User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
New consensus on thyroid eye disease prompts some debate
A new consensus statement from the American Thyroid Association (ATA) and European Thyroid Association (ETA) offers recommendations for endocrinologists on the management of thyroid eye disease (TED), addressing key questions, including about important novel treatments, that transcend international borders.
The consensus statement is important as new therapies transform the treatment of TED that, notably, have even played a key role in simplifying the name of the disease, which has had numerous other, often confusing names over the years, ranging from thyrotropic exophthalmos to Graves ophthalmopathy, Terry F. Davies, MD, of the thyroid research unit, department of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an editorial published along with the statement in Thyroid.
“The emergence of novel therapies has changed the entire discussion concerning TED and not just its name,” he wrote. “These are early and exciting days in the treatment of TED, which is likely to be a much more manageable disease in the years to come.”
However, Dr. Davies stressed to this news organization that there are still a lot of unanswered questions, particularly when it comes to newer therapies. For example, teprotumumab can cost up to $300,000 for one course of treatment for one patient, the consensus statement notes.
When to consult an ophthalmologist
Graves disease is the most common cause of hyperthyroidism and affects > 1% of the U.S. population. TED is the most common complication of Graves disease that occurs outside of the thyroid gland. TED causes a variety of eye-related signs and symptoms, which can be disfiguring and negatively affect quality of life, and in rare cases, threaten vision.
Key issues covered in the consensus statement include timely diagnosis of TED, assessment of disease activity and severity, initial care and referral for specialty care, and treatment recommendations for moderate to severe TED.
In terms of disease assessment, for instance, the statement authors noted the important distinction in TED “between the two interdependent components of inflammatory activity, manifested by pain, redness, and edema, and disease severity, including proptosis, lid malposition, exposure keratopathy, impaired ocular motility, and optic neuropathy.”
“The presence of multiple features of inflammation usually signifies active disease,” they explained.
For initial care, input from endocrinologists as well as ophthalmologists with experience in TED management is urged, and “an ophthalmologist should be consulted when the diagnosis of TED is uncertain, in cases of moderate to severe TED, and when surgical intervention needs to be considered.”
Furthermore, “urgent referral is required when sight-threatening TED is suspected or confirmed,” the authors noted.
Debate over some treatment recommendations
In terms of therapy, for initial care, “a single course of selenium selenite 100 mcg twice daily for 6 months may be considered for patients with mild, active TED, particularly in regions of selenium insufficiency,” the consensus statement recommends.
Intravenous glucocorticoid (IVGC) therapy is meanwhile recommended as a preferred treatment for active moderate to severe TED specifically when disease activity is the prominent feature in the absence of significant proptosis or diplopia.
For patients with active moderate to severe TED who are glucocorticoid-resistant, the authors noted that rituximab and tocilizumab may be considered and that teprotumumab has not been evaluated in this setting.
Teprotumumab, if available, is a preferred therapy for patients with active moderate to severe TED who have significant proptosis.
There is, however, some debate over the issue, editorial author Dr. Davies told this news organization.
“It is still argued over how bad the eyes need to be before recommending this new treatment,” he said. “I think the answer is in the proptosis – the amount of bulging present rather than just inflammation,” Dr. Davies said.
“There is also a real clinical problem in that we have no specific biomarker for the disease, however, high levels of TSH receptor antibody are often a good indicator of eye disease.”
The authors cautioned, however, that clinical trials with medical therapies have been limited by inclusion criteria and other factors, and biologics have meanwhile increased the cost of treatment “many-fold” compared with conventional agents.
Therefore, “clinicians should balance the demonstrated efficacy of recently introduced therapies [such as teprotumumab] against the absence of experience on sustained long-term efficacy, safety, and cost-effectiveness,” they noted.
Importantly, “one course consisting of eight infusions of teprotumumab has a retail cost of approximately $300,000, depending on patient weight, [which is] approximately 2,000 times that of IVGC,” they noted.
“The process involved in selecting therapy with these drugs and other drugs includes a consideration of both short- and long-term efficacy, adverse effects that are both known and unknown, the likelihood of disease aggravation or relapse after a previously beneficial response, and the relative cost and availability,” said Henry B. Burch, MD, who is at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, in Bethesda, Md., and is on the consensus statement task force.
To help with those decisions, the consensus statement provides comprehensive tables that compare drug efficacy for key outcomes including inflammation, proptosis, diplopia, and quality of life, and importantly, comparisons also of drug costs and potential adverse effects for each of the current TED therapies.
Consensus statement not a guideline
The groups noted that the consensus statement is not meant to be a clinical practice guideline and was not written to “establish a standard of care, replace sound clinical judgment, or capture all nuances likely to be present in any particular patient,” and “specific outcomes are not guaranteed.”
What the statement is intended for is to “provide a concise and timely appraisal of a rapidly changing therapeutic arena” for practicing endocrinologists, they explained.
Overall, the authors recommend an individualized management approach, based on factors ranging from disease severity, duration, its impact on daily living, patient age, comorbidities, and importantly, the costs of therapies.
Ultimately, patient satisfaction is essential in TED management, Dr. Burch added.
“Consideration of the impact of TED on patient’s satisfaction with their appearance and visual functioning is a key component in management decisions concerning TED.”A version of this article first appeared on Medscape.com.
A new consensus statement from the American Thyroid Association (ATA) and European Thyroid Association (ETA) offers recommendations for endocrinologists on the management of thyroid eye disease (TED), addressing key questions, including about important novel treatments, that transcend international borders.
The consensus statement is important as new therapies transform the treatment of TED that, notably, have even played a key role in simplifying the name of the disease, which has had numerous other, often confusing names over the years, ranging from thyrotropic exophthalmos to Graves ophthalmopathy, Terry F. Davies, MD, of the thyroid research unit, department of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an editorial published along with the statement in Thyroid.
“The emergence of novel therapies has changed the entire discussion concerning TED and not just its name,” he wrote. “These are early and exciting days in the treatment of TED, which is likely to be a much more manageable disease in the years to come.”
However, Dr. Davies stressed to this news organization that there are still a lot of unanswered questions, particularly when it comes to newer therapies. For example, teprotumumab can cost up to $300,000 for one course of treatment for one patient, the consensus statement notes.
When to consult an ophthalmologist
Graves disease is the most common cause of hyperthyroidism and affects > 1% of the U.S. population. TED is the most common complication of Graves disease that occurs outside of the thyroid gland. TED causes a variety of eye-related signs and symptoms, which can be disfiguring and negatively affect quality of life, and in rare cases, threaten vision.
Key issues covered in the consensus statement include timely diagnosis of TED, assessment of disease activity and severity, initial care and referral for specialty care, and treatment recommendations for moderate to severe TED.
In terms of disease assessment, for instance, the statement authors noted the important distinction in TED “between the two interdependent components of inflammatory activity, manifested by pain, redness, and edema, and disease severity, including proptosis, lid malposition, exposure keratopathy, impaired ocular motility, and optic neuropathy.”
“The presence of multiple features of inflammation usually signifies active disease,” they explained.
For initial care, input from endocrinologists as well as ophthalmologists with experience in TED management is urged, and “an ophthalmologist should be consulted when the diagnosis of TED is uncertain, in cases of moderate to severe TED, and when surgical intervention needs to be considered.”
Furthermore, “urgent referral is required when sight-threatening TED is suspected or confirmed,” the authors noted.
Debate over some treatment recommendations
In terms of therapy, for initial care, “a single course of selenium selenite 100 mcg twice daily for 6 months may be considered for patients with mild, active TED, particularly in regions of selenium insufficiency,” the consensus statement recommends.
Intravenous glucocorticoid (IVGC) therapy is meanwhile recommended as a preferred treatment for active moderate to severe TED specifically when disease activity is the prominent feature in the absence of significant proptosis or diplopia.
For patients with active moderate to severe TED who are glucocorticoid-resistant, the authors noted that rituximab and tocilizumab may be considered and that teprotumumab has not been evaluated in this setting.
Teprotumumab, if available, is a preferred therapy for patients with active moderate to severe TED who have significant proptosis.
There is, however, some debate over the issue, editorial author Dr. Davies told this news organization.
“It is still argued over how bad the eyes need to be before recommending this new treatment,” he said. “I think the answer is in the proptosis – the amount of bulging present rather than just inflammation,” Dr. Davies said.
“There is also a real clinical problem in that we have no specific biomarker for the disease, however, high levels of TSH receptor antibody are often a good indicator of eye disease.”
The authors cautioned, however, that clinical trials with medical therapies have been limited by inclusion criteria and other factors, and biologics have meanwhile increased the cost of treatment “many-fold” compared with conventional agents.
Therefore, “clinicians should balance the demonstrated efficacy of recently introduced therapies [such as teprotumumab] against the absence of experience on sustained long-term efficacy, safety, and cost-effectiveness,” they noted.
Importantly, “one course consisting of eight infusions of teprotumumab has a retail cost of approximately $300,000, depending on patient weight, [which is] approximately 2,000 times that of IVGC,” they noted.
“The process involved in selecting therapy with these drugs and other drugs includes a consideration of both short- and long-term efficacy, adverse effects that are both known and unknown, the likelihood of disease aggravation or relapse after a previously beneficial response, and the relative cost and availability,” said Henry B. Burch, MD, who is at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, in Bethesda, Md., and is on the consensus statement task force.
To help with those decisions, the consensus statement provides comprehensive tables that compare drug efficacy for key outcomes including inflammation, proptosis, diplopia, and quality of life, and importantly, comparisons also of drug costs and potential adverse effects for each of the current TED therapies.
Consensus statement not a guideline
The groups noted that the consensus statement is not meant to be a clinical practice guideline and was not written to “establish a standard of care, replace sound clinical judgment, or capture all nuances likely to be present in any particular patient,” and “specific outcomes are not guaranteed.”
What the statement is intended for is to “provide a concise and timely appraisal of a rapidly changing therapeutic arena” for practicing endocrinologists, they explained.
Overall, the authors recommend an individualized management approach, based on factors ranging from disease severity, duration, its impact on daily living, patient age, comorbidities, and importantly, the costs of therapies.
Ultimately, patient satisfaction is essential in TED management, Dr. Burch added.
“Consideration of the impact of TED on patient’s satisfaction with their appearance and visual functioning is a key component in management decisions concerning TED.”A version of this article first appeared on Medscape.com.
A new consensus statement from the American Thyroid Association (ATA) and European Thyroid Association (ETA) offers recommendations for endocrinologists on the management of thyroid eye disease (TED), addressing key questions, including about important novel treatments, that transcend international borders.
The consensus statement is important as new therapies transform the treatment of TED that, notably, have even played a key role in simplifying the name of the disease, which has had numerous other, often confusing names over the years, ranging from thyrotropic exophthalmos to Graves ophthalmopathy, Terry F. Davies, MD, of the thyroid research unit, department of medicine, Icahn School of Medicine at Mount Sinai, New York, said in an editorial published along with the statement in Thyroid.
“The emergence of novel therapies has changed the entire discussion concerning TED and not just its name,” he wrote. “These are early and exciting days in the treatment of TED, which is likely to be a much more manageable disease in the years to come.”
However, Dr. Davies stressed to this news organization that there are still a lot of unanswered questions, particularly when it comes to newer therapies. For example, teprotumumab can cost up to $300,000 for one course of treatment for one patient, the consensus statement notes.
When to consult an ophthalmologist
Graves disease is the most common cause of hyperthyroidism and affects > 1% of the U.S. population. TED is the most common complication of Graves disease that occurs outside of the thyroid gland. TED causes a variety of eye-related signs and symptoms, which can be disfiguring and negatively affect quality of life, and in rare cases, threaten vision.
Key issues covered in the consensus statement include timely diagnosis of TED, assessment of disease activity and severity, initial care and referral for specialty care, and treatment recommendations for moderate to severe TED.
In terms of disease assessment, for instance, the statement authors noted the important distinction in TED “between the two interdependent components of inflammatory activity, manifested by pain, redness, and edema, and disease severity, including proptosis, lid malposition, exposure keratopathy, impaired ocular motility, and optic neuropathy.”
“The presence of multiple features of inflammation usually signifies active disease,” they explained.
For initial care, input from endocrinologists as well as ophthalmologists with experience in TED management is urged, and “an ophthalmologist should be consulted when the diagnosis of TED is uncertain, in cases of moderate to severe TED, and when surgical intervention needs to be considered.”
Furthermore, “urgent referral is required when sight-threatening TED is suspected or confirmed,” the authors noted.
Debate over some treatment recommendations
In terms of therapy, for initial care, “a single course of selenium selenite 100 mcg twice daily for 6 months may be considered for patients with mild, active TED, particularly in regions of selenium insufficiency,” the consensus statement recommends.
Intravenous glucocorticoid (IVGC) therapy is meanwhile recommended as a preferred treatment for active moderate to severe TED specifically when disease activity is the prominent feature in the absence of significant proptosis or diplopia.
For patients with active moderate to severe TED who are glucocorticoid-resistant, the authors noted that rituximab and tocilizumab may be considered and that teprotumumab has not been evaluated in this setting.
Teprotumumab, if available, is a preferred therapy for patients with active moderate to severe TED who have significant proptosis.
There is, however, some debate over the issue, editorial author Dr. Davies told this news organization.
“It is still argued over how bad the eyes need to be before recommending this new treatment,” he said. “I think the answer is in the proptosis – the amount of bulging present rather than just inflammation,” Dr. Davies said.
“There is also a real clinical problem in that we have no specific biomarker for the disease, however, high levels of TSH receptor antibody are often a good indicator of eye disease.”
The authors cautioned, however, that clinical trials with medical therapies have been limited by inclusion criteria and other factors, and biologics have meanwhile increased the cost of treatment “many-fold” compared with conventional agents.
Therefore, “clinicians should balance the demonstrated efficacy of recently introduced therapies [such as teprotumumab] against the absence of experience on sustained long-term efficacy, safety, and cost-effectiveness,” they noted.
Importantly, “one course consisting of eight infusions of teprotumumab has a retail cost of approximately $300,000, depending on patient weight, [which is] approximately 2,000 times that of IVGC,” they noted.
“The process involved in selecting therapy with these drugs and other drugs includes a consideration of both short- and long-term efficacy, adverse effects that are both known and unknown, the likelihood of disease aggravation or relapse after a previously beneficial response, and the relative cost and availability,” said Henry B. Burch, MD, who is at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, in Bethesda, Md., and is on the consensus statement task force.
To help with those decisions, the consensus statement provides comprehensive tables that compare drug efficacy for key outcomes including inflammation, proptosis, diplopia, and quality of life, and importantly, comparisons also of drug costs and potential adverse effects for each of the current TED therapies.
Consensus statement not a guideline
The groups noted that the consensus statement is not meant to be a clinical practice guideline and was not written to “establish a standard of care, replace sound clinical judgment, or capture all nuances likely to be present in any particular patient,” and “specific outcomes are not guaranteed.”
What the statement is intended for is to “provide a concise and timely appraisal of a rapidly changing therapeutic arena” for practicing endocrinologists, they explained.
Overall, the authors recommend an individualized management approach, based on factors ranging from disease severity, duration, its impact on daily living, patient age, comorbidities, and importantly, the costs of therapies.
Ultimately, patient satisfaction is essential in TED management, Dr. Burch added.
“Consideration of the impact of TED on patient’s satisfaction with their appearance and visual functioning is a key component in management decisions concerning TED.”A version of this article first appeared on Medscape.com.
Advanced Primary Care program boosts COVID-19 results
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
The better outcomes were seen in higher vaccination rates and fewer infections, hospitalizations, and deaths from the disease, according to study authors, led by Emily Gruber, MBA, MPH, with the Maryland Primary Care Program, Maryland Department of Health in Baltimore.
The results were published online in JAMA Network Open.
The study population was divided into MDPCP participants (n = 208,146) and a matched cohort (n = 37,203) of beneficiaries not attributed to MDPCP practices but who met eligibility criteria for study participation from Jan. 1, 2020, through Dec. 31, 2021.
More vaccinations, more antibody treatments
Researchers broke down the comparisons of better outcomes: 84.47% of MDPCP beneficiaries were fully vaccinated vs. 77.93% of nonparticipating beneficiaries (P less than .001). COVID-19–positive program beneficiaries also received monoclonal antibody treatment more often (8.45% vs. 6.11%; P less than .001).
Plus, program participants received more care via telehealth (62.95% vs. 54.53%; P less than .001) compared with those not participating.
Regarding secondary outcomes, MDPCP beneficiaries had lower rates of COVID cases (6.55% vs. 7.09%; P less than .001), lower rates of COVID-19 hospitalizations (1.81% vs. 2.06%; P = .001), and lower rates of death due to COVID-19 (0.56% vs. 0.77%; P less than .001).
Program components
Enrollment in the MDPCP is voluntary, and primary care practices can apply each year to be part of the program.
The model integrates primary care and public health in the pandemic response. It was created by the Maryland Department of Health (MDH) and the Centers for Medicare & Medicaid Services (CMS).
It expands the role of primary care to include services such as expanded care management, integrated behavioral health, data-driven care, and screenings and referrals to address social needs.
Coauthor Howard Haft, MD, MMM, with the Maryland Department of Public Health, said in an interview that among the most important factors in the program’s success were giving providers vaccines to distribute and then giving providers data on how many patients are vaccinated, and who’s not vaccinated but at high risk, and how those rates compare to other practices.
As to whether this could be a widespread model, Dr. Haft said, “It’s highly replicable.”
“Every state in the nation overall has all of these resources. It’s a matter of having the operational and political will to put those resources together. Almost every state has the technological ability to use their health information exchange to help tie pieces together.”
Vaccines and testing made available to providers
Making ample vaccines and testing available to providers in their offices helped patients get those services in a place they trust, Dr. Haft said.
The model also included a payment system for providers that included a significant amount of non–visit-based payments when many locations were closed in the height of the pandemic.
“That helped financially,” as did providing free telehealth platforms to practices with training on how to use them, Dr. Haft said.
‘Innovative and important’
Renu Tipirneni, MD, an assistant professor of internal medicine at the University of Michigan and at the Institute for Healthcare Policy and Innovation in Ann Arbor, said Maryland is out front putting into practice what practices nationwide aspire to do – coordinating physical and mental health and social needs and integrating primary and public health. Dr. Tipirneni, who was not involved with the study, said she was impressed the researchers were able to show statistically significant improvement with COVID-19 outcomes in the first 2 years.
“In terms of health outcomes, we often have to wait longer to see good outcomes,” she said. “It’s a really innovative and important model.”
She said states can learn from each other and this model is an example.
Integrating primary care and public health and addressing social needs may be the biggest challenges for states, she said, as those realms typically have been siloed.
“But they may be the key components to achieving these outcomes,” she said.
Take-home message
The most important benefit of the program is that data suggest it saves lives, according to Dr. Haft. While the actual difference between COVID deaths in the program and nonprogram groups was small, multiplying that savings across the nation shows substantial potential benefit, he explained.
“At a time when we were losing lives at an unconscionable rate, we were able to make a difference in saving lives,” Dr. Haft said.
Authors report no relevant financial disclosures.
The study received financial support from the Maryland Department of Health.
Dr. Tiperneni is helping evaluate Michigan’s Medicaid contract.
FROM JAMA NETWORK OPEN
Do collagen supplements benefit the skin?
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2022
Berdazimer gel under review at FDA for treating molluscum contagiosum
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
, the manufacturer announced.
If the submission is accepted by the FDA, the topical product could be approved in the first quarter of 2024, according to a press release from Novan, the manufacturer. If approved, it would be the first-in-class topical treatment for MC, the common, contagious viral skin infection that affects approximately six million individuals in the United States each year, most of them children aged 1-14 years, the statement noted. No FDA-approved therapies currently exist for the condition, which causes unsightly lesions on the face, trunk, limbs, and axillae that may persist untreated for a period of years.
The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a nitric oxide–releasing agent. A 3.4% formulation is in development for the topical treatment of acne, according to the company.
The submission for FDA approval is based on data from the B-SIMPLE4 study, a phase 3 randomized trial of nearly 900 individuals with MC aged 6 months and older (mean age, 6.6 years), with 3-70 raised lesions. Participants were randomized to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily for 12 weeks. The results were published in JAMA Dermatology.
The primary outcome was complete clearance of all lesions. At 12 weeks, 32.4% of patients in the berdazimer group achieved this outcome vs. 19.7% of those in the vehicle group (P < .001). Overall adverse event rates were low in both groups; 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events across both groups were application-site pain and erythema, and most of these were mild or moderate.
Latest steps toward reducing U.S. insulin cost begin in 2023
As of Jan. 1, 2023, the new provision tucked into the Inflation Reduction Act, signed into law by President Biden in August 2022, means that beneficiaries who take insulin via pen or syringe, covered under Medicare part D (prescription drugs), fall under the $35/month co-pay cap.
On July 1, 2023, the same out-of-pocket limit will also apply to those who take insulin via pump, which falls under Medicare part B (durable medical equipment).
The bill originally included the co-pay cap for people with private insurance as well, but that was stripped out as part of the reconciliation process and didn’t garner the necessary 60 Senate votes to keep it in prior to passage.
However, since 2019, 22 U.S. states have passed their own co-pay caps for people with state-regulated private insurance, ranging from $25 to $100 for a 30-day supply. A few states also cap the cost of diabetes devices as well.
Moreover, federal legislation could still address co-pay caps for people with private insurance, as well as include provisions to help those without insurance to afford insulin, Niels Knutson, director of government relations for the type 1 diabetes advocacy organization JDRF, told this news organization.
“There’s a whole menu of ideas on how to address the issue of insulin affordability. Most pathways to solving this on the federal level will require 60 votes in the Senate. There is universal recognition that this is a problem. The challenge becomes: is everybody on the same page for how to fix it,” Mr. Knutson said.
JDRF is supporting the bipartisan Improving Needed Safeguards for Users of Lifesaving Insulin Now (INSULIN) Act, introduced in June 2022 by U.S. Senators Jeanne Shaheen (D-NH) and Susan Collins (R-ME), who co-chair the Senate Diabetes Caucus. The bill includes a co-pay cap and also provisions to encourage insulin manufacturers to reduce their list prices.
“The bill is unique in that it adds a pathway to reduce the cost of insulin for everybody, regardless of whether they have insurance or not ... We see the Insulin Act as being the best path forward and the most viable path to have the biggest impact for the most people,” Mr. Knutson explained.
At the same time, JDRF is also supporting a nonprofit pharmaceutical company called Civica, which plans to bring biosimilar versions of the insulin analogs glargine, lispro, and aspart to the U.S. market by 2024 at a cost of no more than $30 for a vial and $50 for a box of prefilled pens. The state of California is expected to partner with Civica as well.
“This is just another access point for insulin, especially for folks who are uninsured, that would make a big impact,” Mr. Knutson said.
Other entities that have announced intentions to bring lower-cost insulin to the United States market include the Korean firm Undbio and billionaire entrepreneur Mark Cuban, through his company Cost Plus Drugs.
“Insulin is such a clear and present crisis that we need to address,” Mr. Knutson said. “You’re seeing this problem being recognized and solutions from all different angles coming at it.”
A version of this article first appeared on Medscape.com.
As of Jan. 1, 2023, the new provision tucked into the Inflation Reduction Act, signed into law by President Biden in August 2022, means that beneficiaries who take insulin via pen or syringe, covered under Medicare part D (prescription drugs), fall under the $35/month co-pay cap.
On July 1, 2023, the same out-of-pocket limit will also apply to those who take insulin via pump, which falls under Medicare part B (durable medical equipment).
The bill originally included the co-pay cap for people with private insurance as well, but that was stripped out as part of the reconciliation process and didn’t garner the necessary 60 Senate votes to keep it in prior to passage.
However, since 2019, 22 U.S. states have passed their own co-pay caps for people with state-regulated private insurance, ranging from $25 to $100 for a 30-day supply. A few states also cap the cost of diabetes devices as well.
Moreover, federal legislation could still address co-pay caps for people with private insurance, as well as include provisions to help those without insurance to afford insulin, Niels Knutson, director of government relations for the type 1 diabetes advocacy organization JDRF, told this news organization.
“There’s a whole menu of ideas on how to address the issue of insulin affordability. Most pathways to solving this on the federal level will require 60 votes in the Senate. There is universal recognition that this is a problem. The challenge becomes: is everybody on the same page for how to fix it,” Mr. Knutson said.
JDRF is supporting the bipartisan Improving Needed Safeguards for Users of Lifesaving Insulin Now (INSULIN) Act, introduced in June 2022 by U.S. Senators Jeanne Shaheen (D-NH) and Susan Collins (R-ME), who co-chair the Senate Diabetes Caucus. The bill includes a co-pay cap and also provisions to encourage insulin manufacturers to reduce their list prices.
“The bill is unique in that it adds a pathway to reduce the cost of insulin for everybody, regardless of whether they have insurance or not ... We see the Insulin Act as being the best path forward and the most viable path to have the biggest impact for the most people,” Mr. Knutson explained.
At the same time, JDRF is also supporting a nonprofit pharmaceutical company called Civica, which plans to bring biosimilar versions of the insulin analogs glargine, lispro, and aspart to the U.S. market by 2024 at a cost of no more than $30 for a vial and $50 for a box of prefilled pens. The state of California is expected to partner with Civica as well.
“This is just another access point for insulin, especially for folks who are uninsured, that would make a big impact,” Mr. Knutson said.
Other entities that have announced intentions to bring lower-cost insulin to the United States market include the Korean firm Undbio and billionaire entrepreneur Mark Cuban, through his company Cost Plus Drugs.
“Insulin is such a clear and present crisis that we need to address,” Mr. Knutson said. “You’re seeing this problem being recognized and solutions from all different angles coming at it.”
A version of this article first appeared on Medscape.com.
As of Jan. 1, 2023, the new provision tucked into the Inflation Reduction Act, signed into law by President Biden in August 2022, means that beneficiaries who take insulin via pen or syringe, covered under Medicare part D (prescription drugs), fall under the $35/month co-pay cap.
On July 1, 2023, the same out-of-pocket limit will also apply to those who take insulin via pump, which falls under Medicare part B (durable medical equipment).
The bill originally included the co-pay cap for people with private insurance as well, but that was stripped out as part of the reconciliation process and didn’t garner the necessary 60 Senate votes to keep it in prior to passage.
However, since 2019, 22 U.S. states have passed their own co-pay caps for people with state-regulated private insurance, ranging from $25 to $100 for a 30-day supply. A few states also cap the cost of diabetes devices as well.
Moreover, federal legislation could still address co-pay caps for people with private insurance, as well as include provisions to help those without insurance to afford insulin, Niels Knutson, director of government relations for the type 1 diabetes advocacy organization JDRF, told this news organization.
“There’s a whole menu of ideas on how to address the issue of insulin affordability. Most pathways to solving this on the federal level will require 60 votes in the Senate. There is universal recognition that this is a problem. The challenge becomes: is everybody on the same page for how to fix it,” Mr. Knutson said.
JDRF is supporting the bipartisan Improving Needed Safeguards for Users of Lifesaving Insulin Now (INSULIN) Act, introduced in June 2022 by U.S. Senators Jeanne Shaheen (D-NH) and Susan Collins (R-ME), who co-chair the Senate Diabetes Caucus. The bill includes a co-pay cap and also provisions to encourage insulin manufacturers to reduce their list prices.
“The bill is unique in that it adds a pathway to reduce the cost of insulin for everybody, regardless of whether they have insurance or not ... We see the Insulin Act as being the best path forward and the most viable path to have the biggest impact for the most people,” Mr. Knutson explained.
At the same time, JDRF is also supporting a nonprofit pharmaceutical company called Civica, which plans to bring biosimilar versions of the insulin analogs glargine, lispro, and aspart to the U.S. market by 2024 at a cost of no more than $30 for a vial and $50 for a box of prefilled pens. The state of California is expected to partner with Civica as well.
“This is just another access point for insulin, especially for folks who are uninsured, that would make a big impact,” Mr. Knutson said.
Other entities that have announced intentions to bring lower-cost insulin to the United States market include the Korean firm Undbio and billionaire entrepreneur Mark Cuban, through his company Cost Plus Drugs.
“Insulin is such a clear and present crisis that we need to address,” Mr. Knutson said. “You’re seeing this problem being recognized and solutions from all different angles coming at it.”
A version of this article first appeared on Medscape.com.
Gene associated with vision loss also linked to COVID: Study
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
age-related macular degeneration.
The findings show that COVID and AMD were associated with variations in what is called the PDGFB gene, which has a role in new blood vessel formation and is linked to abnormal blood vessel changes that occur in AMD. The study was published in the Journal of Clinical Medicine. The analysis included genetic data from more than 16,000 people with AMD, more than 50,000 people with COVID, plus control groups.
Age-related macular degeneration is a vision problem that occurs when a part of the retina – the macula – is damaged, according to the American Academy of Ophthalmology. The result is that central vision is lost, but peripheral vision remains normal, so it is difficult to see fine details. For example, a person with AMD can see a clock’s numbers but not its hands.
“Our analysis lends credence to previously reported clinical studies that found those with AMD have a higher risk for COVID-19 infection and severe disease, and that this increased risk may have a genetic basis,” Boston University researcher Lindsay Farrer, PhD, chief of biomedical genetics, explained in a news release.
Previous research has shown that people with AMD have a 25% increased risk of respiratory failure or death due to COVID, which is higher than other well-known risk factors such as type 2 diabetes (21%) or obesity (13%), according to the news release.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF CLINICAL MEDICINE
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Science reveals link between gut health and exercise motivation
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
FROM NATURE
Lifestyle changes may reduce colorectal cancer risk
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.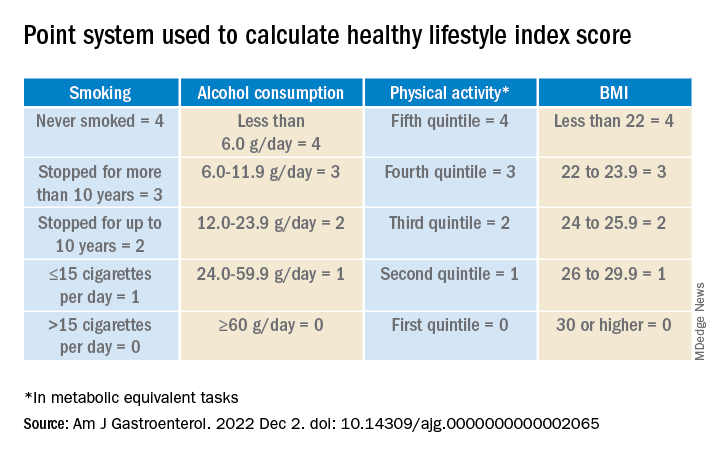
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Long COVID clinical trials may offer shortcut to new treatments
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.



