User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
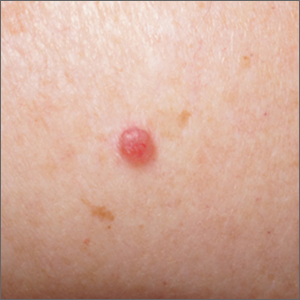
Pink shoulder lesion
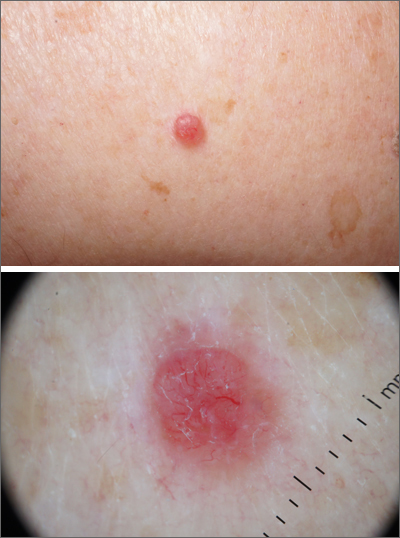
A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.

A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References

A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
Study finds high rate of psychiatric burden in cosmetic dermatology patients
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Have you heard the one about the emergency dept. that called 911?
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Medicaid coverage of HPV vaccine in adults: Implications in dermatology
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Does subclinical hyperthyroidism raise fracture risk?
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Four-drug combo gets BP down in one step: QUARTET-USA
Use of a combination antihypertensive product containing quarter doses of four different drugs could be an effective strategy to get patients to target blood pressures in one step, a new study suggests.
The study, QUARTET-USA, showed a reduction in BP of almost 5 mm Hg more than the comparator of one antihypertensive agent at standard dose over the 12-week follow-up period in patients with mild to moderate hypertension.
The QUARTET-USA study was presented at the American Heart Association scientific sessions by Mark Huffman, MD, professor of medicine at Washington University in St. Louis.
It builds on a previous trial, QUARTET, conducted in Australia, which first showed benefits with this approach.
In the new U.S. study, which was considerably smaller than the Australian trial, the four-drug combination, including candesartan, amlodipine, indapamide, and bisoprolol, led to a –4.8/–4.9 mm Hg greater reduction in BP from baseline to 12 weeks, compared with standard-dose candesartan monotherapy.
Differences in systolic BP were not statistically significant, which is likely because of limited power related to the sample size, Dr. Huffman noted.
Adverse events were more common in the four-drug intervention group, but the rate of discontinuation was higher in the comparator group. No severe adverse events were deemed related to the study drug.
“The direction and magnitude of [the] blood pressure–lowering effect were similar between the previous Australian study and this American study, despite different populations with lower baseline blood pressure in the current study, thus strengthening the case for this new approach,” Dr. Huffman concluded.
“The two studies together show that the approach of using four drugs in quarter doses is more effective in lowering blood pressure than a single standard dose antihypertensive agent and has an acceptable safely profile,” he said in an interview.
He said the four-drug combination could be an effective way of getting patients to target without multiple appointments.
“If you think about how many visits to the doctor’s office it takes to get patients to goal blood pressures, this combination gets patients down to new guideline target levels in one step, whereas in the SPRINT trial it took three or more visits to get down to these levels. And in practice we lose people – they don’t come back,” he said.
Dr. Huffman explained that the rationale for the study was the persistently low hypertension control rate, which demonstrates the need for a new approach.
The previous Australian QUARTET study suggested that ultra–low-dose combination therapy has a favorable balance between blood pressure–lowering effect, tolerability, and adherence.
That study, conducted in 591 patients and reported in 2021, demonstrated a greater BP-lowering effect with a four-drug combination at quarter doses (irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) at 12 weeks, compared with irbesartan 150 mg daily. Systolic BP was reduced by more than 6.9 mm Hg and diastolic BP by 5.8 mm Hg than full-dose irbesartan alone, both significant differences.
The current study, QUARTET-USA, aimed to see if a similar strategy could produce comparable results in a U.S. population.
The U.S. study included 62 patients from the Access Community Health Network, Chicago, who were either treatment naive with BPs above 140/90 mm Hg, or already taking antihypertensive monotherapy with BPs above 130/85 mm Hg.
The mean systolic BP at baseline was 138 mm Hg and the mean diastolic pressure was 84 mm Hg.
Study participants were mainly from ethnic minorities (90% Hispanic or Black) and over half were from low-income households (annual household income less than $25,000).
They were randomly assigned to daily administration of a four-drug combination at quarter doses (candesartan 2 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) or a full dose of candesartan 8 mg (the comparator arm).
Amlodipine 5 mg daily could be added on to treatment if BP remained above 130/80 mm Hg at 6 weeks. This occurred in 18% of the study group versus 53% of the comparator group.
Results showed that at 12 weeks the adjusted mean change in systolic BP weeks was –4.8 mm Hg (95% CI,–10.7 to 1.2), and the adjusted mean change in diastolic BP was –4.9 mm Hg (95% CI, –8.6 to –1.1) in the four-drug combination group, compared with the comparator arm.
Average BPs at the end of 12-week study period were 121 mm Hg systolic and 73 mm Hg diastolic in the four-drug intervention group, compared with 124 mm Hg systolic and 77 mm Hg diastolic in the comparator group.
Any adverse events that were possibly related to drug therapy occurred in 25% of the intervention group versus 10% of the comparator group. But adverse events leading to discontinuation occurred in 6.3% of the study group versus 26.7% of patients in the comparator arm.
“New approaches are needed to achieve lower blood pressure targets, especially for patients and communities with a high burden of hypertension and hypertension-related diseases. QUARTET-USA was the first trial of a four-drug, ultra–low-dose, blood pressure–lowering combination therapy in the U.S.,” Dr. Huffman said.
“We showed reductions in blood pressure similar in magnitude to those in the Australian study. It is useful to know that the direction of the effect is similar across varied populations. Now that we have that signal of efficacy and tolerability, we can move to actually getting it into the hands of patients and providers,” he added.
Noting that further studies will be required to attain marketing authorization, Dr. Huffman suggested that a pharmaceutical company would need to complete that process.
“These are promising results for companies who may be interested in partnering,” he said.
‘A more efficient approach’
LaPrincess C. Brewer, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minn., and discussant of the study, said the QUARTET-USA study suggests the four-drug, low-dose combination shows promise in lowering BP, compared with the standard dose, and while the reduction in systolic BP was not quite significant, it was clinically meaningful.
“Most U.S. adults with hypertension do not have it under control. This is due to unfavorable social and structural determinants of health which limit adherence to antihypertensive medication,” Dr. Brewer noted.
From a patient point of view, the multiple visits needed to attain goals are a burden and there is also the issue of clinical inertia and lack of medication intensification by clinicians, she commented.
“Of patients with uncontrolled hypertension, 40% are taking just one antihypertensive medication, so up-front, low-dose combination therapy is likely a more efficient approach,” she said.
“This study builds the evidence base for the need for tailored interventions that address the social determinants of health and the intentional prioritization of diverse population in clinical trials,” Dr. Brewer concluded.
QUARTET was an investigator-initiated study, Dr. Huffman reported a pending patent for a heart failure polypill. The George Institute for Global Health, Sydney, Australia, where Huffman has a secondary appointment, has a patent, license, and has received investment funding with intent to commercialize fixed-dose combination therapy. Dr. Brewer reported research support from the National Institutes of Health, Centers for Disease Control and Prevention, American Heart Association, and Bristol-Meyers Squibb Foundation.
A version of this article first appeared on Medscape.com.
Use of a combination antihypertensive product containing quarter doses of four different drugs could be an effective strategy to get patients to target blood pressures in one step, a new study suggests.
The study, QUARTET-USA, showed a reduction in BP of almost 5 mm Hg more than the comparator of one antihypertensive agent at standard dose over the 12-week follow-up period in patients with mild to moderate hypertension.
The QUARTET-USA study was presented at the American Heart Association scientific sessions by Mark Huffman, MD, professor of medicine at Washington University in St. Louis.
It builds on a previous trial, QUARTET, conducted in Australia, which first showed benefits with this approach.
In the new U.S. study, which was considerably smaller than the Australian trial, the four-drug combination, including candesartan, amlodipine, indapamide, and bisoprolol, led to a –4.8/–4.9 mm Hg greater reduction in BP from baseline to 12 weeks, compared with standard-dose candesartan monotherapy.
Differences in systolic BP were not statistically significant, which is likely because of limited power related to the sample size, Dr. Huffman noted.
Adverse events were more common in the four-drug intervention group, but the rate of discontinuation was higher in the comparator group. No severe adverse events were deemed related to the study drug.
“The direction and magnitude of [the] blood pressure–lowering effect were similar between the previous Australian study and this American study, despite different populations with lower baseline blood pressure in the current study, thus strengthening the case for this new approach,” Dr. Huffman concluded.
“The two studies together show that the approach of using four drugs in quarter doses is more effective in lowering blood pressure than a single standard dose antihypertensive agent and has an acceptable safely profile,” he said in an interview.
He said the four-drug combination could be an effective way of getting patients to target without multiple appointments.
“If you think about how many visits to the doctor’s office it takes to get patients to goal blood pressures, this combination gets patients down to new guideline target levels in one step, whereas in the SPRINT trial it took three or more visits to get down to these levels. And in practice we lose people – they don’t come back,” he said.
Dr. Huffman explained that the rationale for the study was the persistently low hypertension control rate, which demonstrates the need for a new approach.
The previous Australian QUARTET study suggested that ultra–low-dose combination therapy has a favorable balance between blood pressure–lowering effect, tolerability, and adherence.
That study, conducted in 591 patients and reported in 2021, demonstrated a greater BP-lowering effect with a four-drug combination at quarter doses (irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) at 12 weeks, compared with irbesartan 150 mg daily. Systolic BP was reduced by more than 6.9 mm Hg and diastolic BP by 5.8 mm Hg than full-dose irbesartan alone, both significant differences.
The current study, QUARTET-USA, aimed to see if a similar strategy could produce comparable results in a U.S. population.
The U.S. study included 62 patients from the Access Community Health Network, Chicago, who were either treatment naive with BPs above 140/90 mm Hg, or already taking antihypertensive monotherapy with BPs above 130/85 mm Hg.
The mean systolic BP at baseline was 138 mm Hg and the mean diastolic pressure was 84 mm Hg.
Study participants were mainly from ethnic minorities (90% Hispanic or Black) and over half were from low-income households (annual household income less than $25,000).
They were randomly assigned to daily administration of a four-drug combination at quarter doses (candesartan 2 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) or a full dose of candesartan 8 mg (the comparator arm).
Amlodipine 5 mg daily could be added on to treatment if BP remained above 130/80 mm Hg at 6 weeks. This occurred in 18% of the study group versus 53% of the comparator group.
Results showed that at 12 weeks the adjusted mean change in systolic BP weeks was –4.8 mm Hg (95% CI,–10.7 to 1.2), and the adjusted mean change in diastolic BP was –4.9 mm Hg (95% CI, –8.6 to –1.1) in the four-drug combination group, compared with the comparator arm.
Average BPs at the end of 12-week study period were 121 mm Hg systolic and 73 mm Hg diastolic in the four-drug intervention group, compared with 124 mm Hg systolic and 77 mm Hg diastolic in the comparator group.
Any adverse events that were possibly related to drug therapy occurred in 25% of the intervention group versus 10% of the comparator group. But adverse events leading to discontinuation occurred in 6.3% of the study group versus 26.7% of patients in the comparator arm.
“New approaches are needed to achieve lower blood pressure targets, especially for patients and communities with a high burden of hypertension and hypertension-related diseases. QUARTET-USA was the first trial of a four-drug, ultra–low-dose, blood pressure–lowering combination therapy in the U.S.,” Dr. Huffman said.
“We showed reductions in blood pressure similar in magnitude to those in the Australian study. It is useful to know that the direction of the effect is similar across varied populations. Now that we have that signal of efficacy and tolerability, we can move to actually getting it into the hands of patients and providers,” he added.
Noting that further studies will be required to attain marketing authorization, Dr. Huffman suggested that a pharmaceutical company would need to complete that process.
“These are promising results for companies who may be interested in partnering,” he said.
‘A more efficient approach’
LaPrincess C. Brewer, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minn., and discussant of the study, said the QUARTET-USA study suggests the four-drug, low-dose combination shows promise in lowering BP, compared with the standard dose, and while the reduction in systolic BP was not quite significant, it was clinically meaningful.
“Most U.S. adults with hypertension do not have it under control. This is due to unfavorable social and structural determinants of health which limit adherence to antihypertensive medication,” Dr. Brewer noted.
From a patient point of view, the multiple visits needed to attain goals are a burden and there is also the issue of clinical inertia and lack of medication intensification by clinicians, she commented.
“Of patients with uncontrolled hypertension, 40% are taking just one antihypertensive medication, so up-front, low-dose combination therapy is likely a more efficient approach,” she said.
“This study builds the evidence base for the need for tailored interventions that address the social determinants of health and the intentional prioritization of diverse population in clinical trials,” Dr. Brewer concluded.
QUARTET was an investigator-initiated study, Dr. Huffman reported a pending patent for a heart failure polypill. The George Institute for Global Health, Sydney, Australia, where Huffman has a secondary appointment, has a patent, license, and has received investment funding with intent to commercialize fixed-dose combination therapy. Dr. Brewer reported research support from the National Institutes of Health, Centers for Disease Control and Prevention, American Heart Association, and Bristol-Meyers Squibb Foundation.
A version of this article first appeared on Medscape.com.
Use of a combination antihypertensive product containing quarter doses of four different drugs could be an effective strategy to get patients to target blood pressures in one step, a new study suggests.
The study, QUARTET-USA, showed a reduction in BP of almost 5 mm Hg more than the comparator of one antihypertensive agent at standard dose over the 12-week follow-up period in patients with mild to moderate hypertension.
The QUARTET-USA study was presented at the American Heart Association scientific sessions by Mark Huffman, MD, professor of medicine at Washington University in St. Louis.
It builds on a previous trial, QUARTET, conducted in Australia, which first showed benefits with this approach.
In the new U.S. study, which was considerably smaller than the Australian trial, the four-drug combination, including candesartan, amlodipine, indapamide, and bisoprolol, led to a –4.8/–4.9 mm Hg greater reduction in BP from baseline to 12 weeks, compared with standard-dose candesartan monotherapy.
Differences in systolic BP were not statistically significant, which is likely because of limited power related to the sample size, Dr. Huffman noted.
Adverse events were more common in the four-drug intervention group, but the rate of discontinuation was higher in the comparator group. No severe adverse events were deemed related to the study drug.
“The direction and magnitude of [the] blood pressure–lowering effect were similar between the previous Australian study and this American study, despite different populations with lower baseline blood pressure in the current study, thus strengthening the case for this new approach,” Dr. Huffman concluded.
“The two studies together show that the approach of using four drugs in quarter doses is more effective in lowering blood pressure than a single standard dose antihypertensive agent and has an acceptable safely profile,” he said in an interview.
He said the four-drug combination could be an effective way of getting patients to target without multiple appointments.
“If you think about how many visits to the doctor’s office it takes to get patients to goal blood pressures, this combination gets patients down to new guideline target levels in one step, whereas in the SPRINT trial it took three or more visits to get down to these levels. And in practice we lose people – they don’t come back,” he said.
Dr. Huffman explained that the rationale for the study was the persistently low hypertension control rate, which demonstrates the need for a new approach.
The previous Australian QUARTET study suggested that ultra–low-dose combination therapy has a favorable balance between blood pressure–lowering effect, tolerability, and adherence.
That study, conducted in 591 patients and reported in 2021, demonstrated a greater BP-lowering effect with a four-drug combination at quarter doses (irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) at 12 weeks, compared with irbesartan 150 mg daily. Systolic BP was reduced by more than 6.9 mm Hg and diastolic BP by 5.8 mm Hg than full-dose irbesartan alone, both significant differences.
The current study, QUARTET-USA, aimed to see if a similar strategy could produce comparable results in a U.S. population.
The U.S. study included 62 patients from the Access Community Health Network, Chicago, who were either treatment naive with BPs above 140/90 mm Hg, or already taking antihypertensive monotherapy with BPs above 130/85 mm Hg.
The mean systolic BP at baseline was 138 mm Hg and the mean diastolic pressure was 84 mm Hg.
Study participants were mainly from ethnic minorities (90% Hispanic or Black) and over half were from low-income households (annual household income less than $25,000).
They were randomly assigned to daily administration of a four-drug combination at quarter doses (candesartan 2 mg, amlodipine 1.25 mg, indapamide 0.625 mg, bisoprolol 2.5 mg) or a full dose of candesartan 8 mg (the comparator arm).
Amlodipine 5 mg daily could be added on to treatment if BP remained above 130/80 mm Hg at 6 weeks. This occurred in 18% of the study group versus 53% of the comparator group.
Results showed that at 12 weeks the adjusted mean change in systolic BP weeks was –4.8 mm Hg (95% CI,–10.7 to 1.2), and the adjusted mean change in diastolic BP was –4.9 mm Hg (95% CI, –8.6 to –1.1) in the four-drug combination group, compared with the comparator arm.
Average BPs at the end of 12-week study period were 121 mm Hg systolic and 73 mm Hg diastolic in the four-drug intervention group, compared with 124 mm Hg systolic and 77 mm Hg diastolic in the comparator group.
Any adverse events that were possibly related to drug therapy occurred in 25% of the intervention group versus 10% of the comparator group. But adverse events leading to discontinuation occurred in 6.3% of the study group versus 26.7% of patients in the comparator arm.
“New approaches are needed to achieve lower blood pressure targets, especially for patients and communities with a high burden of hypertension and hypertension-related diseases. QUARTET-USA was the first trial of a four-drug, ultra–low-dose, blood pressure–lowering combination therapy in the U.S.,” Dr. Huffman said.
“We showed reductions in blood pressure similar in magnitude to those in the Australian study. It is useful to know that the direction of the effect is similar across varied populations. Now that we have that signal of efficacy and tolerability, we can move to actually getting it into the hands of patients and providers,” he added.
Noting that further studies will be required to attain marketing authorization, Dr. Huffman suggested that a pharmaceutical company would need to complete that process.
“These are promising results for companies who may be interested in partnering,” he said.
‘A more efficient approach’
LaPrincess C. Brewer, MD, assistant professor of medicine at the Mayo Clinic, Rochester, Minn., and discussant of the study, said the QUARTET-USA study suggests the four-drug, low-dose combination shows promise in lowering BP, compared with the standard dose, and while the reduction in systolic BP was not quite significant, it was clinically meaningful.
“Most U.S. adults with hypertension do not have it under control. This is due to unfavorable social and structural determinants of health which limit adherence to antihypertensive medication,” Dr. Brewer noted.
From a patient point of view, the multiple visits needed to attain goals are a burden and there is also the issue of clinical inertia and lack of medication intensification by clinicians, she commented.
“Of patients with uncontrolled hypertension, 40% are taking just one antihypertensive medication, so up-front, low-dose combination therapy is likely a more efficient approach,” she said.
“This study builds the evidence base for the need for tailored interventions that address the social determinants of health and the intentional prioritization of diverse population in clinical trials,” Dr. Brewer concluded.
QUARTET was an investigator-initiated study, Dr. Huffman reported a pending patent for a heart failure polypill. The George Institute for Global Health, Sydney, Australia, where Huffman has a secondary appointment, has a patent, license, and has received investment funding with intent to commercialize fixed-dose combination therapy. Dr. Brewer reported research support from the National Institutes of Health, Centers for Disease Control and Prevention, American Heart Association, and Bristol-Meyers Squibb Foundation.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Lego introduces first character with vitiligo
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The
The character appears with the customizable array of players to assemble for a table football team.
It’s the latest representation of the disease as toymakers diversify their lines.
In May 2022, Mattel released a Ken doll with vitiligo after a Barbie with vitiligo was released in 2020. Rainbow High and other toy makers also have character versions.
The Lego addition follows a big summer medically for vitiligo as the first treatment was approved for repigmentation. In July, a cream formulation of ruxolitinib (Opzelura), a Janus kinase inhibitor, became the first repigmentation treatment approved by the Food and Drug Administration for nonsegmental vitiligo, the most common form of the disease.
Vitiligo is estimated to affect 1.9 million–2.8 million adults in the United States and more than 100 million people worldwide. It cuts across races and genders and can be psychologically painful for many who live with it.
John E. Harris, MD, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester, wrote about the Lego character in his blog “Speaking of Vitiligo ...” saying: “I could not be more excited. This new minifigure also serves as a way to educate both children and adults who are not familiar with vitiligo about the disease.”
He noted that until recently vitiligo representation in kids’ toys has been limited. “By adding diversity such as representations of vitiligo in toys, it can help remove stigmas associated with vitiligo and give children more options that they can relate to.”
Erika Page of Richmond, Va., who founded and edits the vitiligo blog “Living Dappled,” told this news organization she was thrilled to see the new Lego character.
“Growing up I didn’t know anyone who looked like me, let alone a toy or a character,” she said. The message the representations send is important not just for the kids but for the parents of kids with vitiligo who want to help their kids in any way they can.
Ms. Page was diagnosed with vitiligo at age 7 and struggled emotionally in her high school and college years when she often looked in the mirror, saw “giraffe-like” spots, and cried. Over time she lost 100% of her pigment to the condition and today at age 33, lives with universal vitiligo or overall very pale skin.
She founded the Living Dappled blog 6 years ago to help people with the disease feel less alone. The Lego character will also help with that, she said.
“Growing up with vitiligo was so isolating and you felt so different,” Ms. Page said. “Today we see billboards and models and dolls and now Legos that look like us. I hope this is a first of many to come for Lego.”
Dr. Harris and Ms. Page declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practical pearls guide treatment of psoriasis in tricky areas
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Pediatric celiac disease incidence varies across U.S., Europe
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GASTROENTEROLOGY
Children and COVID: New cases increase for second straight week
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.