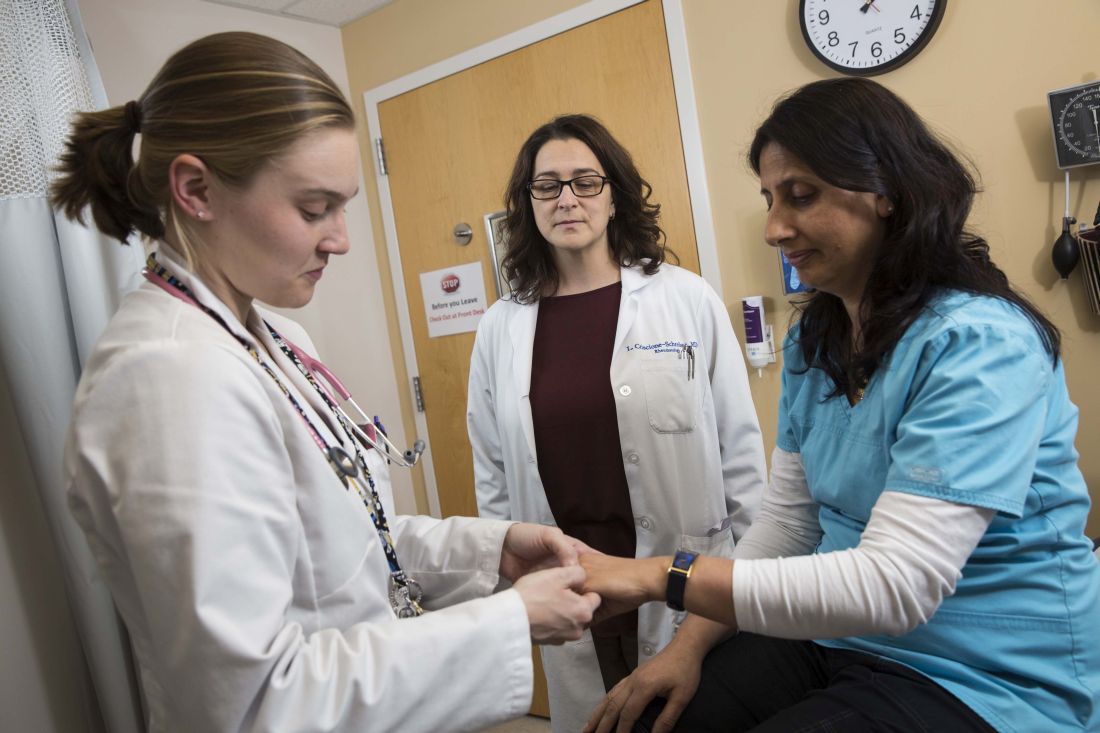User login
FDA approves topical oxymetazoline for rosacea
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
FDA approves ibrutinib for refractory MZL
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
hematologynews@frontlinemedcom.com
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
hematologynews@frontlinemedcom.com
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
hematologynews@frontlinemedcom.com
On Twitter @HematologyNews1
Prominent clinical guidelines fall short of conflict of interest standards
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
Two recent clinical practice guidelines – one for cholesterol management and another for treatment of chronic hepatitis C – did not meet the Institute of Medicine’s standards for limiting commercial conflicts of interest, according to results of a new analysis.
In research published online Jan. 17, Akilah A. Jefferson, MD, and Steven D. Pearson, MD, both of the National Institutes of Health in Bethesda, Md., re-examined conflict of interest disclosures for the 2013 American College of Cardiology and American Heart Association cholesterol management guideline, as well as for the American Association for the Study of Liver Diseases and Infectious Diseases Society of America’s joint 2014 guideline related to novel drug treatments for chronic hepatitis C virus (HCV) infection.
The IOM standards for conflicts of interest in guidelines, introduced in 2011, require that less than half the members of any guideline writing committee have a commercial conflict, which can include consultancies, board memberships, and stock in manufacturers of devices or treatments. Guideline writing committee chairs and cochairs should have no commercial conflicts of interest, according to the IOM.
For the cholesterol guidelines, the investigators found that while the committee members fell below the threshold with only 44% reporting commercial conflicts, one writing chair had multiple conflicts until about 1 year before guideline development began, an analysis of concurrent publications suggested. For the HCV guidelines, meanwhile, 72% of the committee members reported commercial conflicts, along with four out of six committee cochairs. An analysis of concurrent publications revealed incomplete disclosure of conflicts among authors of both guidelines (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8439).
“Management of levels of commercial [conflict of interest] among guideline committees remains an important problem 5 years after the IOM standards were published,” the investigators wrote. They recommended “broader and more explicit adoption” of the IOM’s framework for conflict of interest.
The study notes that the HCV guideline met all nine of the additional IOM guideline development and evidence standards, while the cholesterol guideline met five of those standards.
The study was funded by an NIH grant. Dr. Pearson reported receiving research funding from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: In total, 72% of members of the hepatitis C virus guideline committee disclosed conflicts of interest, while 44% of members of the cholesterol guideline committee reported commercial conflicts.
Data source: A retrospective review of the ACA/AHA’s cholesterol guideline and the AASLD/IDSA’s hepatitis C virus guideline.
Disclosures: The research was funded by a grant from the National Institutes of Health. Dr. Pearson reported research grants from foundations and membership dues paid by insurance and pharmaceutical companies. No other disclosures were reported.
Two-thirds of patient advocacy groups receive industry funding
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
Industry funding strengthens and extends the much-needed patient voice in health care, but at what cost? During the EpiPen scandal, the manufacturer-sponsored advocacy groups were largely silent about price gouging. Recently, a drug company–funded “patient advocacy” campaign called “Even the Score” helped win regulatory approval for the thrice rejected controversial female sex drug flibanserin.
Just as the industry funding of clinical trials has been associated with more favorable findings, patient groups also face risks of bias when accepting money from companies seeking to expand markets for their new tests and treatments.
This new work demonstrates an urgent need for patient advocacy organizations to explicitly focus much more on representing the interests of patients and citizens, rather than serving – inadvertently or otherwise – the interests of their industry sponsors. In the meantime, we need much greater transparency about industry funding, including prominently displayed disclosures of dollar amounts and proportions of total funding on group websites, as well as addition of patient advocacy groups to the Open Payments program established by the Sunshine Act, which would mean mandatory disclosure of funding by sponsors.
Ray Moynihan, PhD, and Lisa Bero, PhD, are at the University of Sydney, New South Wales, Australia. Their comments are adapted from an editorial. They reported having no relevant financial disclosures. (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.9179 ).
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
About two-thirds of patient advocacy organizations report receiving funds from for-profit firms, including pharmaceutical, device and biotechnology manufacturers, according to new survey results.
The study, published online Jan. 17 in JAMA Internal Medicine, sought responses from a randomly chosen sample of 439 executives at patient advocacy organizations (PAOs), of whom 66% (n = 289) responded. The median annual revenue for the groups surveyed was nearly $300,000, and 67% (n = 165) reported receiving at least some industry funding. Of those, 12% reported that more than half their annual funding comes from industry sources.
A total of 22 PAO leaders surveyed (8%) reported that they perceived pressure to conform their organizational interests to those of corporate donors (JAMA Intern Med. 2017 Jan 17. doi: 10.1001/jamainternmed.2016.8443).
The vast majority of respondents (82%) said they found conflicts of interest to be relevant to PAOs, and most reported having a written policy on conflicts. More than half said they viewed their own organizations’ conflict of interest policies as sound.
The findings warrant a broad re-examination of PAO conflict-of-interest policies and disclosures, and a detailed examination of the specific ways in which PAOs are influenced or pressured, wrote Susannah L. Rose, PhD, of the Cleveland Clinic, and her colleagues.
PAOs do not routinely and publicly disclose all their sources of funding on websites, tax returns, or annual reports, and there are media investigations into some groups “regarding their ties to industry and the integrity of their activities,” the investigators wrote.
Dr. Rose and her colleagues recommended that PAOs disclose detailed information about industry funding on their websites and on Open Payments, a government website. The researchers acknowledged that their study relied on self-reported data, and though it was designed to obscure the identity of respondents and their organizations, the possibility of response bias exists.
Harvard University’s Edmond J. Safra Center for Ethics funded the study. One coauthor, Steven Joffe, MD, MPH, disclosed past funding from Genzyme Sanofi. No other conflicts were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: A total of 67% of PAOs receive industry support, with 12% reporting funds comprising more than half their yearly funding.
Data source: A survey study sent to executives of 439 PAOs chosen at random; the response rate was 66%.
Disclosures: An ethics center at Harvard University sponsored the study; one coauthor reported prior funding from Genzyme Sanofi.
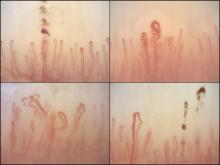
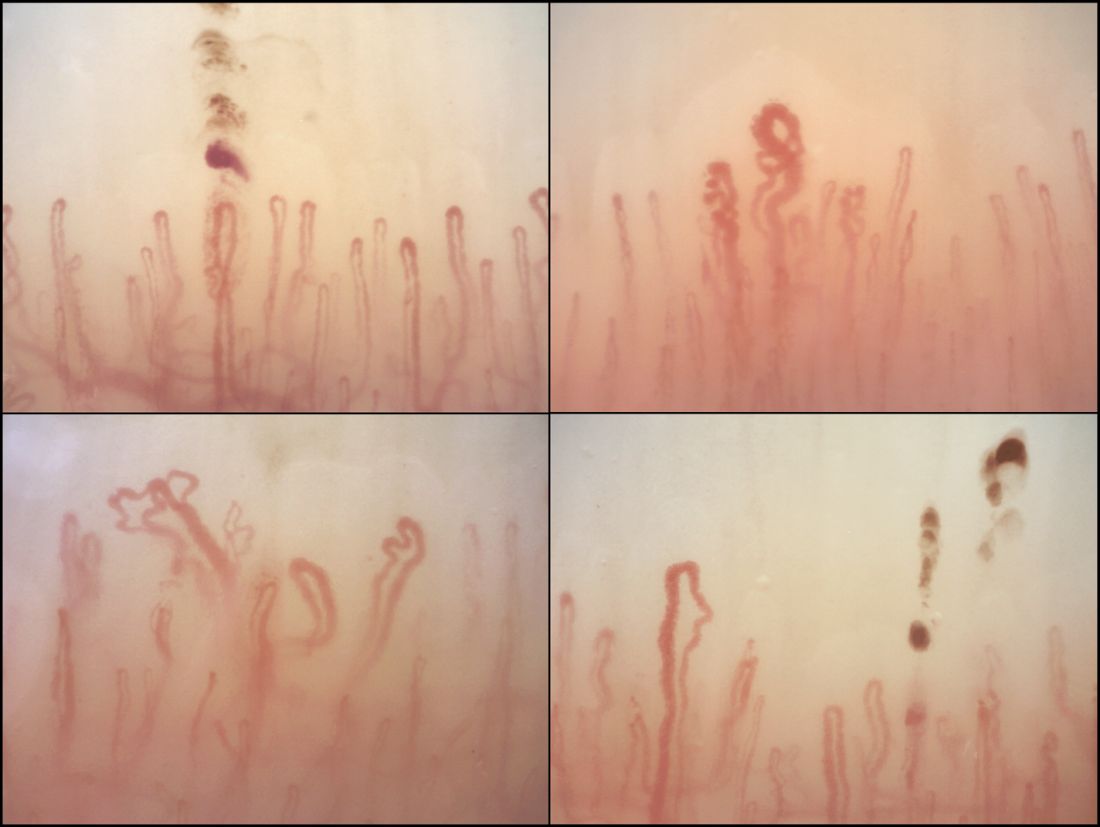
Nailfold analysis can predict cardiopulmonary complications in systemic sclerosis
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: Across the major autoantibody subtypes seen in an SSc cohort, NVC pattern showed a stable association with presence of interstitial lung disease (OR, 1.3-1.4) or elevated systolic pulmonary artery pressure (OR, 2.2-2.4).
Data source: A cross-section of 287 patients in a Dutch SSc cohort.
Disclosures: The study was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Enthesitis seen in 35% of PsA patients
About one-third of people with psoriatic arthritis have clinical enthesitis, according to results from a prospective cohort study of more than 800 patients.
Enthesitis, or soreness and inflammation at sites where soft tissue attaches to bone, is considered to be common in psoriatic arthritis (PsA), but its true prevalence has been difficult to define in this population, according to a group of researchers led by Ari Polachek, MD, of the University of Toronto.
Previous studies attempting to quantify enthesitis prevalence in PsA populations have found it to be as low as 8% to more than 50% of patients affected, Dr. Polachek and his colleagues noted, but such disparities can likely be attributed to the use of different enthesitis measures. To define enthesitis in the current study, the investigators used the SPondyloArthritis Research Consortium Canada (SPARCC) enthesitis index, which they called “valid and reliable, particularly for patients with PsA.”
Dr. Polachek and his colleagues detected enthesitis in 281 (35%) of 803 patents who had been recruited during 2008-2014 at a single clinic dedicated to PsA. The enthesitis diagnoses were established for at least one site on an initial visit (n = 128) or during a mean 3 years’ follow-up (n = 192).
The investigators reported that the annual incidence of enthesitis in this population was 0.9% (Arthritis Care Res. 2016 Dec 20. doi: 10.1002/acr.23174). About half of the patients in the cohort had only one site affected, and one-third had two sites affected. The most common of these sites were the Achilles tendon, plantar fascia, and the lateral epicondyle, Dr. Polachek and colleagues reported. They also found several factors associated with enthesitis: higher inflamed joint count (odds ratio, 1.06; P = .0002), less clinical damage (OR, 0.9; P = .04), more pain (OR, 1.15; P = .01), and presence of tenosynovitis (OR, 5.3; P less than .0001) or dactylitis (OR, 2.5; P = .02).
Significant risk factors for enthesitis included higher body mass index (hazard ratio, 1.04; P = .02), more actively inflamed joints (HR, 1.04; P = .0004), and younger age (HR, 0.98; P = .02). Among the patients in the cohort, 57% were male, the mean age was 50.8 years, and mean PsA disease duration was 12.3 years. Median time to resolution of enthesitis was 7.5 months, with 70% of patients improving without changes to their treatment.
The University of Toronto PsA research program receives funding from Krembil Foundation. Dr. Polachek disclosed grant funding from Janssen Canada. No other authors reported conflicts of interest.
About one-third of people with psoriatic arthritis have clinical enthesitis, according to results from a prospective cohort study of more than 800 patients.
Enthesitis, or soreness and inflammation at sites where soft tissue attaches to bone, is considered to be common in psoriatic arthritis (PsA), but its true prevalence has been difficult to define in this population, according to a group of researchers led by Ari Polachek, MD, of the University of Toronto.
Previous studies attempting to quantify enthesitis prevalence in PsA populations have found it to be as low as 8% to more than 50% of patients affected, Dr. Polachek and his colleagues noted, but such disparities can likely be attributed to the use of different enthesitis measures. To define enthesitis in the current study, the investigators used the SPondyloArthritis Research Consortium Canada (SPARCC) enthesitis index, which they called “valid and reliable, particularly for patients with PsA.”
Dr. Polachek and his colleagues detected enthesitis in 281 (35%) of 803 patents who had been recruited during 2008-2014 at a single clinic dedicated to PsA. The enthesitis diagnoses were established for at least one site on an initial visit (n = 128) or during a mean 3 years’ follow-up (n = 192).
The investigators reported that the annual incidence of enthesitis in this population was 0.9% (Arthritis Care Res. 2016 Dec 20. doi: 10.1002/acr.23174). About half of the patients in the cohort had only one site affected, and one-third had two sites affected. The most common of these sites were the Achilles tendon, plantar fascia, and the lateral epicondyle, Dr. Polachek and colleagues reported. They also found several factors associated with enthesitis: higher inflamed joint count (odds ratio, 1.06; P = .0002), less clinical damage (OR, 0.9; P = .04), more pain (OR, 1.15; P = .01), and presence of tenosynovitis (OR, 5.3; P less than .0001) or dactylitis (OR, 2.5; P = .02).
Significant risk factors for enthesitis included higher body mass index (hazard ratio, 1.04; P = .02), more actively inflamed joints (HR, 1.04; P = .0004), and younger age (HR, 0.98; P = .02). Among the patients in the cohort, 57% were male, the mean age was 50.8 years, and mean PsA disease duration was 12.3 years. Median time to resolution of enthesitis was 7.5 months, with 70% of patients improving without changes to their treatment.
The University of Toronto PsA research program receives funding from Krembil Foundation. Dr. Polachek disclosed grant funding from Janssen Canada. No other authors reported conflicts of interest.
About one-third of people with psoriatic arthritis have clinical enthesitis, according to results from a prospective cohort study of more than 800 patients.
Enthesitis, or soreness and inflammation at sites where soft tissue attaches to bone, is considered to be common in psoriatic arthritis (PsA), but its true prevalence has been difficult to define in this population, according to a group of researchers led by Ari Polachek, MD, of the University of Toronto.
Previous studies attempting to quantify enthesitis prevalence in PsA populations have found it to be as low as 8% to more than 50% of patients affected, Dr. Polachek and his colleagues noted, but such disparities can likely be attributed to the use of different enthesitis measures. To define enthesitis in the current study, the investigators used the SPondyloArthritis Research Consortium Canada (SPARCC) enthesitis index, which they called “valid and reliable, particularly for patients with PsA.”
Dr. Polachek and his colleagues detected enthesitis in 281 (35%) of 803 patents who had been recruited during 2008-2014 at a single clinic dedicated to PsA. The enthesitis diagnoses were established for at least one site on an initial visit (n = 128) or during a mean 3 years’ follow-up (n = 192).
The investigators reported that the annual incidence of enthesitis in this population was 0.9% (Arthritis Care Res. 2016 Dec 20. doi: 10.1002/acr.23174). About half of the patients in the cohort had only one site affected, and one-third had two sites affected. The most common of these sites were the Achilles tendon, plantar fascia, and the lateral epicondyle, Dr. Polachek and colleagues reported. They also found several factors associated with enthesitis: higher inflamed joint count (odds ratio, 1.06; P = .0002), less clinical damage (OR, 0.9; P = .04), more pain (OR, 1.15; P = .01), and presence of tenosynovitis (OR, 5.3; P less than .0001) or dactylitis (OR, 2.5; P = .02).
Significant risk factors for enthesitis included higher body mass index (hazard ratio, 1.04; P = .02), more actively inflamed joints (HR, 1.04; P = .0004), and younger age (HR, 0.98; P = .02). Among the patients in the cohort, 57% were male, the mean age was 50.8 years, and mean PsA disease duration was 12.3 years. Median time to resolution of enthesitis was 7.5 months, with 70% of patients improving without changes to their treatment.
The University of Toronto PsA research program receives funding from Krembil Foundation. Dr. Polachek disclosed grant funding from Janssen Canada. No other authors reported conflicts of interest.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Prevalence of enthesitis was 35% in a cohort of PsA patients; significant risk factors included high BMI, higher disease activity, and younger age.
Data source: About 800 PsA patients treated at a university clinic during 2008-2014; mean follow-up was 3.3 years.
Disclosures: The Krembil Foundation indirectly supported the study, whose lead author disclosed receipt of a grant from a pharmaceutical manufacturer.
Pain after hernia repair shows improvement at 6 months
Patients who have undergone open ventral abdominal hernia repair see significant improvements in some self-reported pain measures at 6 or more months after surgery, according to results from a new study.
The investigators, led by Eugene Park, MD, of Northwestern University, Chicago, suggested that the timing of the improvements may have to do with the mesh used in the surgeries.
For their research, published in the January issue of The American Journal of Surgery (2017;213:58-63), Dr. Park and his colleagues recruited 77 patients scheduled for midline incisional ventral hernia repair between 2010 and 2013 (mean age, 54 years; 45% female), who completed detailed pain surveys before surgery and during all postoperative visits; 38 patients completed surveys at least 6 months after surgery. All surgeries were performed by one of the study authors, Gregory A. Dumanian, MD, also of Northwestern.
The investigators used pain surveys from the Patient-Reported Outcomes Measurement Information System (PROMIS), which was developed under the National Institutes of Health. The investigators called the PROMIS surveys, which are computer based, a “rigorous and reliable tool” to measure patient feedback in clinical research and healthcare settings. PROMIS is designed to measure, among other things, how pain impacts a patient’s behavior and interferes with his or her everyday functioning.
Dr. Park and his colleagues reported that the patients with at least 6 months of follow-up saw significant improvement in measures of pain interference (P less than 0.05), though not in pain behavior.
The researchers wrote in their analysis that the mesh used in securing the hernia repair – all patients in the study were treated with some type of mesh – might be why pain scores were seen to improve significantly at around 6 months.
“The changes noted in pain interference at the 4- to 8-month time frame may represent a physiologic change as the mesh solidly integrates and begins to contribute to a patient’s increasing ability to perform tasks.”
The mesh used in the study, the researchers also noted, was narrower than that generally reported for hernia repairs of this type.
Dr. Park and his colleagues described as limitations of their study the relatively small number of patients completing long-term follow-up. Also, the investigators noted, the PROMIS pain interference and pain behavior surveys “were not designed specifically with ventral hernia patients in mind, which may limit the scope of hernia-related symptoms covered” and that data on patients’ use of pain medications was not recorded.
The study authors reported no outside funding or conflicts of interest related to their findings.
Patients who have undergone open ventral abdominal hernia repair see significant improvements in some self-reported pain measures at 6 or more months after surgery, according to results from a new study.
The investigators, led by Eugene Park, MD, of Northwestern University, Chicago, suggested that the timing of the improvements may have to do with the mesh used in the surgeries.
For their research, published in the January issue of The American Journal of Surgery (2017;213:58-63), Dr. Park and his colleagues recruited 77 patients scheduled for midline incisional ventral hernia repair between 2010 and 2013 (mean age, 54 years; 45% female), who completed detailed pain surveys before surgery and during all postoperative visits; 38 patients completed surveys at least 6 months after surgery. All surgeries were performed by one of the study authors, Gregory A. Dumanian, MD, also of Northwestern.
The investigators used pain surveys from the Patient-Reported Outcomes Measurement Information System (PROMIS), which was developed under the National Institutes of Health. The investigators called the PROMIS surveys, which are computer based, a “rigorous and reliable tool” to measure patient feedback in clinical research and healthcare settings. PROMIS is designed to measure, among other things, how pain impacts a patient’s behavior and interferes with his or her everyday functioning.
Dr. Park and his colleagues reported that the patients with at least 6 months of follow-up saw significant improvement in measures of pain interference (P less than 0.05), though not in pain behavior.
The researchers wrote in their analysis that the mesh used in securing the hernia repair – all patients in the study were treated with some type of mesh – might be why pain scores were seen to improve significantly at around 6 months.
“The changes noted in pain interference at the 4- to 8-month time frame may represent a physiologic change as the mesh solidly integrates and begins to contribute to a patient’s increasing ability to perform tasks.”
The mesh used in the study, the researchers also noted, was narrower than that generally reported for hernia repairs of this type.
Dr. Park and his colleagues described as limitations of their study the relatively small number of patients completing long-term follow-up. Also, the investigators noted, the PROMIS pain interference and pain behavior surveys “were not designed specifically with ventral hernia patients in mind, which may limit the scope of hernia-related symptoms covered” and that data on patients’ use of pain medications was not recorded.
The study authors reported no outside funding or conflicts of interest related to their findings.
Patients who have undergone open ventral abdominal hernia repair see significant improvements in some self-reported pain measures at 6 or more months after surgery, according to results from a new study.
The investigators, led by Eugene Park, MD, of Northwestern University, Chicago, suggested that the timing of the improvements may have to do with the mesh used in the surgeries.
For their research, published in the January issue of The American Journal of Surgery (2017;213:58-63), Dr. Park and his colleagues recruited 77 patients scheduled for midline incisional ventral hernia repair between 2010 and 2013 (mean age, 54 years; 45% female), who completed detailed pain surveys before surgery and during all postoperative visits; 38 patients completed surveys at least 6 months after surgery. All surgeries were performed by one of the study authors, Gregory A. Dumanian, MD, also of Northwestern.
The investigators used pain surveys from the Patient-Reported Outcomes Measurement Information System (PROMIS), which was developed under the National Institutes of Health. The investigators called the PROMIS surveys, which are computer based, a “rigorous and reliable tool” to measure patient feedback in clinical research and healthcare settings. PROMIS is designed to measure, among other things, how pain impacts a patient’s behavior and interferes with his or her everyday functioning.
Dr. Park and his colleagues reported that the patients with at least 6 months of follow-up saw significant improvement in measures of pain interference (P less than 0.05), though not in pain behavior.
The researchers wrote in their analysis that the mesh used in securing the hernia repair – all patients in the study were treated with some type of mesh – might be why pain scores were seen to improve significantly at around 6 months.
“The changes noted in pain interference at the 4- to 8-month time frame may represent a physiologic change as the mesh solidly integrates and begins to contribute to a patient’s increasing ability to perform tasks.”
The mesh used in the study, the researchers also noted, was narrower than that generally reported for hernia repairs of this type.
Dr. Park and his colleagues described as limitations of their study the relatively small number of patients completing long-term follow-up. Also, the investigators noted, the PROMIS pain interference and pain behavior surveys “were not designed specifically with ventral hernia patients in mind, which may limit the scope of hernia-related symptoms covered” and that data on patients’ use of pain medications was not recorded.
The study authors reported no outside funding or conflicts of interest related to their findings.
Key clinical point: People undergoing open ventral hernia repair saw significant improvements in self-reported pain starting at about 6 months after their procedures.
Major finding: Reported reductions of pain interference were significant among patients with 6 or more months’ follow-up (P less than .05).
Data source: 77 patients undergoing open ventral hernia repairs who completed validated pain questionnaires pre- and post-surgery; of these, 38 had follow-up of 6 months or longer.
Disclosures: None.
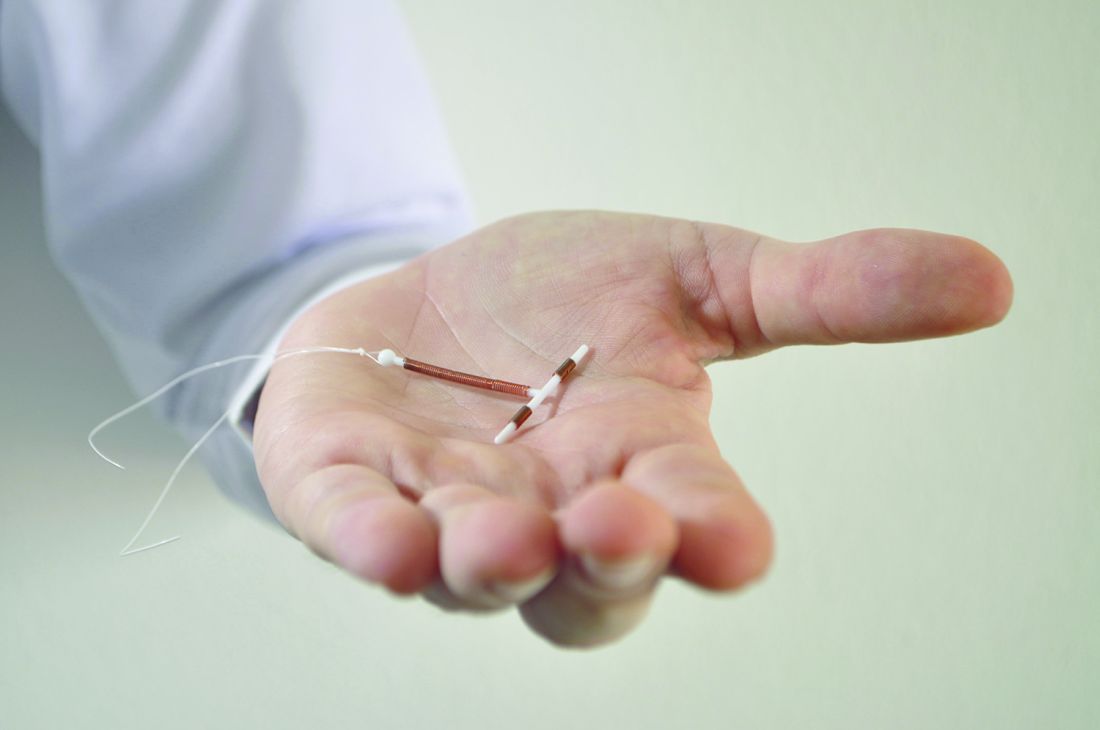
Immediate postpartum LARC requires cross-disciplinary cooperation in the hospital
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Success in establishing an immediate postpartum LARC program involves team-building across hospital disciplines.
Major finding: Factors associated with success included early coordination among financial, administrative, pharmacy, and clinical personnel.
Data source: A qualitative analysis of interviews with 32 employees (clinical and nonclinical) from 10 hospitals in Georgia.
Disclosures: Two authors disclosed relationships with LARC manufacturers.
Curricular milestones in rheumatology: Is granular better?
A new curricular “road map” attempts to lay out precisely what rheumatology fellows are expected to be able to know and do at different time points in their training.
The 80 “curricular milestones” for rheumatology, developed under the direction of the Accreditation Council for Graduate Medical Education, are meant to complement the 23 internal medicine subspecialty reporting milestones already mandated by the ACGME for use in trainee evaluation.
Unlike the reporting milestones, which came into use in 2014, the curricular milestones are not compulsory. Instead they “offer a guide so that trainees and the people teaching them know what’s expected of them,” said Lisa Criscione-Schreiber, MD, of Duke University, Durham, N.C., the lead author of a recent article in Arthritis Care & Research describing the new milestones (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23151).
While the reporting milestones are designed to target broader competencies, the curricular milestones are highly granular. For example, a rheumatology fellow at 12 months is expected to be able to “perform compensated polarized microscopy to examine and interpret synovial fluid,” according to one milestone; before 24 months of training, he or she is expected to be able to teach others to do the same.
Authors of an accompanying editorial (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23150) by Richard Panush, MD, of the University of Southern California in Los Angeles, Eric Hsieh, MD, also of USC, and Nortin Hadler, MD, of the University of North Carolina at Chapel Hill, praised the curricular milestones as meticulous and well conceived, but questioned their complexity and the broader push toward rubric-based training, particularly in the absence of evidence showing it to be superior to established methods.
Ultimately, Dr. Panush acknowledged in an interview, the move to milestones – including the curricular milestones – may prove worthwhile. But it simply “isn’t as sufficiently grounded in science as we would have liked for such a major departure from everything we have done, and with all that implementing the change would imply: the time, the manpower, the cost,” he said.
A leap of faith?
Dr. Criscione-Schreiber, who is Duke’s rheumatology program director, acknowledged that the milestones’ value and utility remain to be determined. “We’re not sure it’s the best way, but it’s a try,” she said. “We’re all in an era of continuous quality improvement in which you change something then test whether it works. The ACGME is collecting data about all this – if they find out it’s burdensome, they have assured program directors that they will change it.”
But Dr. Brown conceded that the editorialists’ concerns were valid. “The ACGME has been the main organization describing parameters by which we train doctors, and it is relatively unopposed. We’ve seen a proliferation of the rubric-based approach, and we are adopting these [curricular milestones] without scientific evidence. I commend the editorial authors in saying we need to find ways to measure this to see if it does improve, but if we wait for that evidence before we move forward, we’ll never get past square one.”
Observation and feedback
A key goal of the milestones is to encourage direct observation of trainees. Dr. Panush and his colleagues, in their editorial, argue that “a wise and astute clinician educator ... can judge when trainees are ready to function independently and have learned how to keep learning and changing without needing menus of evaluations or detailed instruments.”
Dr. Criscione-Schreiber said there’s traditionally been less direct observation in rheumatology training than people would like to think. “It used to be more like what we call the piano analogy. You send them into a room for an hour alone with a piano and some sheet music and they come out saying ‘I can play the piano.’ And you believe them.”
Dr. Brown, meanwhile, compared traditional training approaches to what he called the teabag model. The trainee “was the teabag steeping in the hot water of an academic medical center, and the major criteria for finishing was time,” he said. “I think all of us would recognize that this doesn’t take into consideration different environments, different learning styles and rates of learning.”
Using the milestones
Dr. Panush, in an interview, noted that the implementation of rubric-based education in internal medicine training came with a host of problems resulting in published critiques. He cited a recent article by Ronald Witteles, MD, and Abraham Verghese, MD, both of Stanford (Calif.) University, that criticized the reporting milestones as onerous to implement in large internal medicine programs, leading to excess administrative work and box-ticking (JAMA Intern Med. 2016;176[11]:1599-1600).
But rheumatology fellowship programs, which are generally small, may be less likely to find the curricular milestones burdensome, their champions say. The success of these milestones depends, in large part, on how people choose to integrate them into their training programs.
“The milestones appear a little daunting because they’re new,” Dr. Bolster said, cautioning that they are not designed to be foisted wholesale on trainees. Used judiciously, she said, the curricular milestones can help identify areas of strength and weakness and foster dialogue. “What I do is select a few of them, a couple of times a year, to go over with each fellow,” she said. “I also recommend them for fellows to use as self-reflection, so that they may determine where they are in terms of training.”
Details vs. the big picture
Anne Bass, MD, the rheumatology program director at the Hospital for Special Surgery in New York, who did not take part in either the curricular milestones writing committee or the editorial, said they could be used piecemeal, helping program directors to design portions of their curriculum, or to provide feedback and remediation by pointing out areas of trainee weakness.
But Dr. Bass also said, echoing a concern of the editorialists, that she felt that the milestones’ emphasis on highly specific strengths and weaknesses risked “losing the forest for the trees.”
A trainee who appears weak in one knowledge area is likely to have broader shortcomings, she said.
“If you’re somebody who gets the basic facts, you’re able to interview the patient, do the physical exam, and collect the information, but you’re not really able to apply it yet – that’s probably something global to that trainee that will apply with other areas as well,” not just the milestone you’re measuring, Dr. Bass said.
“The whole point about milestones is it’s showing you where along the continuum you are. But the continuum goes from data collection and description to application, testing, management, teaching – that’s kind of the spectrum, and that development is generally across the board. It’s not usually specific to one area.”
Training rheumatologists like pilots
The authors of the milestones say they’re responding to a sense widely shared in the rheumatology community that training must adapt to the needs of a changing field.
“Medicine is not the same as it was 10 or even 5 years ago, and certainly not as it was 20 years ago. A physician who is going to practice 10 years from now will require a different skill set,” Dr. Criscione-Schreiber said.
This, of course, includes many skills not directly related to patient care but rather successful functioning within current systems of health care delivery.
Dr. Criscione-Schreiber said that amid a coming shortage of rheumatologists, the next generation must be prepared to lead teams.
“We’re going to have clinics with nurses doing much of the education, and a whole team of people taking care of patients, if we have any hope of caring for the population in the U.S. that needs rheumatologists. It’s different from training a one-on-one doctor who sits in the room with a patient for 25 minutes,” she said.
Dr. Brown said he views the milestones effort, whether it proves successful or not, as an attempt to train in a way that ensures all future rheumatologists share the same core skills.
“People ask us all the time which doctor should they see for this or that condition, because they want to see someone really good,” Dr. Brown said. “Contrast that to the last time you got on an airplane. Did you ask a friend which pilot you should fly with? No, because you trusted that the pilot was competent, and had all requisite training, to safely pilot the plane. We want medicine to have many of those qualities and characteristics.”
Human beings are more complicated than airplanes, Dr. Brown acknowledged. “So it’s not perfectly analogous. But if we can more granularly describe training, and what it is we want to achieve, we’d all benefit – to the point where we can confidently tell you to see the rheumatologist trained in an accredited program who’s nearest your home.”
A new curricular “road map” attempts to lay out precisely what rheumatology fellows are expected to be able to know and do at different time points in their training.
The 80 “curricular milestones” for rheumatology, developed under the direction of the Accreditation Council for Graduate Medical Education, are meant to complement the 23 internal medicine subspecialty reporting milestones already mandated by the ACGME for use in trainee evaluation.
Unlike the reporting milestones, which came into use in 2014, the curricular milestones are not compulsory. Instead they “offer a guide so that trainees and the people teaching them know what’s expected of them,” said Lisa Criscione-Schreiber, MD, of Duke University, Durham, N.C., the lead author of a recent article in Arthritis Care & Research describing the new milestones (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23151).
While the reporting milestones are designed to target broader competencies, the curricular milestones are highly granular. For example, a rheumatology fellow at 12 months is expected to be able to “perform compensated polarized microscopy to examine and interpret synovial fluid,” according to one milestone; before 24 months of training, he or she is expected to be able to teach others to do the same.
Authors of an accompanying editorial (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23150) by Richard Panush, MD, of the University of Southern California in Los Angeles, Eric Hsieh, MD, also of USC, and Nortin Hadler, MD, of the University of North Carolina at Chapel Hill, praised the curricular milestones as meticulous and well conceived, but questioned their complexity and the broader push toward rubric-based training, particularly in the absence of evidence showing it to be superior to established methods.
Ultimately, Dr. Panush acknowledged in an interview, the move to milestones – including the curricular milestones – may prove worthwhile. But it simply “isn’t as sufficiently grounded in science as we would have liked for such a major departure from everything we have done, and with all that implementing the change would imply: the time, the manpower, the cost,” he said.
A leap of faith?
Dr. Criscione-Schreiber, who is Duke’s rheumatology program director, acknowledged that the milestones’ value and utility remain to be determined. “We’re not sure it’s the best way, but it’s a try,” she said. “We’re all in an era of continuous quality improvement in which you change something then test whether it works. The ACGME is collecting data about all this – if they find out it’s burdensome, they have assured program directors that they will change it.”
But Dr. Brown conceded that the editorialists’ concerns were valid. “The ACGME has been the main organization describing parameters by which we train doctors, and it is relatively unopposed. We’ve seen a proliferation of the rubric-based approach, and we are adopting these [curricular milestones] without scientific evidence. I commend the editorial authors in saying we need to find ways to measure this to see if it does improve, but if we wait for that evidence before we move forward, we’ll never get past square one.”
Observation and feedback
A key goal of the milestones is to encourage direct observation of trainees. Dr. Panush and his colleagues, in their editorial, argue that “a wise and astute clinician educator ... can judge when trainees are ready to function independently and have learned how to keep learning and changing without needing menus of evaluations or detailed instruments.”
Dr. Criscione-Schreiber said there’s traditionally been less direct observation in rheumatology training than people would like to think. “It used to be more like what we call the piano analogy. You send them into a room for an hour alone with a piano and some sheet music and they come out saying ‘I can play the piano.’ And you believe them.”
Dr. Brown, meanwhile, compared traditional training approaches to what he called the teabag model. The trainee “was the teabag steeping in the hot water of an academic medical center, and the major criteria for finishing was time,” he said. “I think all of us would recognize that this doesn’t take into consideration different environments, different learning styles and rates of learning.”
Using the milestones
Dr. Panush, in an interview, noted that the implementation of rubric-based education in internal medicine training came with a host of problems resulting in published critiques. He cited a recent article by Ronald Witteles, MD, and Abraham Verghese, MD, both of Stanford (Calif.) University, that criticized the reporting milestones as onerous to implement in large internal medicine programs, leading to excess administrative work and box-ticking (JAMA Intern Med. 2016;176[11]:1599-1600).
But rheumatology fellowship programs, which are generally small, may be less likely to find the curricular milestones burdensome, their champions say. The success of these milestones depends, in large part, on how people choose to integrate them into their training programs.
“The milestones appear a little daunting because they’re new,” Dr. Bolster said, cautioning that they are not designed to be foisted wholesale on trainees. Used judiciously, she said, the curricular milestones can help identify areas of strength and weakness and foster dialogue. “What I do is select a few of them, a couple of times a year, to go over with each fellow,” she said. “I also recommend them for fellows to use as self-reflection, so that they may determine where they are in terms of training.”
Details vs. the big picture
Anne Bass, MD, the rheumatology program director at the Hospital for Special Surgery in New York, who did not take part in either the curricular milestones writing committee or the editorial, said they could be used piecemeal, helping program directors to design portions of their curriculum, or to provide feedback and remediation by pointing out areas of trainee weakness.
But Dr. Bass also said, echoing a concern of the editorialists, that she felt that the milestones’ emphasis on highly specific strengths and weaknesses risked “losing the forest for the trees.”
A trainee who appears weak in one knowledge area is likely to have broader shortcomings, she said.
“If you’re somebody who gets the basic facts, you’re able to interview the patient, do the physical exam, and collect the information, but you’re not really able to apply it yet – that’s probably something global to that trainee that will apply with other areas as well,” not just the milestone you’re measuring, Dr. Bass said.
“The whole point about milestones is it’s showing you where along the continuum you are. But the continuum goes from data collection and description to application, testing, management, teaching – that’s kind of the spectrum, and that development is generally across the board. It’s not usually specific to one area.”
Training rheumatologists like pilots
The authors of the milestones say they’re responding to a sense widely shared in the rheumatology community that training must adapt to the needs of a changing field.
“Medicine is not the same as it was 10 or even 5 years ago, and certainly not as it was 20 years ago. A physician who is going to practice 10 years from now will require a different skill set,” Dr. Criscione-Schreiber said.
This, of course, includes many skills not directly related to patient care but rather successful functioning within current systems of health care delivery.
Dr. Criscione-Schreiber said that amid a coming shortage of rheumatologists, the next generation must be prepared to lead teams.
“We’re going to have clinics with nurses doing much of the education, and a whole team of people taking care of patients, if we have any hope of caring for the population in the U.S. that needs rheumatologists. It’s different from training a one-on-one doctor who sits in the room with a patient for 25 minutes,” she said.
Dr. Brown said he views the milestones effort, whether it proves successful or not, as an attempt to train in a way that ensures all future rheumatologists share the same core skills.
“People ask us all the time which doctor should they see for this or that condition, because they want to see someone really good,” Dr. Brown said. “Contrast that to the last time you got on an airplane. Did you ask a friend which pilot you should fly with? No, because you trusted that the pilot was competent, and had all requisite training, to safely pilot the plane. We want medicine to have many of those qualities and characteristics.”
Human beings are more complicated than airplanes, Dr. Brown acknowledged. “So it’s not perfectly analogous. But if we can more granularly describe training, and what it is we want to achieve, we’d all benefit – to the point where we can confidently tell you to see the rheumatologist trained in an accredited program who’s nearest your home.”
A new curricular “road map” attempts to lay out precisely what rheumatology fellows are expected to be able to know and do at different time points in their training.
The 80 “curricular milestones” for rheumatology, developed under the direction of the Accreditation Council for Graduate Medical Education, are meant to complement the 23 internal medicine subspecialty reporting milestones already mandated by the ACGME for use in trainee evaluation.
Unlike the reporting milestones, which came into use in 2014, the curricular milestones are not compulsory. Instead they “offer a guide so that trainees and the people teaching them know what’s expected of them,” said Lisa Criscione-Schreiber, MD, of Duke University, Durham, N.C., the lead author of a recent article in Arthritis Care & Research describing the new milestones (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23151).
While the reporting milestones are designed to target broader competencies, the curricular milestones are highly granular. For example, a rheumatology fellow at 12 months is expected to be able to “perform compensated polarized microscopy to examine and interpret synovial fluid,” according to one milestone; before 24 months of training, he or she is expected to be able to teach others to do the same.
Authors of an accompanying editorial (Arthritis Care Res. 2016 Nov 11. doi: 10.1002/acr.23150) by Richard Panush, MD, of the University of Southern California in Los Angeles, Eric Hsieh, MD, also of USC, and Nortin Hadler, MD, of the University of North Carolina at Chapel Hill, praised the curricular milestones as meticulous and well conceived, but questioned their complexity and the broader push toward rubric-based training, particularly in the absence of evidence showing it to be superior to established methods.
Ultimately, Dr. Panush acknowledged in an interview, the move to milestones – including the curricular milestones – may prove worthwhile. But it simply “isn’t as sufficiently grounded in science as we would have liked for such a major departure from everything we have done, and with all that implementing the change would imply: the time, the manpower, the cost,” he said.
A leap of faith?
Dr. Criscione-Schreiber, who is Duke’s rheumatology program director, acknowledged that the milestones’ value and utility remain to be determined. “We’re not sure it’s the best way, but it’s a try,” she said. “We’re all in an era of continuous quality improvement in which you change something then test whether it works. The ACGME is collecting data about all this – if they find out it’s burdensome, they have assured program directors that they will change it.”
But Dr. Brown conceded that the editorialists’ concerns were valid. “The ACGME has been the main organization describing parameters by which we train doctors, and it is relatively unopposed. We’ve seen a proliferation of the rubric-based approach, and we are adopting these [curricular milestones] without scientific evidence. I commend the editorial authors in saying we need to find ways to measure this to see if it does improve, but if we wait for that evidence before we move forward, we’ll never get past square one.”
Observation and feedback
A key goal of the milestones is to encourage direct observation of trainees. Dr. Panush and his colleagues, in their editorial, argue that “a wise and astute clinician educator ... can judge when trainees are ready to function independently and have learned how to keep learning and changing without needing menus of evaluations or detailed instruments.”
Dr. Criscione-Schreiber said there’s traditionally been less direct observation in rheumatology training than people would like to think. “It used to be more like what we call the piano analogy. You send them into a room for an hour alone with a piano and some sheet music and they come out saying ‘I can play the piano.’ And you believe them.”
Dr. Brown, meanwhile, compared traditional training approaches to what he called the teabag model. The trainee “was the teabag steeping in the hot water of an academic medical center, and the major criteria for finishing was time,” he said. “I think all of us would recognize that this doesn’t take into consideration different environments, different learning styles and rates of learning.”
Using the milestones
Dr. Panush, in an interview, noted that the implementation of rubric-based education in internal medicine training came with a host of problems resulting in published critiques. He cited a recent article by Ronald Witteles, MD, and Abraham Verghese, MD, both of Stanford (Calif.) University, that criticized the reporting milestones as onerous to implement in large internal medicine programs, leading to excess administrative work and box-ticking (JAMA Intern Med. 2016;176[11]:1599-1600).
But rheumatology fellowship programs, which are generally small, may be less likely to find the curricular milestones burdensome, their champions say. The success of these milestones depends, in large part, on how people choose to integrate them into their training programs.
“The milestones appear a little daunting because they’re new,” Dr. Bolster said, cautioning that they are not designed to be foisted wholesale on trainees. Used judiciously, she said, the curricular milestones can help identify areas of strength and weakness and foster dialogue. “What I do is select a few of them, a couple of times a year, to go over with each fellow,” she said. “I also recommend them for fellows to use as self-reflection, so that they may determine where they are in terms of training.”
Details vs. the big picture
Anne Bass, MD, the rheumatology program director at the Hospital for Special Surgery in New York, who did not take part in either the curricular milestones writing committee or the editorial, said they could be used piecemeal, helping program directors to design portions of their curriculum, or to provide feedback and remediation by pointing out areas of trainee weakness.
But Dr. Bass also said, echoing a concern of the editorialists, that she felt that the milestones’ emphasis on highly specific strengths and weaknesses risked “losing the forest for the trees.”
A trainee who appears weak in one knowledge area is likely to have broader shortcomings, she said.
“If you’re somebody who gets the basic facts, you’re able to interview the patient, do the physical exam, and collect the information, but you’re not really able to apply it yet – that’s probably something global to that trainee that will apply with other areas as well,” not just the milestone you’re measuring, Dr. Bass said.
“The whole point about milestones is it’s showing you where along the continuum you are. But the continuum goes from data collection and description to application, testing, management, teaching – that’s kind of the spectrum, and that development is generally across the board. It’s not usually specific to one area.”
Training rheumatologists like pilots
The authors of the milestones say they’re responding to a sense widely shared in the rheumatology community that training must adapt to the needs of a changing field.
“Medicine is not the same as it was 10 or even 5 years ago, and certainly not as it was 20 years ago. A physician who is going to practice 10 years from now will require a different skill set,” Dr. Criscione-Schreiber said.
This, of course, includes many skills not directly related to patient care but rather successful functioning within current systems of health care delivery.
Dr. Criscione-Schreiber said that amid a coming shortage of rheumatologists, the next generation must be prepared to lead teams.
“We’re going to have clinics with nurses doing much of the education, and a whole team of people taking care of patients, if we have any hope of caring for the population in the U.S. that needs rheumatologists. It’s different from training a one-on-one doctor who sits in the room with a patient for 25 minutes,” she said.
Dr. Brown said he views the milestones effort, whether it proves successful or not, as an attempt to train in a way that ensures all future rheumatologists share the same core skills.
“People ask us all the time which doctor should they see for this or that condition, because they want to see someone really good,” Dr. Brown said. “Contrast that to the last time you got on an airplane. Did you ask a friend which pilot you should fly with? No, because you trusted that the pilot was competent, and had all requisite training, to safely pilot the plane. We want medicine to have many of those qualities and characteristics.”
Human beings are more complicated than airplanes, Dr. Brown acknowledged. “So it’s not perfectly analogous. But if we can more granularly describe training, and what it is we want to achieve, we’d all benefit – to the point where we can confidently tell you to see the rheumatologist trained in an accredited program who’s nearest your home.”
FROM ARTHRITIS CARE & RESEARCH
Child, adolescent autism patients visiting EDs in higher numbers
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
EXPERT ANALYSIS FROM AACAP 2016