User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
World falls short on HBV, HCV elimination targets
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
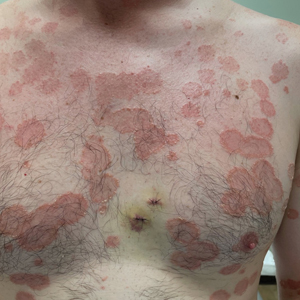
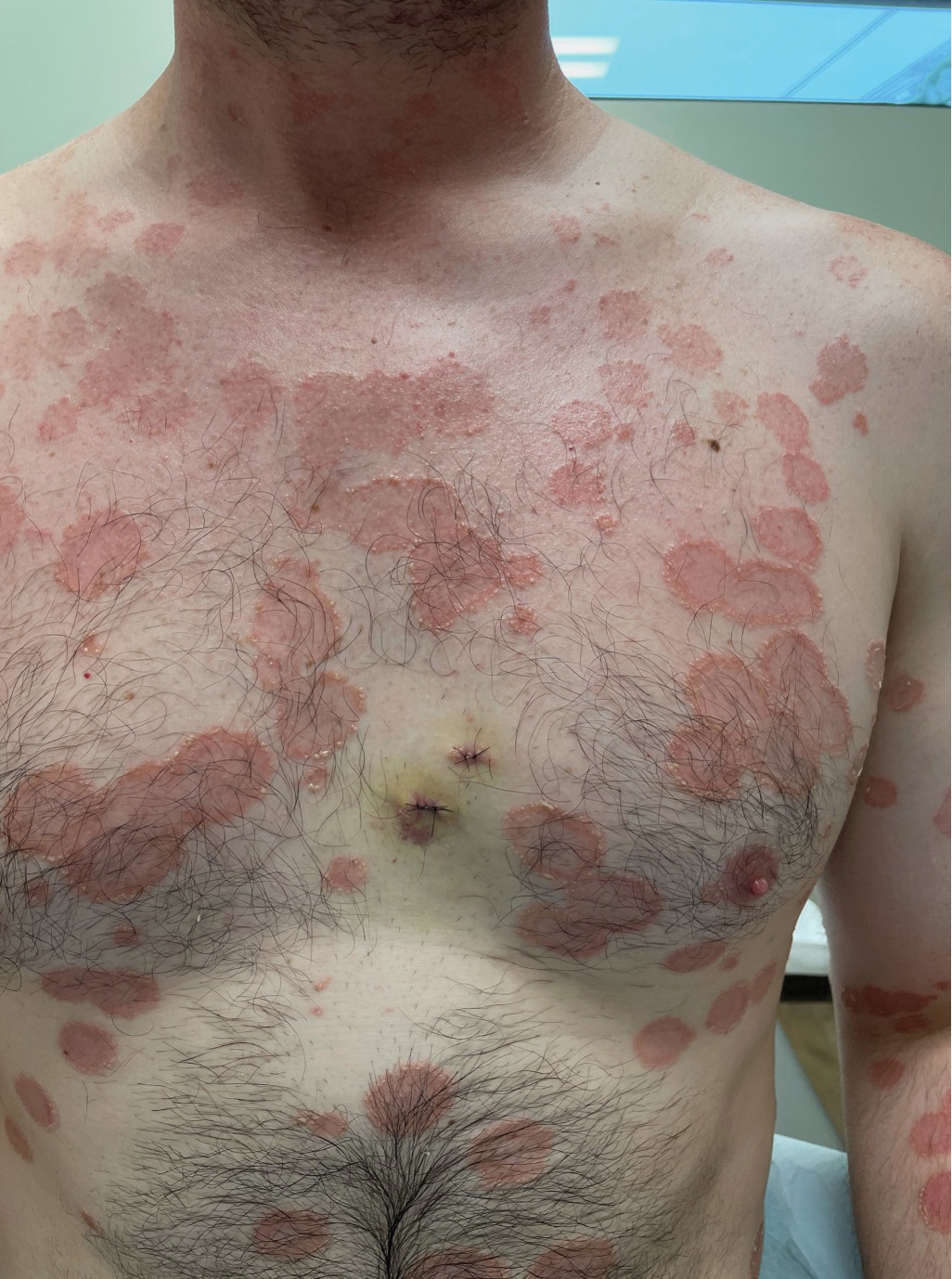
Painful and Pruritic Eruptions on the Entire Body
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
A 36-year-old man presented with painful tender blisters and rashes on the entire body, including the ears and tongue. The rash began as a few pinpointed red dots on the abdomen, which subsequently increased in size and spread over the last week. He initially felt red and flushed and noticed new lesions appearing throughout the day. He did not attempt any specific treatment for these lesions. The patient tested positive for COVID-19 four months prior to the skin eruption. He denied systemic symptoms, smoking, or recent travel. He had no history of skin cancer, skin disorders, HIV, or hepatitis. He had no known medication allergies. Physical examination revealed multiple disseminated pustules on the ears, superficial ulcerations on the tongue, and blisters on the right lip. Few lesions were tender to the touch and drained clear fluid. Bacterial, viral, HIV, herpes, and rapid plasma reagin culture and laboratory screenings were negative. He was started on valaciclovir and cephalexin; however, no improvement was noticed. Punch biopsies were taken from the blisters on the chest and perilesional area.
Dietary supplements hyped as LDL cholesterol lowering are a bust: SPORT
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AHA 2022
‘Lucid dying’: EEG backs near-death experience during CPR
“These recalled experiences and brain wave changes may be the first signs of the so-called ‘near-death’ experience, and we have captured them for the first time in a large study,” lead investigator Sam Parnia, MD, PhD, with NYU Langone Health, said in a news release.
Identifying measurable electrical signs of lucid and heightened brain activity during CPR, coupled with stories of recalled near-death experiences, suggests that the human sense of self and consciousness, much like other biological body functions, may not stop completely around the time of death, Dr. Parnia added.
He presented the findings Nov. 6 at a resuscitation science symposium at the American Heart Association scientific sessions.
The AWARE II study
“For years, some people in cardiac arrest have reported being lucid, often with a heightened sense of consciousness, while seemingly unconscious and on the brink of death,” Dr. Parnia noted in an interview.
“Yet, no one’s ever be able to prove it and a lot of people have dismissed these experiences, thinking it’s all just a trick on the brain,” Dr. Parnia said.
In a first-of-its-kind study, Dr. Parnia and colleagues examined consciousness and its underlying electrocortical biomarkers during CPR for in-hospital cardiac arrest (IHCA).
They incorporated independent audiovisual testing of awareness with continuous real-time EEG and cerebral oxygenation (rSO2) monitoring into CPR.
Only 53 of the 567 IHCA patients survived (9.3%). Among the 28 (52.8%) IHCA survivors who completed interviews, 11 (39.3%) reported unique, lucid experiences during resuscitation.
These experiences included a perception of separation from one’s body, observing events without pain or distress, and an awareness and meaningful evaluation of life, including of their actions, intentions, and thoughts toward others.
“These lucid experiences of death are not hallucinations or delusions. They cannot be considered a trick of a disordered or dying brain, but rather a unique human experience that emerges on the brink of death,” Dr. Parnia said.
And what’s “fascinating,” he added, is that despite marked cerebral ischemia (mean regional oxygen saturation [rSO2] 43%), near-normal/physiologic EEG activity (gamma, delta, theta, alpha, and beta rhythms) consistent with consciousness and a possible resumption of a network-level of cognitive and neuronal activity emerged for as long as 35-60 minutes into CPR.
Some of these brain waves normally occur when people are conscious and performing higher mental functions, including thinking, memory retrieval, and conscious perception, he said.
‘Seismic shift’ in understanding of death
This is the first time such biomarkers of consciousness have been identified during cardiac arrest and CPR, Dr. Parnia said.
He said further study is needed to more precisely define biomarkers of what is considered to be clinical consciousness and the recalled experience of death, and to monitor the long-term psychological effects of resuscitation after cardiac arrest.
“Our understanding of death has gone through a seismic shift in the last few years,” he said.
“The biological discoveries around death and the postmortem period are completely different to the social conventions that we have about death. That is, we perceive of death as being the end, but actually what we’re finding is that brain cells don’t die immediately. They die very slowly over many hours of time,” Dr. Parnia noted.
Reached for comment, Ajmal Zemmar, MD, PhD, of University of Louisville (Ky.), noted that several studies, including this one, “challenge the traditional way that we think of death – that when the heart stops beating that’s when we die.”
The observation that during cardiac arrest and CPR, the brain waves are still normal for up to an hour is “fairly remarkable,” Dr. Zemmar told this news organization.
“However, whether there is conscious perception or not is very hard to answer,” he cautioned.
“This type of research tries to bridge the objective EEG recordings with the subjective description you get from the patient, but it’s hard to know when conscious perception stops,” he said.
Funding and support for the study were provided by NYU Langone Health, The John Templeton Foundation, and the UK Resuscitation Council, and National Institutes for Health Research. Dr. Parnia and Dr. Zemmar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“These recalled experiences and brain wave changes may be the first signs of the so-called ‘near-death’ experience, and we have captured them for the first time in a large study,” lead investigator Sam Parnia, MD, PhD, with NYU Langone Health, said in a news release.
Identifying measurable electrical signs of lucid and heightened brain activity during CPR, coupled with stories of recalled near-death experiences, suggests that the human sense of self and consciousness, much like other biological body functions, may not stop completely around the time of death, Dr. Parnia added.
He presented the findings Nov. 6 at a resuscitation science symposium at the American Heart Association scientific sessions.
The AWARE II study
“For years, some people in cardiac arrest have reported being lucid, often with a heightened sense of consciousness, while seemingly unconscious and on the brink of death,” Dr. Parnia noted in an interview.
“Yet, no one’s ever be able to prove it and a lot of people have dismissed these experiences, thinking it’s all just a trick on the brain,” Dr. Parnia said.
In a first-of-its-kind study, Dr. Parnia and colleagues examined consciousness and its underlying electrocortical biomarkers during CPR for in-hospital cardiac arrest (IHCA).
They incorporated independent audiovisual testing of awareness with continuous real-time EEG and cerebral oxygenation (rSO2) monitoring into CPR.
Only 53 of the 567 IHCA patients survived (9.3%). Among the 28 (52.8%) IHCA survivors who completed interviews, 11 (39.3%) reported unique, lucid experiences during resuscitation.
These experiences included a perception of separation from one’s body, observing events without pain or distress, and an awareness and meaningful evaluation of life, including of their actions, intentions, and thoughts toward others.
“These lucid experiences of death are not hallucinations or delusions. They cannot be considered a trick of a disordered or dying brain, but rather a unique human experience that emerges on the brink of death,” Dr. Parnia said.
And what’s “fascinating,” he added, is that despite marked cerebral ischemia (mean regional oxygen saturation [rSO2] 43%), near-normal/physiologic EEG activity (gamma, delta, theta, alpha, and beta rhythms) consistent with consciousness and a possible resumption of a network-level of cognitive and neuronal activity emerged for as long as 35-60 minutes into CPR.
Some of these brain waves normally occur when people are conscious and performing higher mental functions, including thinking, memory retrieval, and conscious perception, he said.
‘Seismic shift’ in understanding of death
This is the first time such biomarkers of consciousness have been identified during cardiac arrest and CPR, Dr. Parnia said.
He said further study is needed to more precisely define biomarkers of what is considered to be clinical consciousness and the recalled experience of death, and to monitor the long-term psychological effects of resuscitation after cardiac arrest.
“Our understanding of death has gone through a seismic shift in the last few years,” he said.
“The biological discoveries around death and the postmortem period are completely different to the social conventions that we have about death. That is, we perceive of death as being the end, but actually what we’re finding is that brain cells don’t die immediately. They die very slowly over many hours of time,” Dr. Parnia noted.
Reached for comment, Ajmal Zemmar, MD, PhD, of University of Louisville (Ky.), noted that several studies, including this one, “challenge the traditional way that we think of death – that when the heart stops beating that’s when we die.”
The observation that during cardiac arrest and CPR, the brain waves are still normal for up to an hour is “fairly remarkable,” Dr. Zemmar told this news organization.
“However, whether there is conscious perception or not is very hard to answer,” he cautioned.
“This type of research tries to bridge the objective EEG recordings with the subjective description you get from the patient, but it’s hard to know when conscious perception stops,” he said.
Funding and support for the study were provided by NYU Langone Health, The John Templeton Foundation, and the UK Resuscitation Council, and National Institutes for Health Research. Dr. Parnia and Dr. Zemmar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“These recalled experiences and brain wave changes may be the first signs of the so-called ‘near-death’ experience, and we have captured them for the first time in a large study,” lead investigator Sam Parnia, MD, PhD, with NYU Langone Health, said in a news release.
Identifying measurable electrical signs of lucid and heightened brain activity during CPR, coupled with stories of recalled near-death experiences, suggests that the human sense of self and consciousness, much like other biological body functions, may not stop completely around the time of death, Dr. Parnia added.
He presented the findings Nov. 6 at a resuscitation science symposium at the American Heart Association scientific sessions.
The AWARE II study
“For years, some people in cardiac arrest have reported being lucid, often with a heightened sense of consciousness, while seemingly unconscious and on the brink of death,” Dr. Parnia noted in an interview.
“Yet, no one’s ever be able to prove it and a lot of people have dismissed these experiences, thinking it’s all just a trick on the brain,” Dr. Parnia said.
In a first-of-its-kind study, Dr. Parnia and colleagues examined consciousness and its underlying electrocortical biomarkers during CPR for in-hospital cardiac arrest (IHCA).
They incorporated independent audiovisual testing of awareness with continuous real-time EEG and cerebral oxygenation (rSO2) monitoring into CPR.
Only 53 of the 567 IHCA patients survived (9.3%). Among the 28 (52.8%) IHCA survivors who completed interviews, 11 (39.3%) reported unique, lucid experiences during resuscitation.
These experiences included a perception of separation from one’s body, observing events without pain or distress, and an awareness and meaningful evaluation of life, including of their actions, intentions, and thoughts toward others.
“These lucid experiences of death are not hallucinations or delusions. They cannot be considered a trick of a disordered or dying brain, but rather a unique human experience that emerges on the brink of death,” Dr. Parnia said.
And what’s “fascinating,” he added, is that despite marked cerebral ischemia (mean regional oxygen saturation [rSO2] 43%), near-normal/physiologic EEG activity (gamma, delta, theta, alpha, and beta rhythms) consistent with consciousness and a possible resumption of a network-level of cognitive and neuronal activity emerged for as long as 35-60 minutes into CPR.
Some of these brain waves normally occur when people are conscious and performing higher mental functions, including thinking, memory retrieval, and conscious perception, he said.
‘Seismic shift’ in understanding of death
This is the first time such biomarkers of consciousness have been identified during cardiac arrest and CPR, Dr. Parnia said.
He said further study is needed to more precisely define biomarkers of what is considered to be clinical consciousness and the recalled experience of death, and to monitor the long-term psychological effects of resuscitation after cardiac arrest.
“Our understanding of death has gone through a seismic shift in the last few years,” he said.
“The biological discoveries around death and the postmortem period are completely different to the social conventions that we have about death. That is, we perceive of death as being the end, but actually what we’re finding is that brain cells don’t die immediately. They die very slowly over many hours of time,” Dr. Parnia noted.
Reached for comment, Ajmal Zemmar, MD, PhD, of University of Louisville (Ky.), noted that several studies, including this one, “challenge the traditional way that we think of death – that when the heart stops beating that’s when we die.”
The observation that during cardiac arrest and CPR, the brain waves are still normal for up to an hour is “fairly remarkable,” Dr. Zemmar told this news organization.
“However, whether there is conscious perception or not is very hard to answer,” he cautioned.
“This type of research tries to bridge the objective EEG recordings with the subjective description you get from the patient, but it’s hard to know when conscious perception stops,” he said.
Funding and support for the study were provided by NYU Langone Health, The John Templeton Foundation, and the UK Resuscitation Council, and National Institutes for Health Research. Dr. Parnia and Dr. Zemmar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Sacral nerve stimulation may aid female sexual dysfunction
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
FROM DIE GYNÄKOLOGIE
Promising new antibiotic emerges for treating UTIs
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
Link between PCOS and increased risk of pancreatic cancer?
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) may be at a higher risk of developing pancreatic cancer, say researchers reporting a single-center case-control study.
A diagnosis of PCOS was associated with a 1.9-fold higher risk of pancreatic cancer after adjusting for age, race, ethnicity, estrogen level, and diabetes.
This is the second study to find such an association.
“Our study findings combined with those from the 2019 Swedish Registry study offer compelling evidence that PCOS may be a novel risk factor for pancreatic cancer,” said corresponding author Mengmeng Du, ScD, department of epidemiology and biostatistics, Memorial Sloan Kettering Cancer Center.
“These data suggest some individuals may have unknown metabolic derangements that may underlie the development of both conditions,” the team concluded.
The findings were published in JAMA Oncology.
Approached for comment, Srinivas Gaddam, MD, MPH, associate director of pancreatic biliary research medicine, Cedars-Sinai, suggested that the findings may pave the way for a better understanding of the two diseases, but he emphasized that more research is needed.
“I think there’s more research to be done because now we’re seeing more younger women get pancreatic cancer,” Dr. Gaddam said. “So that makes it interesting whether PCOS itself contributes to pancreatic cancer. I still think the jury is out there.”
Dr. Gaddam drew attention to the confidence interval for the finding – the adjusted odds ratio was 1.88 (95% confidence interval, 1.02-3.46). “Because their odds ratio includes 1, I’m left with the question as to whether or not this is truly associated. I’m not certain that we can draw any conclusions based on this,” he commented.
The investigators acknowledge that they did “not observe statistically significant interactions” and comment that “prospective studies are needed to examine underlying biologic mechanisms and confirm our findings.”
For the study, the team used data from the Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. They identified patients with pancreatic cancer who also self-reported a diagnosis of PCOS.
The investigators compared data from 446 women with pathologically or cytologically confirmed pancreatic adenocarcinoma with 209 women who had no history of cancer. The mean age at cancer diagnosis or enrollment was 63.8 years among patients with pancreatic cancer and 57.7 years in the control group.
The study found that having PCOS nearly doubled a person’s risk of developing pancreatic cancer.
When adjusted for type 2 diabetes diagnosis, the odds ratio fell slightly to 1.78 (95% CI, 0.95-3.34).
Dr. Du, along with lead author Noah Peeri, PhD, were surprised that even after adjusting for body mass index and the presence of type 2 diabetes, PCOS remained strongly associated with pancreatic cancer risk.
“We originally thought type 2 diabetes may drive this association, given more than half of those with PCOS develop type 2 diabetes by age 40, according to the CDC, and type 2 diabetes has also been linked with increased pancreatic cancer risk,” said Dr. Du.
“While the association was slightly weaker and no longer statistically significant after we controlled for type 2 diabetes, the magnitude of the association remained largely unchanged,” he said.
Dr. Peeri believes that some of the factors that have been causally related to PCOS may increase an individual’s pancreatic cancer risk.
“PCOS itself does not likely cause pancreatic cancer, but metabolic problems (for example, improper breakdown of insulin) and chronic inflammation can contribute to both PCOS and pancreatic cancer risk,” Dr. Peeri said.
He concluded that the study results “suggest other underlying metabolic dysfunction may increase an individual’s pancreatic cancer risk.”
An important limitation of this study was that women in the study self-reported PCOS and may have incorrectly recalled their diagnosis. However, the authors believe it is unlikely that that had a bearing on the study findings.
The study was supported by National Cancer Institute grants, the Geoffrey Beene Foundation, and the Arnold and Arlene Goldstein Family Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
CDC warns of early uptick in respiratory disease
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
New trial suggests CV benefit with EPA: RESPECT-EPA
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Chinese herbal medicine may offer benefits in STEMI: CTS-AMI
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
AT AHA 2022