User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
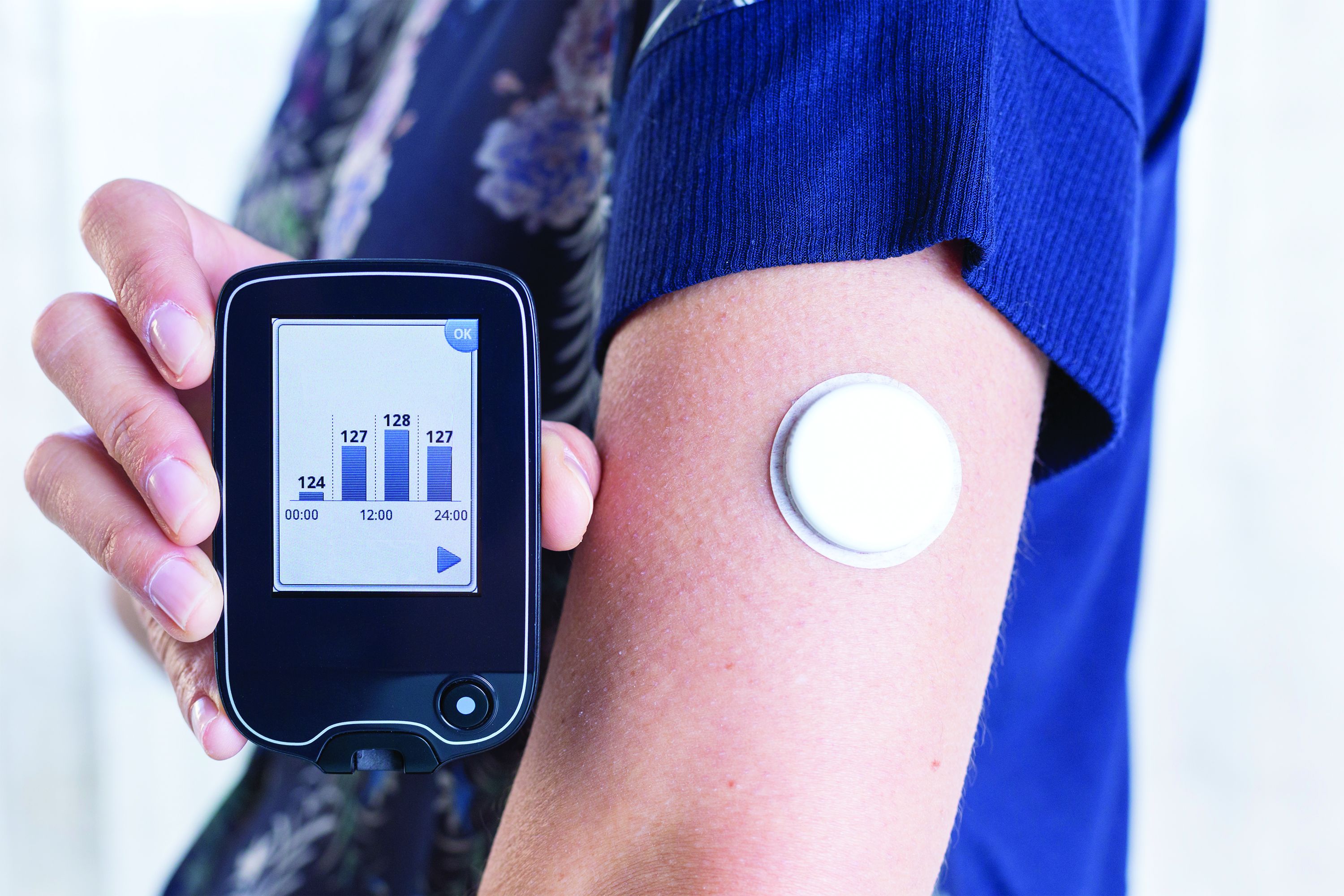
Has the time come for glucose monitors for people without diabetes?
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
AT ADA 2022
Third COVID booster benefits cancer patients
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
though this population still suffers higher risks than those of the general population, according to a new large-scale observational study out of the United Kingdom.
People living with lymphoma and those who underwent recent systemic anti-cancer treatment or radiotherapy are at the highest risk, according to study author Lennard Y.W. Lee, PhD. “Our study is the largest evaluation of a coronavirus third dose vaccine booster effectiveness in people living with cancer in the world. For the first time we have quantified the benefits of boosters for COVID-19 in cancer patients,” said Dr. Lee, UK COVID Cancer program lead and a medical oncologist at the University of Oxford, England.
The research was published in the November issue of the European Journal of Cancer.
Despite the encouraging numbers, those with cancer continue to have a more than threefold increased risk of both hospitalization and death from coronavirus compared to the general population. “More needs to be done to reduce this excess risk, like prophylactic antibody therapies,” Dr. Lee said.
Third dose efficacy was lower among cancer patients who had been diagnosed within the past 12 months, as well as those with lymphoma, and those who had undergone systemic anti-cancer therapy or radiotherapy within the past 12 months.
The increased vulnerability among individuals with cancer is likely due to compromised immune systems. “Patients with cancer often have impaired B and T cell function and this study provides the largest global clinical study showing the definitive meaningful clinical impact of this,” Dr. Lee said. The greater risk among those with lymphoma likely traces to aberrant white cells or immunosuppressant regimens, he said.
“Vaccination probably should be used in combination with new forms of prevention and in Europe the strategy of using prophylactic antibodies is going to provide additional levels of protection,” Dr. Lee said.
Overall, the study reveals the challenges that cancer patients face in a pandemic that remains a critical health concern, one that can seriously affect quality of life. “Many are still shielding, unable to see family or hug loved ones. Furthermore, looking beyond the direct health risks, there is also the mental health impact. Shielding for nearly 3 years is very difficult. It is important to realize that behind this large-scale study, which is the biggest in the world, there are real people. The pandemic still goes on for them as they remain at higher risk from COVID-19 and we must be aware of the impact on them,” Dr. Lee said.
The study included data from the United Kingdom’s third dose booster vaccine program, representing 361,098 individuals who participated from December 2020 through December 2021. It also include results from all coronavirus tests conducted in the United Kingdom during that period. Among the participants, 97.8% got the Pfizer-BioNTech vaccine as a booster, while 1.5% received the Moderna vaccine. Overall, 8,371,139 individuals received a third dose booster, including 230,666 living with cancer. The researchers used a test-negative case-controlled analysis to estimate vaccine efficacy.
The booster shot had a 59.1% efficacy against breakthrough infections, 62.8% efficacy against symptomatic infections, 80.5% efficacy versus coronavirus hospitalization, and 94.5% efficacy against coronavirus death. Patients with solid tumors benefited from higher efficacy versus breakthrough infections 66.0% versus 53.2%) and symptomatic infections (69.6% versus 56.0%).
Patients with lymphoma experienced just a 10.5% efficacy of the primary dose vaccine versus breakthrough infections and 13.6% versus symptomatic infections, and this did not improve with a third dose. The benefit was greater for hospitalization (23.2%) and death (80.1%).
Despite the additional protection of a third dose, patients with cancer had a higher risk than the population control for coronavirus hospitalization (odds ratio, 3.38; P < .000001) and death (odds ratio, 3.01; P < .000001).
Dr. Lee has no relevant financial disclosures.
FROM THE EUROPEAN JOURNAL OF CANCER
Serum dupilumab levels do not predict clinical response
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
FROM JAMA DERMATOLOGY
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Monkeypox in children appears rare and relatively mild
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The clitoris steps into the spotlight with major scientific discovery
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
The patients of Jill Krapf, MD, are often too embarrassed to tell her about discomfort in their clitoris.
“I ask all of my patients about clitoral pain, and it is often the first time they have ever been asked about this,” says Dr. Krapf, the associate director of the Center for Vulvovaginal Disorders, a private clinic in Washington and New York.
Dr. Krapf is an ob.gyn. who specializes in female sexual pain that involves the pelvis, vagina, and vulva.
Many of the conditions Dr. Krapf treats don’t have outward symptoms that appear abnormal, but internally, there are damaged or irritated nerves that can result in hypersensitivity, unwanted arousal, or pain.
“Most recent research indicates that even a herniated disk or tear in the spine can lead to clitoral or vulvar symptoms, just like sciatica pain that shoots down the leg is related to issues in the spine,” Dr. Krapf says.
Dr. Krapf was excited to read of a new discovery: Dr. Krapf and other doctors are hopeful that the attention to the clitoris will spark more interest and comprehensive education among people in their field. They also hope it will empower patients to seek medical help if they are having issues with their clitoris.
“Female sexual health has historically been underfunded, especially compared with male sexual health, like erectile dysfunction,” Dr. Krapf says. “Optimizing vulvar and vaginal health is not only necessary for sexual well-being.”
Blair Peters, MD, a plastic surgeon who specializes in gender-affirming care, led the study, which was presented at the Sexual Medicine Society of North America conference in October. Dr. Peters says he hopes that the new information decreases stigma that the clitoris is not worthy of the same medical attention that other organs of the body receive.
When the clitoris doesn’t properly function, there can be harm to a person’s physical and mental health. Paying attention to discomfort in the clitoris, and seeking medical attention, can help catch and prevent some urinary and vaginal infections.
“The fact that it took until 2022 for someone to do this work speaks to how little attention the clitoris has received,” says Dr. Peters, an assistant professor of surgery at the Oregon Health and Science University School of Medicine, Portland.
What’s inside?
Dr. Peters and his colleagues completed the study by taking clitoral nerve tissue from seven adult transgender men who had received gender-affirming genital surgery. The tissues were dyed and magnified 1,000 times under a microscope so the researchers could count nerve fibers.
Dr. Peters says the finding is important because many surgeries take place in the groin region – like hip replacements, episiotomies during childbirth, and pelvic mesh procedures – and the revived attention to the clitoris may help health care providers know where nerves are so that injuries from medical mistakes are prevented.
“Nerves are at risk of damage if it’s not understood where they are at all times,” he says.
Dr. Peters hopes the new finding will help create new surgical techniques for nerve repair and offer insight for gender-affirming phalloplasty, which is the surgical construction of a penis often for transmasculine people.
Ownership of the body part
When it comes to the clitoris, no one type of doctor has specialized in the sex organ.
Urologists, gynecologists, plastic surgeons, and sex therapists all address potential problems that can arise with the clitoris and its surrounding body parts. But specialists like Dr. Krapf are few and far between.
It wasn’t until 2005 that Australian urologist Helen O’Connell found that the clitoris is filled with erectile and non-erectile tissues that are often hidden in anatomy drawings by fat and bone. And it wasn’t until the early 2000s that researchers began delving in earnest into the anatomy of the clitoris and how it functions.
And a 2018 study showed that if more doctors examined the clitoris, they could identify issues like adhesions or infections in the area, most of which can be treated without surgery.
A body part built for pleasure
Randi Levinson, a sex, marriage, and family therapist in Los Angeles, sees patients who have less sensation in the clitoris or pain while having sex, many of whom have recently given birth or are going through menopause.
Women often become embarrassed when they can’t orgasm, or have less sensation in the clitoris, but tend to avoid seeking medical advice, she says. Normalizing discussions about women’s pleasure and the vast anatomy that supports it may help some of her patients.
“The more normal it is to talk about and explore women’s pleasure, the less shame women will have when getting help when they aren’t experiencing pleasure,” Ms. Levinson says. “I have many ... clients who experience pain and discomfort with sex [after pregnancy] and no longer feel pleasure and are concerned that something is wrong with them.”
A version of this article first appeared on WebMD.com.
Breaking the itch-scratch cycle with mindfulness
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
FROM IDS 2022
Liver disease-related deaths rise during pandemic
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Between 2019 and 2021, ALD-related deaths increased by 17.6% and NAFLD-related deaths increased by 14.5%, Yee Hui Yeo, MD, a resident physician and hepatology-focused investigator at Cedars-Sinai Medical Center in Los Angeles, said at a preconference press briefing.
“Even before the pandemic, the mortality rates for these two diseases have been increasing, with NAFLD having an even steeper increasing trend,” he said. “During the pandemic, these two diseases had a significant surge.”
Recent U.S. liver disease death rates
Dr. Yeo and colleagues analyzed data from the Center for Disease Control and Prevention’s National Vital Statistic System to estimate the age-standardized mortality rates (ASMR) of liver disease between 2010 and 2021, including ALD, NAFLD, hepatitis B, and hepatitis C. Using prediction modeling analyses based on trends from 2010 to 2019, they predicted mortality rates for 2020-2021 and compared them with the observed rates to quantify the differences related to the pandemic.
Between 2010 and 2021, there were about 626,000 chronic liver disease–related deaths, including about 343,000 ALD-related deaths, 204,000 hepatitis C–related deaths, 58,000 NAFLD-related deaths, and 21,000 hepatitis B–related deaths.
For ALD-related deaths, the annual percentage change was 3.5% for 2010-2019 and 17.6% for 2019-2021. The observed ASMR in 2020 was significantly higher than predicted, at 15.7 deaths per 100,000 people versus 13.0 predicted from the 2010-2019 rate. The trend continued in 2021, with 17.4 deaths per 100,000 people versus 13.4 in the previous decade.
The highest numbers of ALD-related deaths during the COVID-19 pandemic occurred in Alaska, Montana, Wyoming, Colorado, New Mexico, and South Dakota.
For NAFLD-related deaths, the annual percentage change was 7.6% for 2010-2014, 11.8% for 2014-2019, and 14.5% for 2019-2021. The observed ASMR was also higher than predicted, at 3.1 deaths per 100,000 people versus 2.6 in 2020, as well as 3.4 versus 2.8 in 2021.
The highest numbers of NAFLD-related deaths during the COVID-19 pandemic occurred in Oklahoma, Indiana, Kentucky, Tennessee, and West Virginia.
Hepatitis B and C gains lost in pandemic
In contrast, the annual percentage change in was –1.9% for hepatitis B and –2.8% for hepatitis C. After new treatment for hepatitis C emerged in 2013-2014, mortality rates were –7.8% for 2014-2019, Dr. Yeo noted.
“However, during the pandemic, we saw that this decrease has become a nonsignificant change,” he said. “That means our progress of the past 5 or 6 years has already stopped during the pandemic.”
By race and ethnicity, the increase in ALD-related mortality was most pronounced in non-Hispanic White, non-Hispanic Black, and Alaska Native/American Indian populations, Dr. Yeo said. Alaska Natives and American Indians had the highest annual percentage change, at 18%, followed by non-Hispanic Whites at 11.7% and non-Hispanic Blacks at 10.8%. There were no significant differences in race and ethnicity for NAFLD-related deaths, although all groups had major increases in recent years.
Biggest rise in young adults
By age, the increase in ALD-related mortality was particularly severe for ages 25-44, with an annual percentage change of 34.6% in 2019-2021, as compared with 13.7% for ages 45-64 and 12.6% for ages 65 and older.
For NAFLD-related deaths, another major increase was observed among ages 25-44, with an annual percentage change of 28.1% for 2019-2021, as compared with 12% for ages 65 and older and 7.4% for ages 45-64.
By sex, the ASMR increase in NAFLD-related mortality was steady throughout 2010-2021 for both men and women. In contrast, ALD-related death increased sharply between 2019 and 2021, with an annual percentage change of 19.1% for women and 16.7% for men.
“The increasing trend in mortality rates for ALD and NAFLD has been quite alarming, with disparities in age, race, and ethnicity,” Dr. Yeo said.
The study received no funding support. Some authors disclosed research funding, advisory board roles, and consulting fees with various pharmaceutical companies.
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Between 2019 and 2021, ALD-related deaths increased by 17.6% and NAFLD-related deaths increased by 14.5%, Yee Hui Yeo, MD, a resident physician and hepatology-focused investigator at Cedars-Sinai Medical Center in Los Angeles, said at a preconference press briefing.
“Even before the pandemic, the mortality rates for these two diseases have been increasing, with NAFLD having an even steeper increasing trend,” he said. “During the pandemic, these two diseases had a significant surge.”
Recent U.S. liver disease death rates
Dr. Yeo and colleagues analyzed data from the Center for Disease Control and Prevention’s National Vital Statistic System to estimate the age-standardized mortality rates (ASMR) of liver disease between 2010 and 2021, including ALD, NAFLD, hepatitis B, and hepatitis C. Using prediction modeling analyses based on trends from 2010 to 2019, they predicted mortality rates for 2020-2021 and compared them with the observed rates to quantify the differences related to the pandemic.
Between 2010 and 2021, there were about 626,000 chronic liver disease–related deaths, including about 343,000 ALD-related deaths, 204,000 hepatitis C–related deaths, 58,000 NAFLD-related deaths, and 21,000 hepatitis B–related deaths.
For ALD-related deaths, the annual percentage change was 3.5% for 2010-2019 and 17.6% for 2019-2021. The observed ASMR in 2020 was significantly higher than predicted, at 15.7 deaths per 100,000 people versus 13.0 predicted from the 2010-2019 rate. The trend continued in 2021, with 17.4 deaths per 100,000 people versus 13.4 in the previous decade.
The highest numbers of ALD-related deaths during the COVID-19 pandemic occurred in Alaska, Montana, Wyoming, Colorado, New Mexico, and South Dakota.
For NAFLD-related deaths, the annual percentage change was 7.6% for 2010-2014, 11.8% for 2014-2019, and 14.5% for 2019-2021. The observed ASMR was also higher than predicted, at 3.1 deaths per 100,000 people versus 2.6 in 2020, as well as 3.4 versus 2.8 in 2021.
The highest numbers of NAFLD-related deaths during the COVID-19 pandemic occurred in Oklahoma, Indiana, Kentucky, Tennessee, and West Virginia.
Hepatitis B and C gains lost in pandemic
In contrast, the annual percentage change in was –1.9% for hepatitis B and –2.8% for hepatitis C. After new treatment for hepatitis C emerged in 2013-2014, mortality rates were –7.8% for 2014-2019, Dr. Yeo noted.
“However, during the pandemic, we saw that this decrease has become a nonsignificant change,” he said. “That means our progress of the past 5 or 6 years has already stopped during the pandemic.”
By race and ethnicity, the increase in ALD-related mortality was most pronounced in non-Hispanic White, non-Hispanic Black, and Alaska Native/American Indian populations, Dr. Yeo said. Alaska Natives and American Indians had the highest annual percentage change, at 18%, followed by non-Hispanic Whites at 11.7% and non-Hispanic Blacks at 10.8%. There were no significant differences in race and ethnicity for NAFLD-related deaths, although all groups had major increases in recent years.
Biggest rise in young adults
By age, the increase in ALD-related mortality was particularly severe for ages 25-44, with an annual percentage change of 34.6% in 2019-2021, as compared with 13.7% for ages 45-64 and 12.6% for ages 65 and older.
For NAFLD-related deaths, another major increase was observed among ages 25-44, with an annual percentage change of 28.1% for 2019-2021, as compared with 12% for ages 65 and older and 7.4% for ages 45-64.
By sex, the ASMR increase in NAFLD-related mortality was steady throughout 2010-2021 for both men and women. In contrast, ALD-related death increased sharply between 2019 and 2021, with an annual percentage change of 19.1% for women and 16.7% for men.
“The increasing trend in mortality rates for ALD and NAFLD has been quite alarming, with disparities in age, race, and ethnicity,” Dr. Yeo said.
The study received no funding support. Some authors disclosed research funding, advisory board roles, and consulting fees with various pharmaceutical companies.
according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Between 2019 and 2021, ALD-related deaths increased by 17.6% and NAFLD-related deaths increased by 14.5%, Yee Hui Yeo, MD, a resident physician and hepatology-focused investigator at Cedars-Sinai Medical Center in Los Angeles, said at a preconference press briefing.
“Even before the pandemic, the mortality rates for these two diseases have been increasing, with NAFLD having an even steeper increasing trend,” he said. “During the pandemic, these two diseases had a significant surge.”
Recent U.S. liver disease death rates
Dr. Yeo and colleagues analyzed data from the Center for Disease Control and Prevention’s National Vital Statistic System to estimate the age-standardized mortality rates (ASMR) of liver disease between 2010 and 2021, including ALD, NAFLD, hepatitis B, and hepatitis C. Using prediction modeling analyses based on trends from 2010 to 2019, they predicted mortality rates for 2020-2021 and compared them with the observed rates to quantify the differences related to the pandemic.
Between 2010 and 2021, there were about 626,000 chronic liver disease–related deaths, including about 343,000 ALD-related deaths, 204,000 hepatitis C–related deaths, 58,000 NAFLD-related deaths, and 21,000 hepatitis B–related deaths.
For ALD-related deaths, the annual percentage change was 3.5% for 2010-2019 and 17.6% for 2019-2021. The observed ASMR in 2020 was significantly higher than predicted, at 15.7 deaths per 100,000 people versus 13.0 predicted from the 2010-2019 rate. The trend continued in 2021, with 17.4 deaths per 100,000 people versus 13.4 in the previous decade.
The highest numbers of ALD-related deaths during the COVID-19 pandemic occurred in Alaska, Montana, Wyoming, Colorado, New Mexico, and South Dakota.
For NAFLD-related deaths, the annual percentage change was 7.6% for 2010-2014, 11.8% for 2014-2019, and 14.5% for 2019-2021. The observed ASMR was also higher than predicted, at 3.1 deaths per 100,000 people versus 2.6 in 2020, as well as 3.4 versus 2.8 in 2021.
The highest numbers of NAFLD-related deaths during the COVID-19 pandemic occurred in Oklahoma, Indiana, Kentucky, Tennessee, and West Virginia.
Hepatitis B and C gains lost in pandemic
In contrast, the annual percentage change in was –1.9% for hepatitis B and –2.8% for hepatitis C. After new treatment for hepatitis C emerged in 2013-2014, mortality rates were –7.8% for 2014-2019, Dr. Yeo noted.
“However, during the pandemic, we saw that this decrease has become a nonsignificant change,” he said. “That means our progress of the past 5 or 6 years has already stopped during the pandemic.”
By race and ethnicity, the increase in ALD-related mortality was most pronounced in non-Hispanic White, non-Hispanic Black, and Alaska Native/American Indian populations, Dr. Yeo said. Alaska Natives and American Indians had the highest annual percentage change, at 18%, followed by non-Hispanic Whites at 11.7% and non-Hispanic Blacks at 10.8%. There were no significant differences in race and ethnicity for NAFLD-related deaths, although all groups had major increases in recent years.
Biggest rise in young adults
By age, the increase in ALD-related mortality was particularly severe for ages 25-44, with an annual percentage change of 34.6% in 2019-2021, as compared with 13.7% for ages 45-64 and 12.6% for ages 65 and older.
For NAFLD-related deaths, another major increase was observed among ages 25-44, with an annual percentage change of 28.1% for 2019-2021, as compared with 12% for ages 65 and older and 7.4% for ages 45-64.
By sex, the ASMR increase in NAFLD-related mortality was steady throughout 2010-2021 for both men and women. In contrast, ALD-related death increased sharply between 2019 and 2021, with an annual percentage change of 19.1% for women and 16.7% for men.
“The increasing trend in mortality rates for ALD and NAFLD has been quite alarming, with disparities in age, race, and ethnicity,” Dr. Yeo said.
The study received no funding support. Some authors disclosed research funding, advisory board roles, and consulting fees with various pharmaceutical companies.
FROM THE LIVER MEETING
More children should be getting flu vaccines
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Tirzepatide lowers weight across all groups with obesity
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022