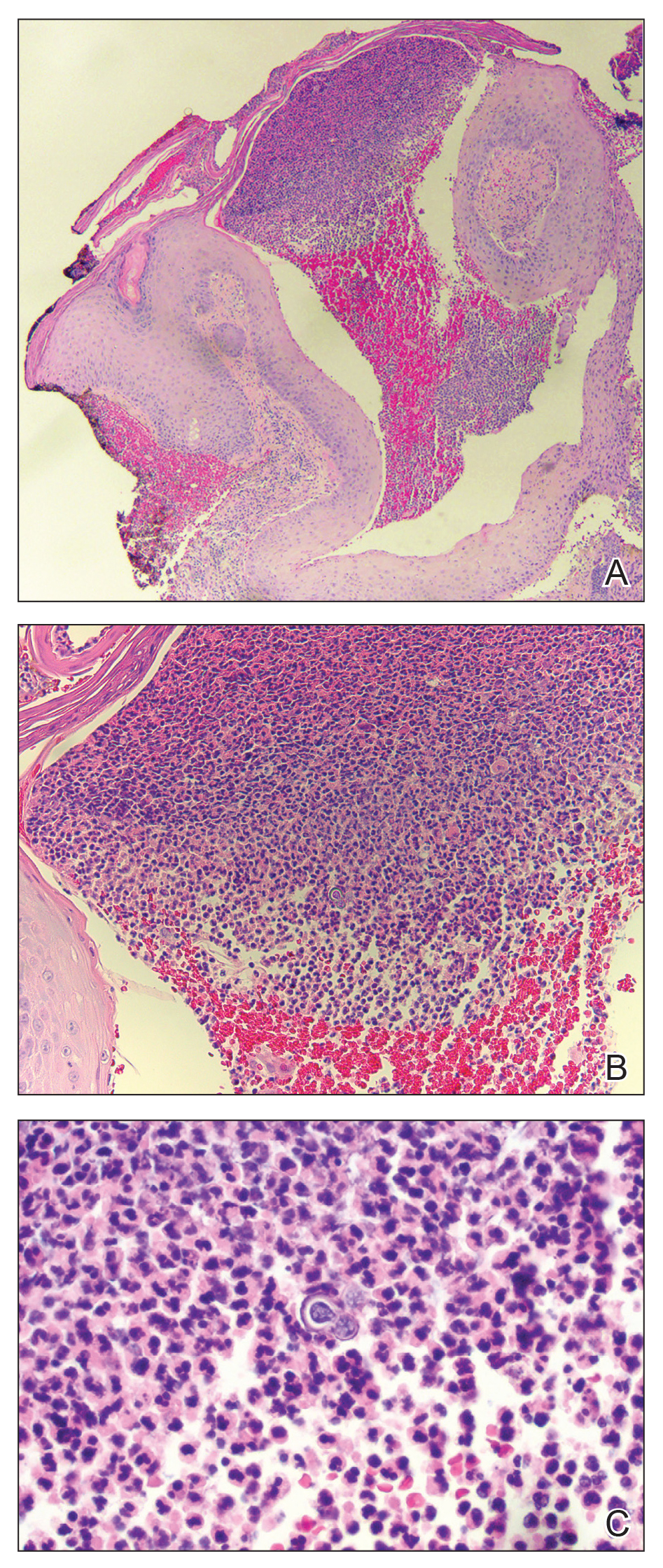
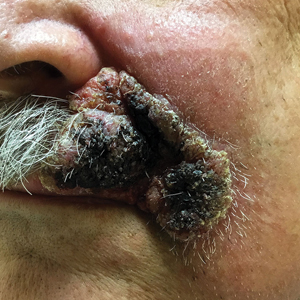
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
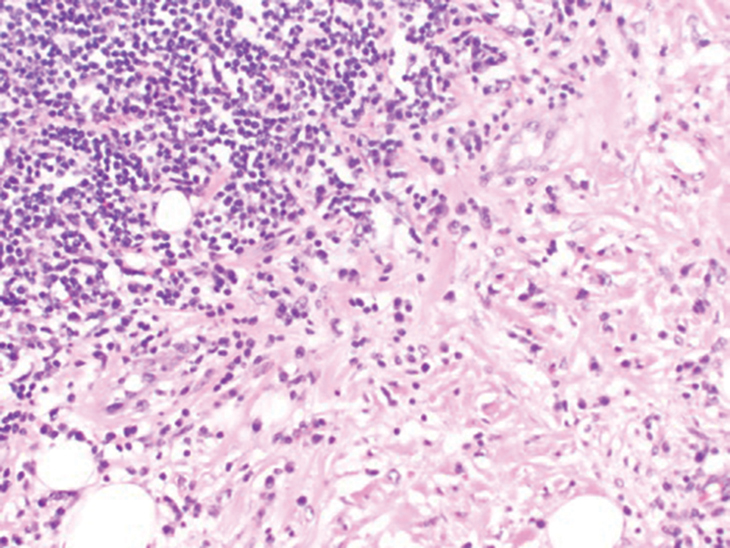
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
Practice Points
- Dermatologists should be aware that testosterone pellet implantation can cause dermatitis overlying the implantation site, which can generalize and differ in morphologic presentation.
- For patients presenting with a suspected case of testosterone pellet–induced dermatitis, a high-dose oral corticosteroid can be deployed as an effective therapy.
Atrophic Lesion on the Abdomen
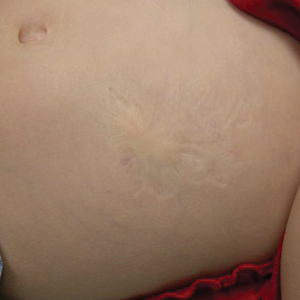
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
An 18-month-old child presented with a 4-cm, atrophic, flesh-colored plaque on the left lateral aspect of the abdomen with overlying wrinkling of the skin. There was no outpouching of the skin or pain associated with the lesion. No other skin abnormalities were noted. The child was born premature at 30 weeks’ gestation (birth weight, 1400 g). The postnatal course was complicated by respiratory distress syndrome requiring prolonged ventilator support. The infant was in the neonatal intensive care unit for 5 months. The atrophic lesion first developed at 5 months of life and remained stable. Although the lesion was not present at birth, the parents noted that it was preceded by an ecchymotic lesion without necrosis that was first noticed at 2 months of life while the patient was in the neonatal intensive care unit.
Tender Soft Tissue Mass on the Base of the Neck
The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6
Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.
Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13
Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16
- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6
Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.
Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13
Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16
The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6
Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.
Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13
Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16
- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
A 47-year-old man presented with a tender soft tissue mass on the upper back with increasing discomfort over the last 4 weeks. He noted that he felt feverish a few times. Physical examination revealed a 3×4-cm area of induration involving the upper mid back with faint erythema of the overlying skin; no drainage was noted. A prominent left posterior cervical lymph node also was appreciated, and a punch biopsy of the mass was performed.
Expanding Verrucous Plaque on the Face
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
A 69-year-old man presented with a slowly expanding, verrucous plaque on the left side of the upper cutaneous lip of 4 months’ duration. The lesion reportedly began as an abscess and had undergone incision and drainage followed by multiple courses of oral antibiotics that were unsuccessful prior to presentation to our clinic. The patient’s hobbies included gardening near his summer home in the mountains of western North Carolina, where he resided when the lesion appeared. Physical examination revealed an approximately 6×4-cm verrucous plaque with central ulceration on the left side of the upper cutaneous and vermilion lip extending to the nasolabial fold. A review of systems was negative for any systemic symptoms. Routine laboratory tests and computed tomography of the head and neck were normal.
High-Grade Ovarian Serous Carcinoma Presenting as Androgenetic Alopecia
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).
Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.
Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).
Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.
Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).
Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.
Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
Practice Points
- Laboratory assessment for possible androgen excess should be performed in patients with female pattern hair loss and include baseline serum total testosterone and dehydroepiandrosterone sulfate.
- Rapid onset or worsening of clinical hyperandrogenism should raise suspicion of malignancy.
- Transvaginal ultrasonography and possible pelvic magnetic resonance imaging are indicated for patients with clinical hyperandrogenism and an isolated testosterone level elevation.
Skin Cancer Management During the COVID-19 Pandemic
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
Practice Points
- Consider the rate of cases and transmission in your area during a pandemic surge when triaging surgical and nonsurgical cases.
- If performing head and neck surgical procedures or cosmetic procedures in which the patient cannot wear a mask, consider testing them 24 to 48 hours before the procedure.
- Follow Centers for Disease Control and Prevention (CDC) guidelines concerning screening asymptomatic patients. Also, follow CDC guidelines on testing patients who have had prior infections.
- Ensure proper personal protective equipment for yourself and staff, including the use of properly fitting N95 respirators and face shields.
Contact Allergy to Nickel: Still #1 After All These Years
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4
Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.
Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Practice Points
- Nickel is the most common cause of contact allergy worldwide. It is ubiquitous in our daily environment, making avoidance challenging.
- Nickel allergic contact dermatitis typically presents in a localized distribution but also can present as systemic contact dermatitis.
- Nickel regulation has been adopted in Europe, but similar legislation does not exist in the United States.