User login
Black diabetics lose limbs at triple the rate of others. Here’s how health care leaders are starting to act.
Prompted by a ProPublica story that detailed how Black Americans with diabetes lose limbs at a rate triple that of others, the American Diabetes Association has included an initiative to prevent unnecessary amputations as part of an unprecedented campaign to reduce racial disparities in diabetes care.
“The ProPublica article raised the consciousness of what the problem is,” said Tracey Brown, the CEO of the ADA. “Every four minutes, someone is losing a limb from diabetic complications. That’s ridiculous. We have got to find a way to drive change.”
The story highlighted obstacles to equitable care for diabetic patients at risk of amputation, from the government’s decision not to endorse screening at-risk patients for vascular disease in the legs, to the inadequate incentives for certain specialists to move to underserved areas, to the health system’s failure to consider limb-saving options before permitting surgeons to apply a blade.
In the weeks that followed publication, several congressional and state legislative offices reached out to the association to ask for guidance on drafting policy to reduce disparities in diabetic amputations. In response, the organization decided to build an agenda around the issue.
The ADA’s Health Equity Now campaign, which addresses the cost of diabetes care, nutrition, discrimination, and more, was motivated by the racial health disparities that have been exposed by COVID-19, which has hit Black Americans with diabetes particularly hard. As part of the project, the association has built a Health Equity Bill of Rights, asserting that all diabetes patients are entitled to affordable drugs, healthy food, the latest medical advances, and other protections.
The right to avoid preventable amputations is the only complication of uncontrolled diabetes that is included in the list. The organization is sharing the document with policymakers, practitioners, and patients as it begins to look toward policy change. It is also encouraging members of the public to ask their governors to support the project.
Dr. Ronald Dalman, president of the Society for Vascular Surgery, said: “I commend the ADA for doubling down on this particular complication of poorly managed diabetes. It’s a long overdue prioritization.” He added that it’s a “moment in time where we can leverage this concern about health care disparities to call out a very specific problem: the prevalence of amputation in certain subsets of the population.”
Dr. Gary Puckrein, head of the National Minority Quality Forum, a nonprofit focused on reducing health care disparities, said that the ADA’s efforts are just a step. “The American health care system was organized during an era when inequality was acceptable and mainstream in American society,” he said. “It’s not that African Americans are sicker, it’s that the health care delivered is unequal.”
He said he hopes that the national conversation on health disparities will mirror the conversation about police violence against Black Americans. “You, in effect, have your knees on their neck in the health care system as well when you don’t provide them with the care that they need.”
Two weeks after publication of the story, Rep. Bennie Thompson, a Democrat from Mississippi, honored Dr. Foluso Fakorede, the main subject of the ProPublica article, for his work in reducing unnecessary amputations in Bolivar County, Mississippi. The acknowledgment, made in the House of Representatives, referenced ProPublica’s findings.
The co-chairs of the Congressional Peripheral Artery Disease Caucus — Rep. Donald M. Payne Jr., a Democrat from New Jersey, and Rep. Gus Bilirakis, a Republican from Florida — have also begun work on a bill to address disparities in amputations, particularly for people with peripheral artery disease, a condition in which clogged arteries in the legs limit the flow of blood.
“The ProPublica article has brought strong awareness and real interest from a variety of parties — from the medical field and from patients and from potentially future patients,” said a spokesman for Payne. “We have been working with Bilirakis and other members to move this forward, with the ultimate goal of introducing legislation.”
Summer Blevins, deputy chief of staff for Bilirakis, added that their legislative ambition “is based on the basic principle that prevention, education and early intervention is best for the patient and also saves money.”
This story was originally published by ProPublica.
Prompted by a ProPublica story that detailed how Black Americans with diabetes lose limbs at a rate triple that of others, the American Diabetes Association has included an initiative to prevent unnecessary amputations as part of an unprecedented campaign to reduce racial disparities in diabetes care.
“The ProPublica article raised the consciousness of what the problem is,” said Tracey Brown, the CEO of the ADA. “Every four minutes, someone is losing a limb from diabetic complications. That’s ridiculous. We have got to find a way to drive change.”
The story highlighted obstacles to equitable care for diabetic patients at risk of amputation, from the government’s decision not to endorse screening at-risk patients for vascular disease in the legs, to the inadequate incentives for certain specialists to move to underserved areas, to the health system’s failure to consider limb-saving options before permitting surgeons to apply a blade.
In the weeks that followed publication, several congressional and state legislative offices reached out to the association to ask for guidance on drafting policy to reduce disparities in diabetic amputations. In response, the organization decided to build an agenda around the issue.
The ADA’s Health Equity Now campaign, which addresses the cost of diabetes care, nutrition, discrimination, and more, was motivated by the racial health disparities that have been exposed by COVID-19, which has hit Black Americans with diabetes particularly hard. As part of the project, the association has built a Health Equity Bill of Rights, asserting that all diabetes patients are entitled to affordable drugs, healthy food, the latest medical advances, and other protections.
The right to avoid preventable amputations is the only complication of uncontrolled diabetes that is included in the list. The organization is sharing the document with policymakers, practitioners, and patients as it begins to look toward policy change. It is also encouraging members of the public to ask their governors to support the project.
Dr. Ronald Dalman, president of the Society for Vascular Surgery, said: “I commend the ADA for doubling down on this particular complication of poorly managed diabetes. It’s a long overdue prioritization.” He added that it’s a “moment in time where we can leverage this concern about health care disparities to call out a very specific problem: the prevalence of amputation in certain subsets of the population.”
Dr. Gary Puckrein, head of the National Minority Quality Forum, a nonprofit focused on reducing health care disparities, said that the ADA’s efforts are just a step. “The American health care system was organized during an era when inequality was acceptable and mainstream in American society,” he said. “It’s not that African Americans are sicker, it’s that the health care delivered is unequal.”
He said he hopes that the national conversation on health disparities will mirror the conversation about police violence against Black Americans. “You, in effect, have your knees on their neck in the health care system as well when you don’t provide them with the care that they need.”
Two weeks after publication of the story, Rep. Bennie Thompson, a Democrat from Mississippi, honored Dr. Foluso Fakorede, the main subject of the ProPublica article, for his work in reducing unnecessary amputations in Bolivar County, Mississippi. The acknowledgment, made in the House of Representatives, referenced ProPublica’s findings.
The co-chairs of the Congressional Peripheral Artery Disease Caucus — Rep. Donald M. Payne Jr., a Democrat from New Jersey, and Rep. Gus Bilirakis, a Republican from Florida — have also begun work on a bill to address disparities in amputations, particularly for people with peripheral artery disease, a condition in which clogged arteries in the legs limit the flow of blood.
“The ProPublica article has brought strong awareness and real interest from a variety of parties — from the medical field and from patients and from potentially future patients,” said a spokesman for Payne. “We have been working with Bilirakis and other members to move this forward, with the ultimate goal of introducing legislation.”
Summer Blevins, deputy chief of staff for Bilirakis, added that their legislative ambition “is based on the basic principle that prevention, education and early intervention is best for the patient and also saves money.”
This story was originally published by ProPublica.
Prompted by a ProPublica story that detailed how Black Americans with diabetes lose limbs at a rate triple that of others, the American Diabetes Association has included an initiative to prevent unnecessary amputations as part of an unprecedented campaign to reduce racial disparities in diabetes care.
“The ProPublica article raised the consciousness of what the problem is,” said Tracey Brown, the CEO of the ADA. “Every four minutes, someone is losing a limb from diabetic complications. That’s ridiculous. We have got to find a way to drive change.”
The story highlighted obstacles to equitable care for diabetic patients at risk of amputation, from the government’s decision not to endorse screening at-risk patients for vascular disease in the legs, to the inadequate incentives for certain specialists to move to underserved areas, to the health system’s failure to consider limb-saving options before permitting surgeons to apply a blade.
In the weeks that followed publication, several congressional and state legislative offices reached out to the association to ask for guidance on drafting policy to reduce disparities in diabetic amputations. In response, the organization decided to build an agenda around the issue.
The ADA’s Health Equity Now campaign, which addresses the cost of diabetes care, nutrition, discrimination, and more, was motivated by the racial health disparities that have been exposed by COVID-19, which has hit Black Americans with diabetes particularly hard. As part of the project, the association has built a Health Equity Bill of Rights, asserting that all diabetes patients are entitled to affordable drugs, healthy food, the latest medical advances, and other protections.
The right to avoid preventable amputations is the only complication of uncontrolled diabetes that is included in the list. The organization is sharing the document with policymakers, practitioners, and patients as it begins to look toward policy change. It is also encouraging members of the public to ask their governors to support the project.
Dr. Ronald Dalman, president of the Society for Vascular Surgery, said: “I commend the ADA for doubling down on this particular complication of poorly managed diabetes. It’s a long overdue prioritization.” He added that it’s a “moment in time where we can leverage this concern about health care disparities to call out a very specific problem: the prevalence of amputation in certain subsets of the population.”
Dr. Gary Puckrein, head of the National Minority Quality Forum, a nonprofit focused on reducing health care disparities, said that the ADA’s efforts are just a step. “The American health care system was organized during an era when inequality was acceptable and mainstream in American society,” he said. “It’s not that African Americans are sicker, it’s that the health care delivered is unequal.”
He said he hopes that the national conversation on health disparities will mirror the conversation about police violence against Black Americans. “You, in effect, have your knees on their neck in the health care system as well when you don’t provide them with the care that they need.”
Two weeks after publication of the story, Rep. Bennie Thompson, a Democrat from Mississippi, honored Dr. Foluso Fakorede, the main subject of the ProPublica article, for his work in reducing unnecessary amputations in Bolivar County, Mississippi. The acknowledgment, made in the House of Representatives, referenced ProPublica’s findings.
The co-chairs of the Congressional Peripheral Artery Disease Caucus — Rep. Donald M. Payne Jr., a Democrat from New Jersey, and Rep. Gus Bilirakis, a Republican from Florida — have also begun work on a bill to address disparities in amputations, particularly for people with peripheral artery disease, a condition in which clogged arteries in the legs limit the flow of blood.
“The ProPublica article has brought strong awareness and real interest from a variety of parties — from the medical field and from patients and from potentially future patients,” said a spokesman for Payne. “We have been working with Bilirakis and other members to move this forward, with the ultimate goal of introducing legislation.”
Summer Blevins, deputy chief of staff for Bilirakis, added that their legislative ambition “is based on the basic principle that prevention, education and early intervention is best for the patient and also saves money.”
This story was originally published by ProPublica.
Compression therapy cuts cellulitis risk in chronic leg edema
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Scalp Wound Closures in Mohs Micrographic Surgery: A Survey of Staples vs Sutures
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).

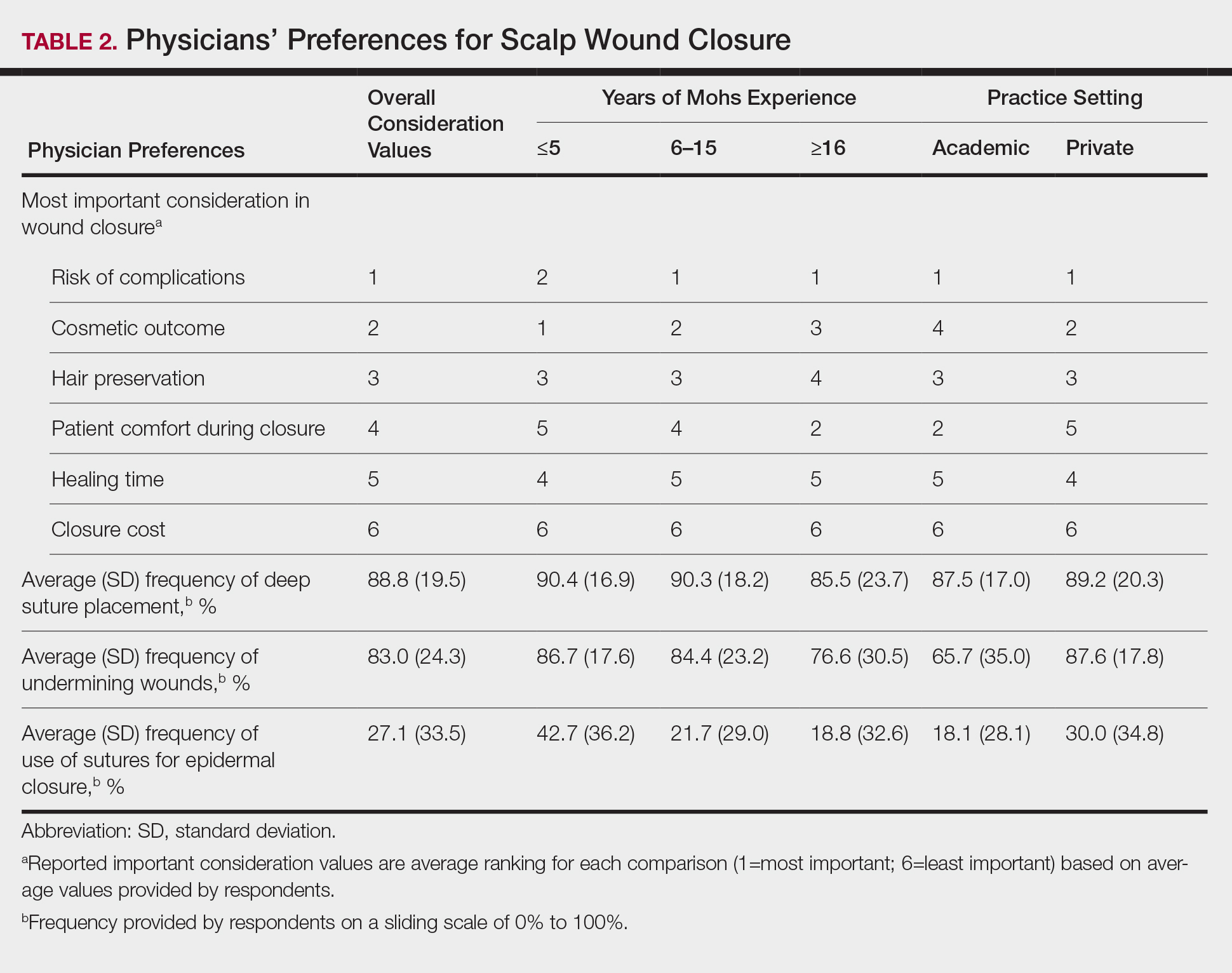
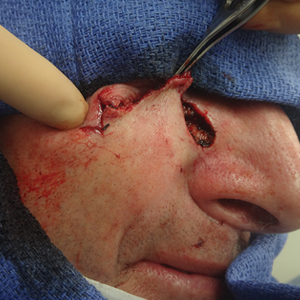
Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.
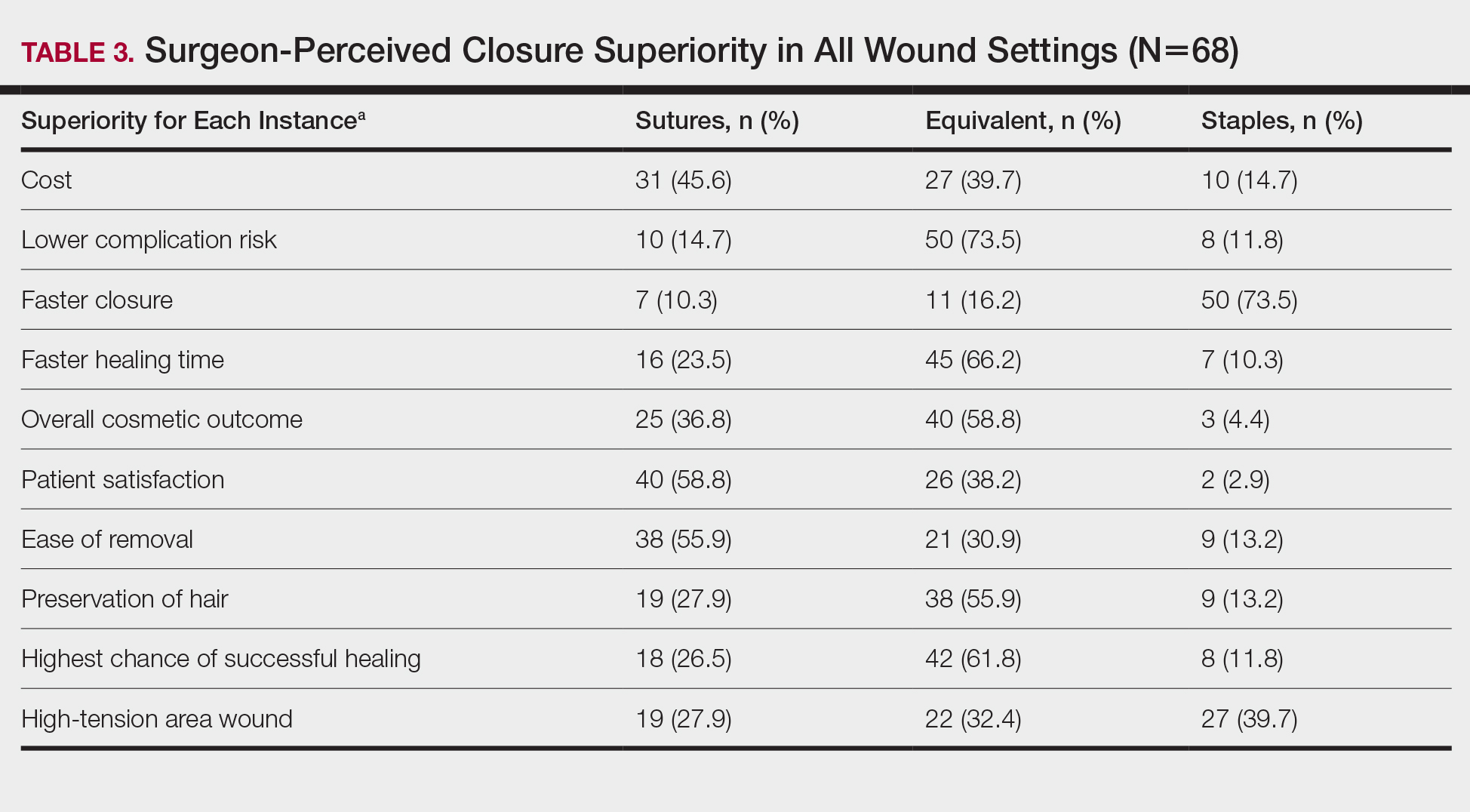
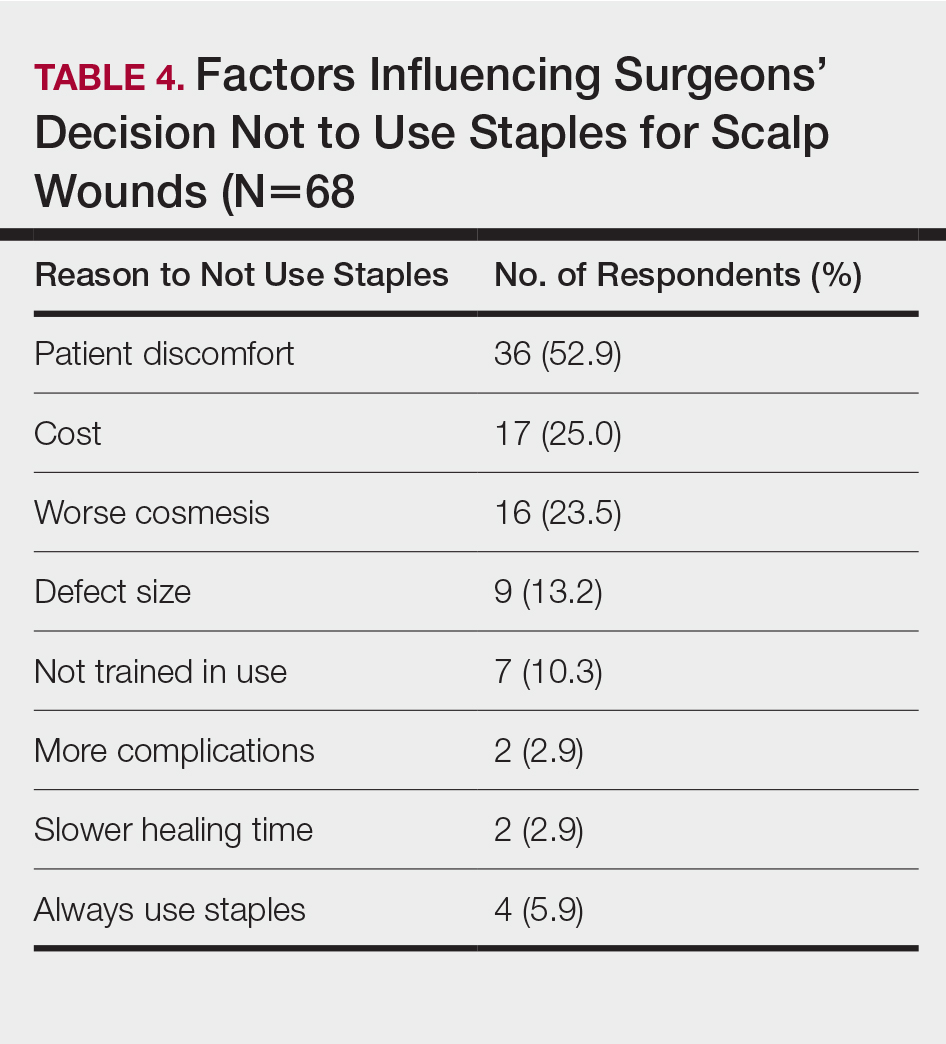
In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).
Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
Limited data exist comparing staples and sutures for scalp closures during Mohs micrographic surgery (MMS). As a result, the closure method for these scalp wounds is based on surgeon preference without established consensus. The purpose of this study was to survey practicing Mohs surgeons on their scalp wound closure preferences as well as the clinical and economic variables that impact their decisions. Understanding practice habits can guide future trial design, with a goal of creating established criterion for MMS scalp wound closures.
Methods
An anonymous survey was distributed from April 2019 to June 2019 to fellowship-trained Mohs surgeons using an electronic mailing list from the American College of Mohs Surgery (ACMS). The 10-question survey was approved by the University of Kansas institutional review board and the executive committee of the ACMS. Surgeons were asked about their preferred method for scalp wound closure as well as clinical and economic variables that impacted those preferences. Respondents indicated their frequency of using deep sutures, epidermal sutures, and wound undermining on a sliding scale of 0% to 100%. Comparisons were made between practice habits, preferences, and surgeon demographics using t tests. Statistical significance was determined as P<.05.
Results
Sixty-eight ACMS fellowship-trained Mohs surgeons completed the survey. The average age of respondents was 45 years; 69.1% (n=47) of respondents were male, and 76.5% (n=52) practiced in a private setting (Table 1). Regardless of epidermal closure type, deep suture placement was used in an average (standard deviation [SD]) of 88.8% (19.5%) of cases overall, which did not statistically differ between years of Mohs experience or practice setting (Table 2). Wound undermining was performed in an average (SD) of 83.0% (24.3%) of cases overall and was more prevalent in private vs academic settings (87.6% [17.8%] vs 65.7% [35.0%]; P<.01). Epidermal sutures were used in an average (SD) of 27.1% (33.5%) of scalp wound cases overall. Surgeons with less experience (≤5 years) used them more frequently (average [SD], 42.7% [36.2%] of cases) than surgeons with more experience (≥16 years; average [SD], 18.8% [32.6%] of cases; P=.037). There was no significant difference between epidermal suture placement rates and practice setting (average [SD], 18.1% [28.1%] of cases for academic providers vs 30.0% [34.8%] of cases with private providers; P=.210).


Clinical and economic factors that were most important during wound closure were ranked (beginning with most important) as the following: risk of complications, cosmetic outcome, hair preservation, patient comfort during closure, healing time, and closure cost. In all demographic cases, risk of complications was ranked 1 or 2 (1=most important; 6=least important) overall; cost was the least important factor overall (Table 2).
Surgeons perceived staples to be superior for speed of closure and for closing wounds in high-tension areas, whereas sutures were perceived as superior when considering cost of closure and ease of removal (Table 3). Successful healing rate, healing time, hair preservation, overall cosmetic outcome, and lower risk of complications were viewed as equivalent when comparing staples and sutures.

In cases in which surgeons did not use staples for closure, the most important factors for opting to not use them were patient discomfort (52.9% [n=36]), cost (25.0% [n=17]), and worse overall cosmetic outcome (23.5% [n=16])(Table 4). The most frequent locations outside of scalp wounds that physicians considered the use of staples for closure were the back (19.1% [n=13]), thigh (10.3% [n=7]), and shoulder (8.8% [n=6]).

Comment
Epidermal closure with sutures was reportedly used in an average of only 27.1% of scalp wound cases, with clinical factors such as cosmetic outcome, risk of complications, and closure time seen as either equivalent or inferior to staples. Our data suggest that surgeon closure perceptions generally are in agreement with established head and neck literature within different medical specialties that favor staple closures, particularly in high-tension areas.1 Interestingly, the most common reasons given for not using staples included patient discomfort, cost, and worse cosmetic outcomes, which are unsubstantiated with head and neck comparative studies.2-4
Although cost was the least important variable for determining closure type in our surveyed cohort, it is likely that the overall cost of closure is frequently underestimated. A higher material cost is noted with staples; however, the largest determinant of overall cost remains the surgeon’s time, which is reduced by factors of 10 or more when closing with staples.2,3 This difference—coupled with the unchanged cosmetic outcome and complication rates—makes staples more advantageous for high-tension scalp wounds.4 Moreover, the stapling technique is more reproducible than suturing, which requires more surgical skill and experience.
Limitations of this study include a lack of directly comparable data for staple and suture scalp wound closures. In addition, the small cohort of respondents in this preliminary study can serve to guide future studies.
Conclusion
Scalp wounds during MMS were most frequently closed using staples vs sutures, with the perception that these methods are equivalent in complication risk, cosmetic outcome, and overall patient satisfaction. These results agree with comparative literature for head and neck surgery and assist with establishing an epidemiologic baseline for future studies comparing their use during MMS.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
- Ritchie AJ, Rocke LG. Staples versus sutures in the closure of scalp wounds: a prospective, double-blind, randomized trial. Injury. 1989;20:217-218.
- Batra J, Bekal RK, Byadgi S, et al. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. J Maxillofac Oral Surg. 2016;15:243-250.
- Kanegaye JT, Vance CW, Chan L, et al. Comparison of skin stapling devices and standard sutures for pediatric scalp lacerations: a randomized study of cost and time benefits. J Pediatr. 1997;130:808-813.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: a prospective, randomized trial. Pediatr Emerg Care. 2002;18:171-173.
Practice Points
- Scalp wounds present a unique challenge for closure during Mohs micrographic surgery due to the scalp's tendency to bleed, limited elasticity, and hair-bearing nature.
- Among fellowship-trained Mohs surgeons, scalp wounds were closed with staples more often than with epidermal sutures.
- Staples and sutures for scalp wounds were perceived to be equivalent in risk of complications, cosmetic outcome, and overall patient satisfaction.
- Compared to epidermal sutures, staples were perceived as advantageous in high-tension areas and for speed of closure.
Z-plasty for Correction of Standing Cutaneous Deformity
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.
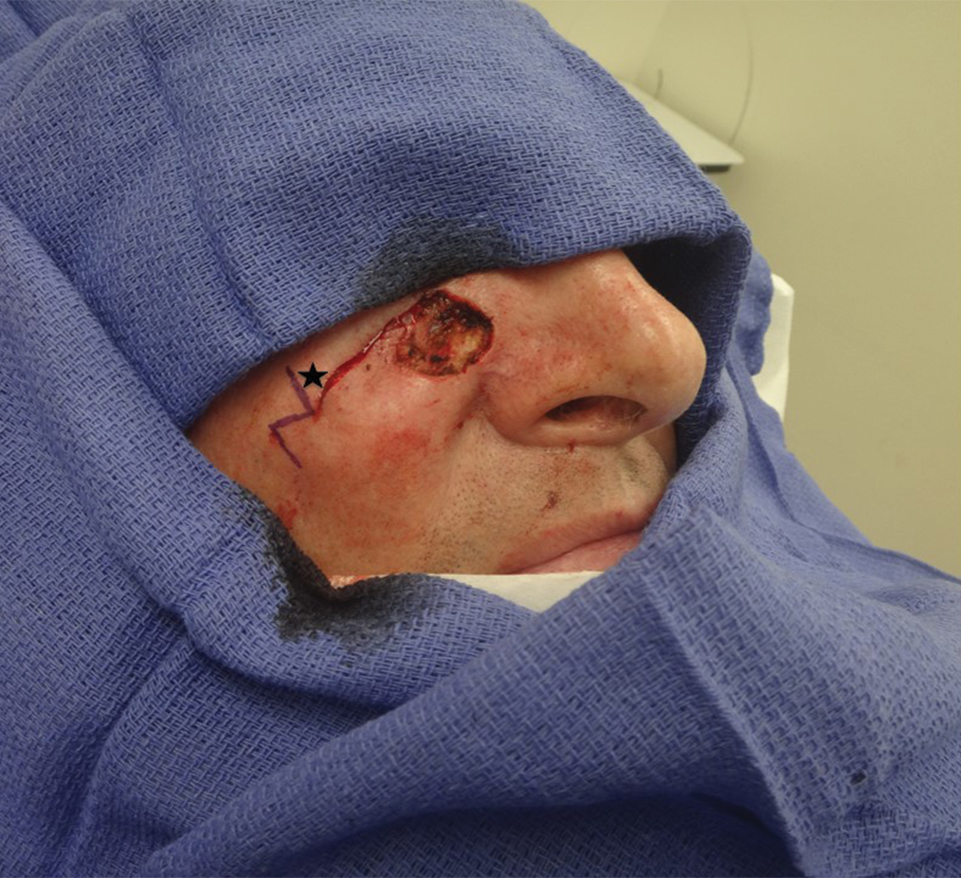

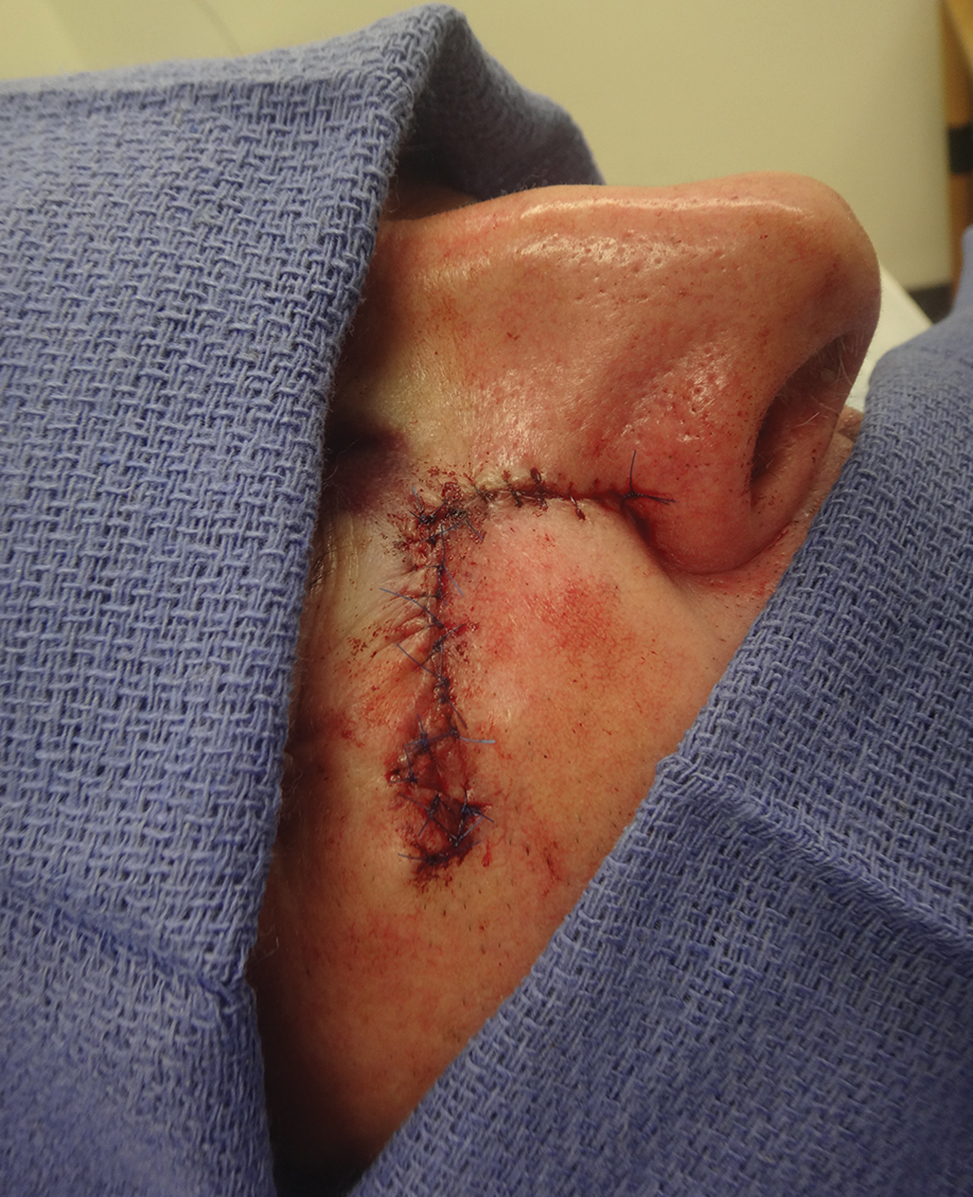

Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.




Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
Practice Gap
Cutaneous head and neck reconstruction following Mohs micrographic surgery frequently presents the surgical dilemma of dog-ear formation during wound closure, often necessitating excision of additional tissue to correct the standing cone, which could pose the risk for an undesirable tension vector as well as encroachment upon additional cosmetic units or sensitive anatomic structures such as a free margin. A classic Z-plasty is a transposition flap (by definition, translocation of tissue laterally about a pivot point) that corrects a dog-ear deformity without skin excision by recruiting tissue from the axis of the standing cone and redistributing it along another.
The Technique
A classic Z-plasty is designed with 3 equal limb lengths (<1 cm each) at 60° angles, abutting the pedicle of the rotation or advancement flap. The limbs can extend away from the pedicle of the flap to minimize vascular compromise. In our patient, the theoretical standing cone was located at the lateral aspect of an O to L advancement flap (Figure 1). The 2 identical triangular flaps were elevated (Figure 2A), transposed around the pivot point (Figure 2B), and inset (Figure 3). The standing cone was corrected by redistribution of tissue without excision of additional tissue, resulting in a softer and thinner scar 2 weeks (Figure 4A) and 4 months (Figure 4B) postoperatively.




Practice Implications
This technique can be used to correct cones following primary wound repairs or flaps. The primary advantage of this technique for dog-ear correction is tissue sparing. Disadvantages include more complex surgical planning and longer scar length compared to excisional corrective techniques. Additionally, Z-plasty requires more time to execute compared to simpler techniques.1,2
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
- Frodel JL, Pawar SS, Wang TD. Z-Plasty. In: Baker SR, ed. Local Flaps in Facial Reconstruction. 3rd ed. Elsevier; 2014:317-338.
- Hundeshagen G, Zapata-Sirvent R, Goverman J, et al. Tissue rearrangements: the power of the Z-plasty. Clin Plast Surg. 2017;44:805-812.
IV gentamicin improves epidermolysis bullosa
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
FROM AAD 2020
What’s Eating You? Megalopyge opercularis
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
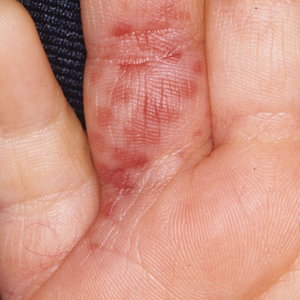
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9
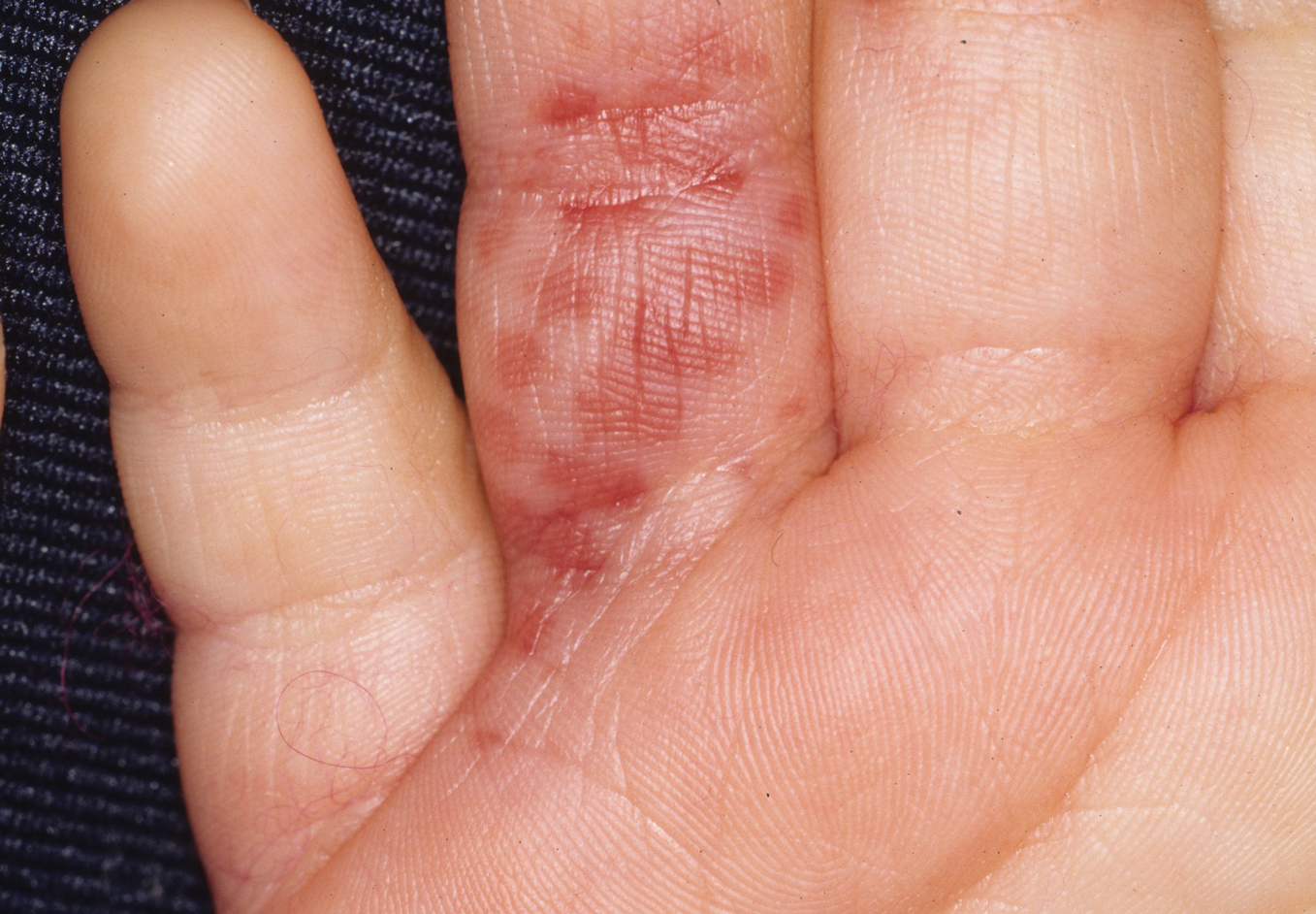
Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Practice Points
- Megalopyge opercularis is the most widely distributed caterpillar species in the Americas, and envenomation by it can occur year-round.
- Skin reactions to M opercularis stings can present as maculopapular dermatitis, eczematous eruptions, or urticarial reactions.
- During the initial presentation, patients experience intense throbbing pain, yet the severity of symptoms depends on the caterpillar’s size and the extent of contact.
- A history of caterpillar exposure helps with diagnosis, and treatment remains empiric.
Sericin, a versatile silk protein, has multiple potential roles in dermatology
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
What’s Eating You? Bark Scorpions (Centruroides exilicauda and Centruroides sculpturatus)
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Practice Points
- Centruroides scorpions can inflict painful stings.
- Children are at greatest risk for systemic toxicity.
Cell therapy closes large wounds in epidermolysis bullosa
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
REPORTING FROM EB WORLD CONGRESS
D.C.-area blacks face increased risk of mortality from SJS/TEN
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.