User login
Combined Treatment of Disfiguring Facial Angiofibromas in Tuberous Sclerosis Complex With Surgical Debulking and Topical Sirolimus
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
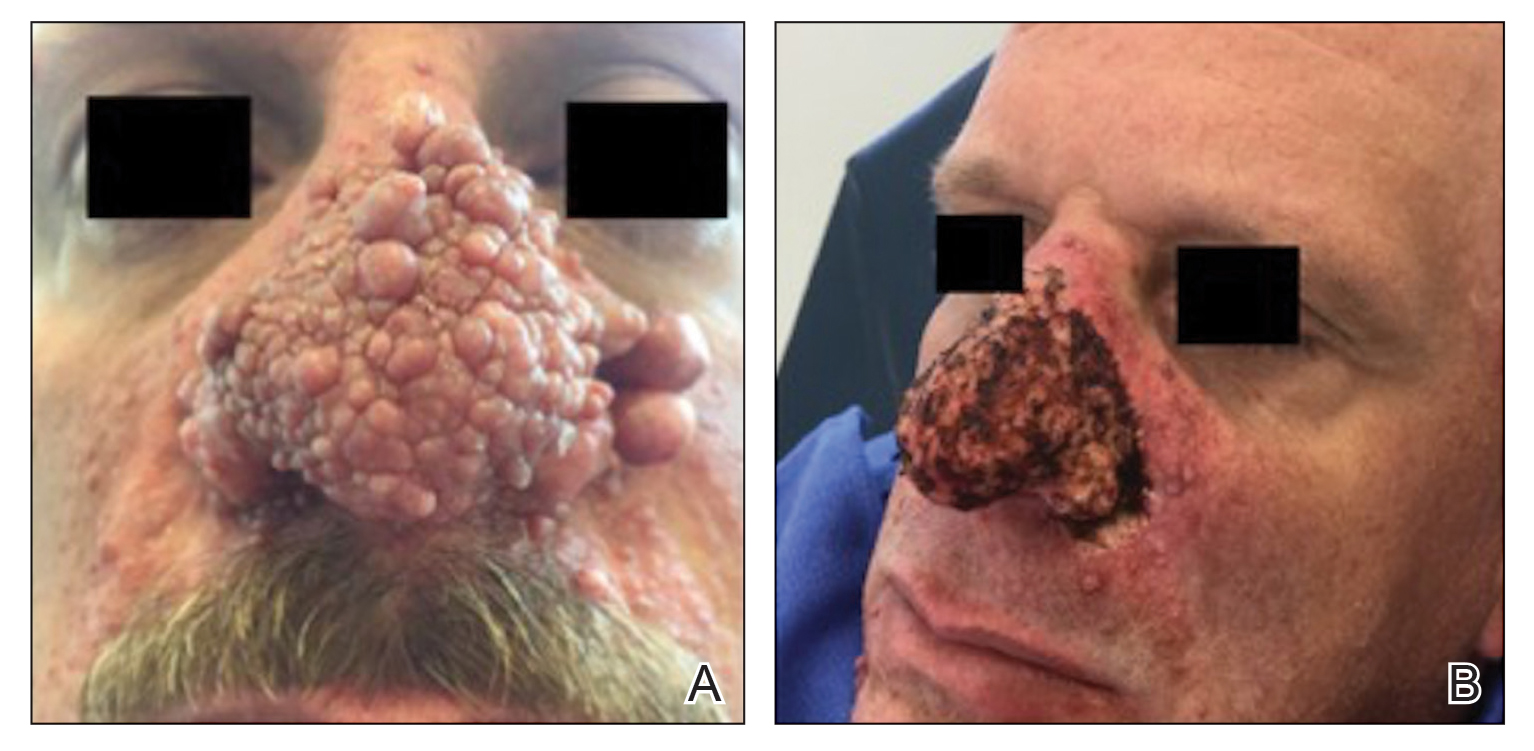
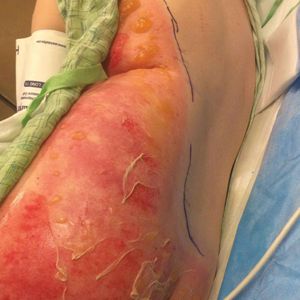
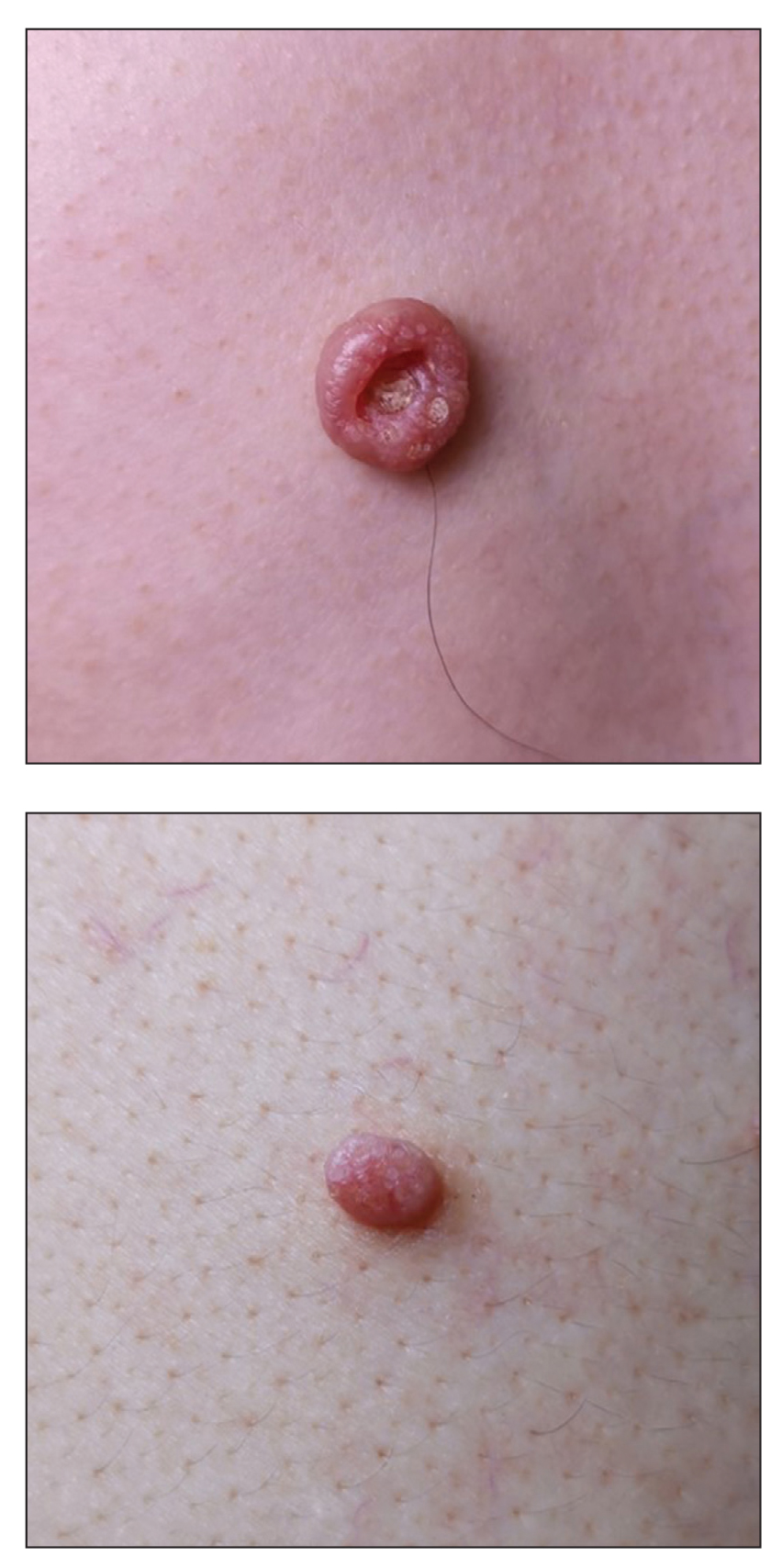
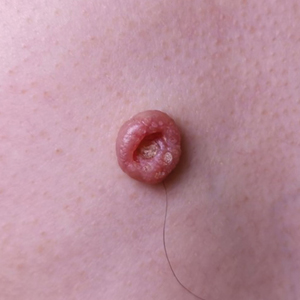
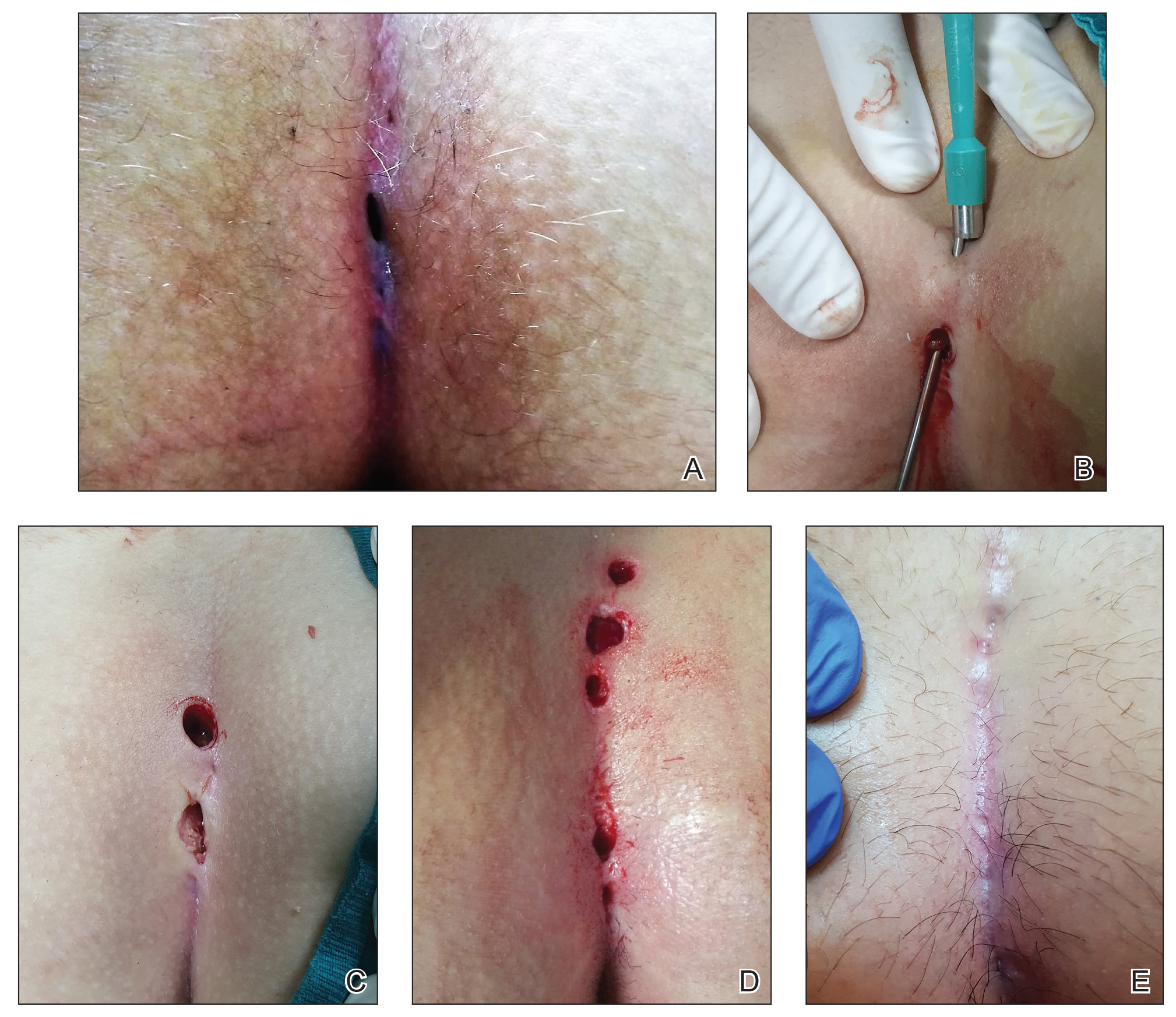
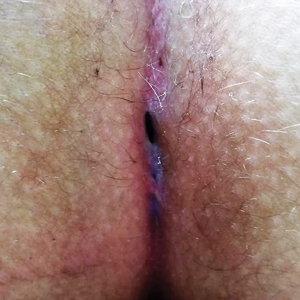
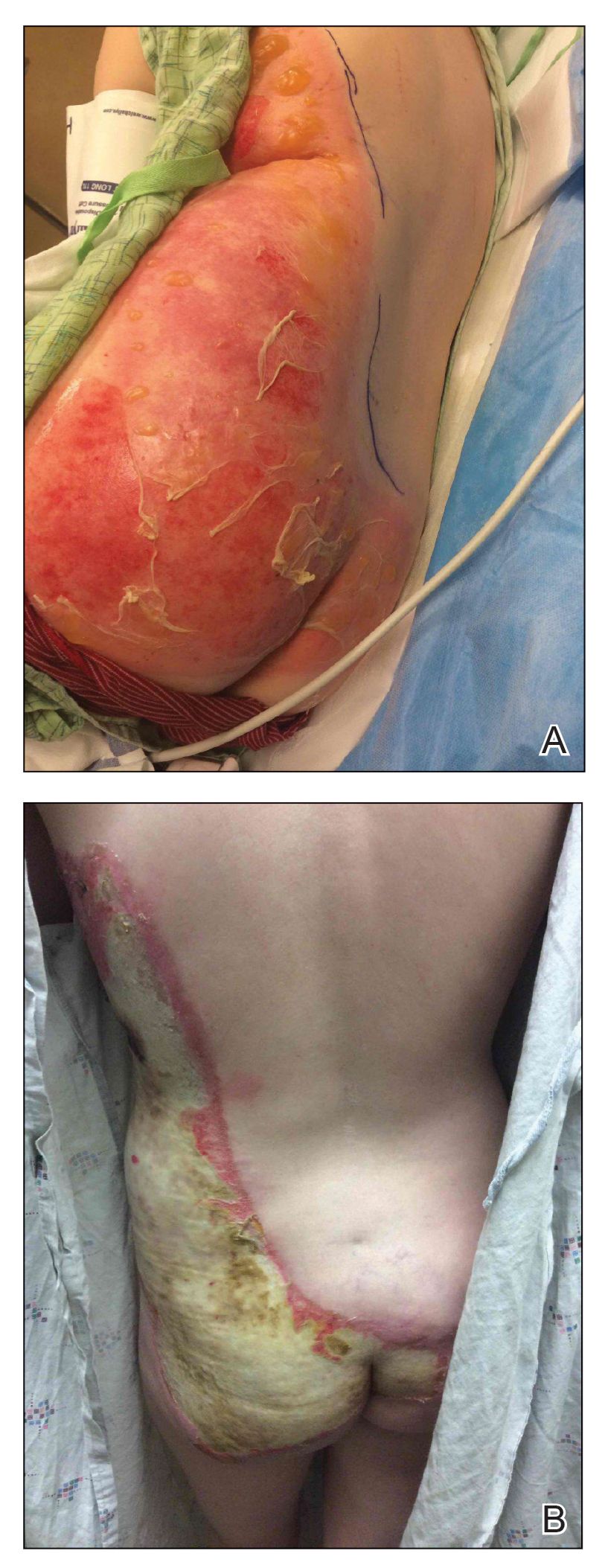
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
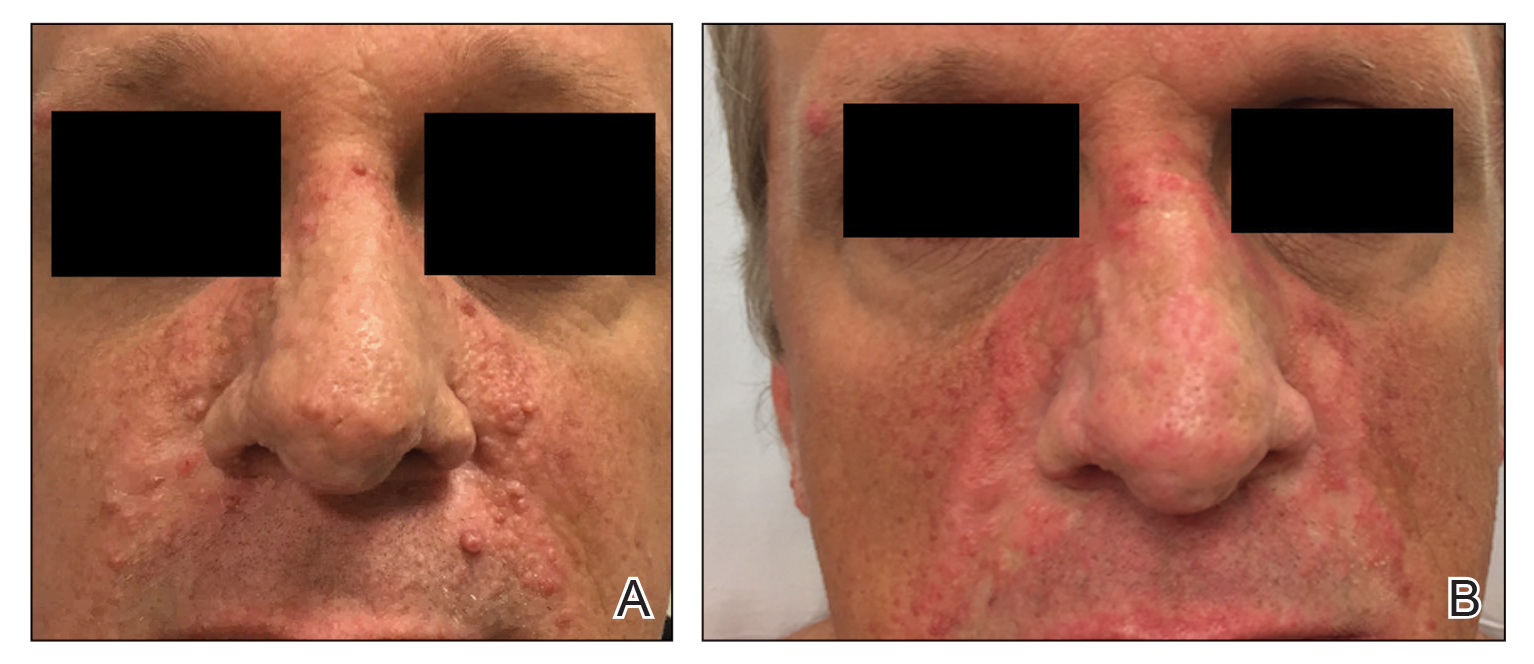
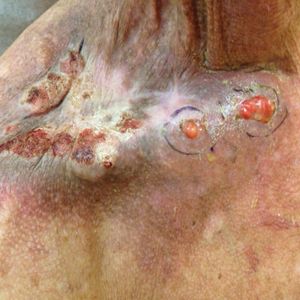
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Doctor in a Bottle: Examining the Increase in Essential Oil Use
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
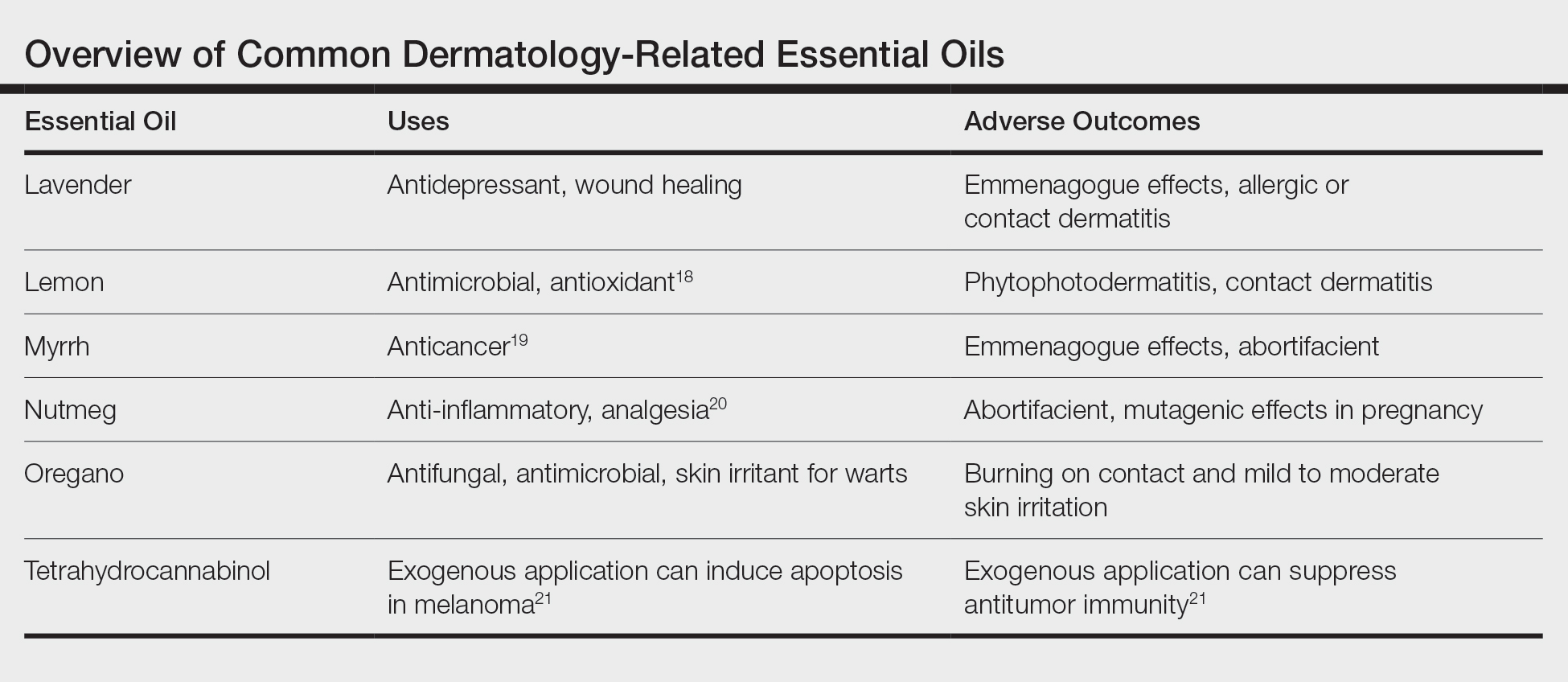
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
Practice Points
- Essential oils are a rising trend of nonprescribed topical supplements used by patients to self-treat.
- Research into historically medicinal essential oils may unlock treatment opportunities in the near future.
- Keeping an open-minded line of communication is critical for divulgence of potential home remedies that could be causing patients harm.
- Understanding the mindset of the essential oil–using community is key to building trust and treating these patients who are often distrusting of Western medicine.
Symmetric Drug-Related Intertriginous and Flexural Exanthema
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.
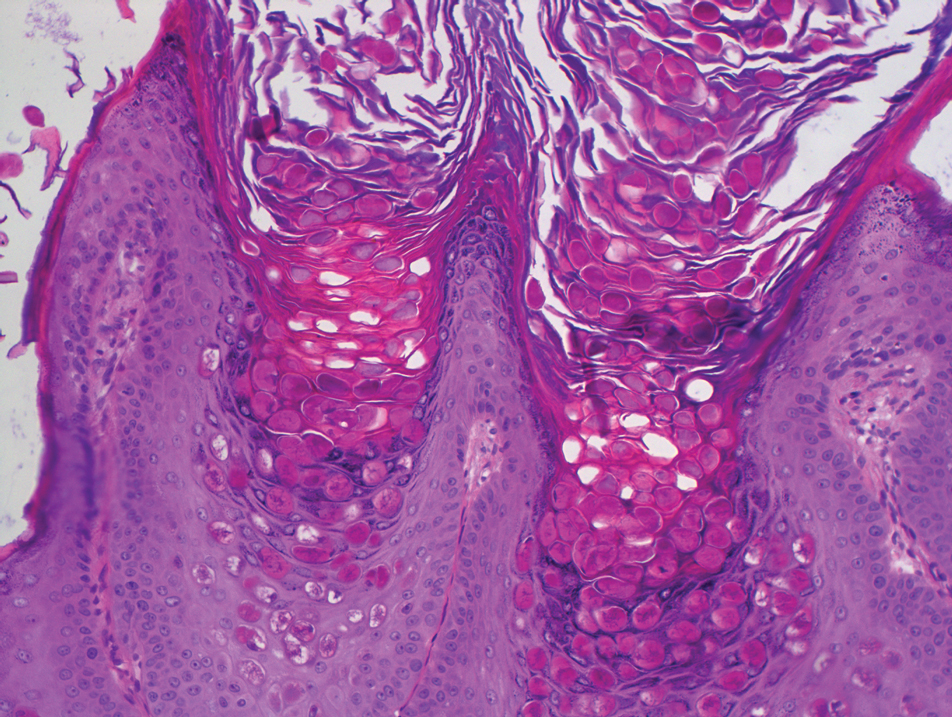
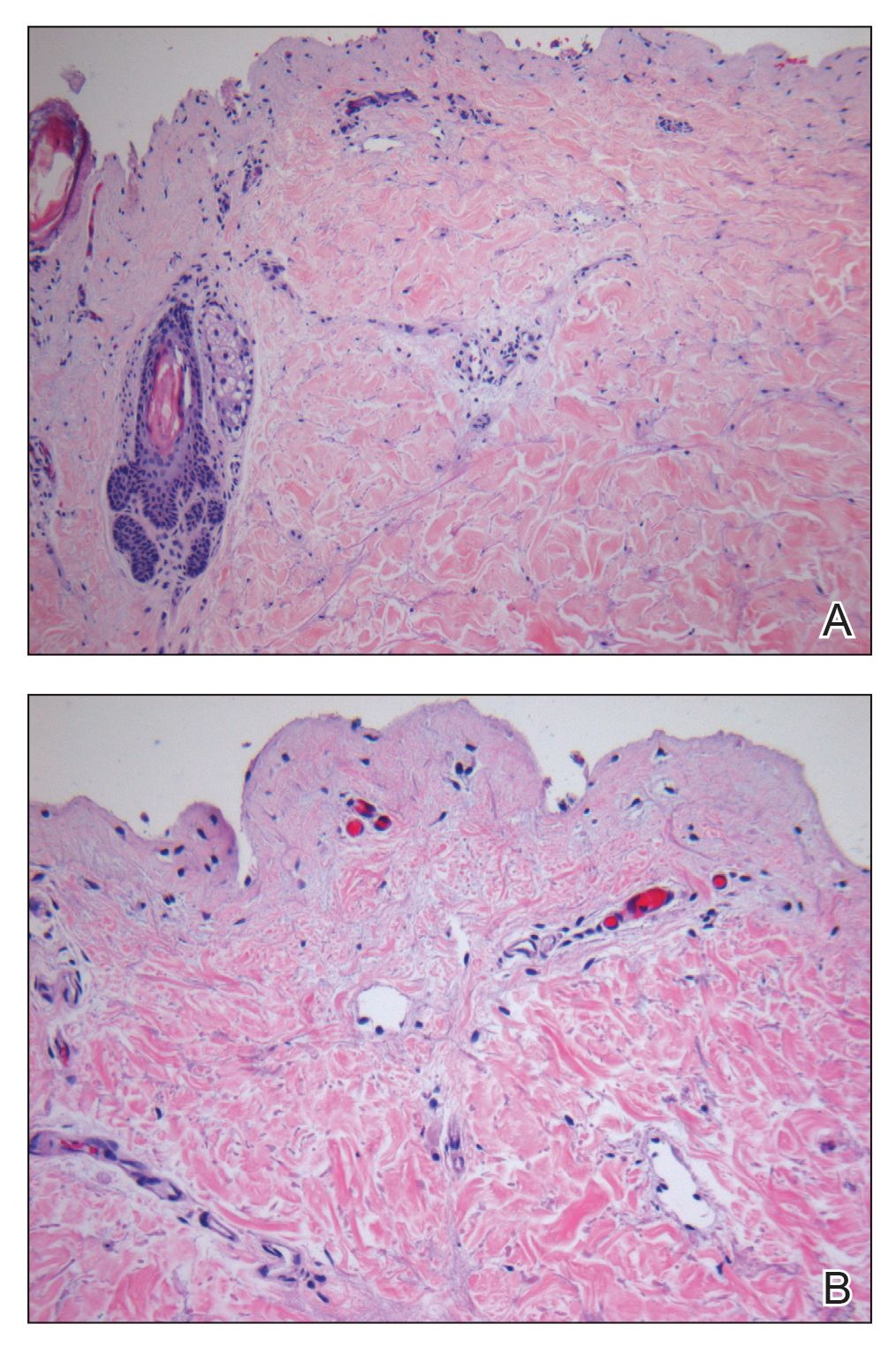
Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4
Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.

Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4

Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.

Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4

Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
Practice Points
- Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) appears in the absence of systemic signs and symptoms such as fever, which may help differentiate it from infectious causes.
- β-Lactam antibiotics, particularly amoxicillin, are common offenders in the pathogenesis of SDRIFE, but new drug relationships frequently are being described.
- Symmetric drug-related intertriginous and flexural exanthema commonly follows a benign course but warrants respect, as it may have devastating potential.
Foreign-Body Reaction to Orthopedic Hardware a Decade After Implantation
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
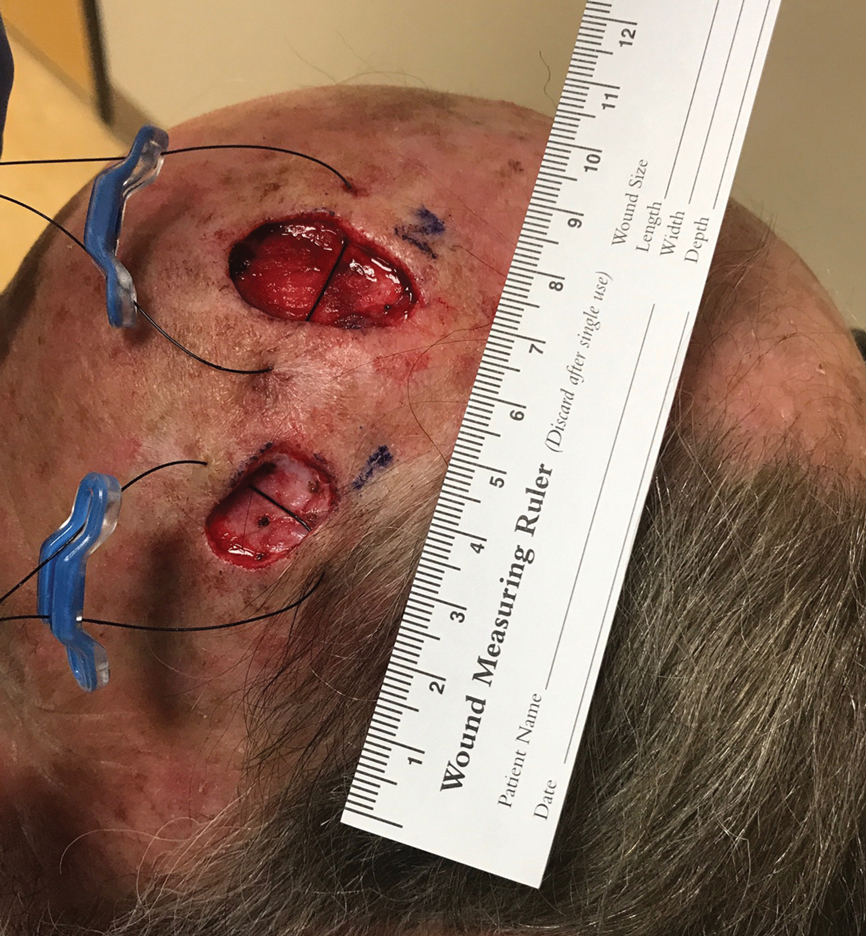
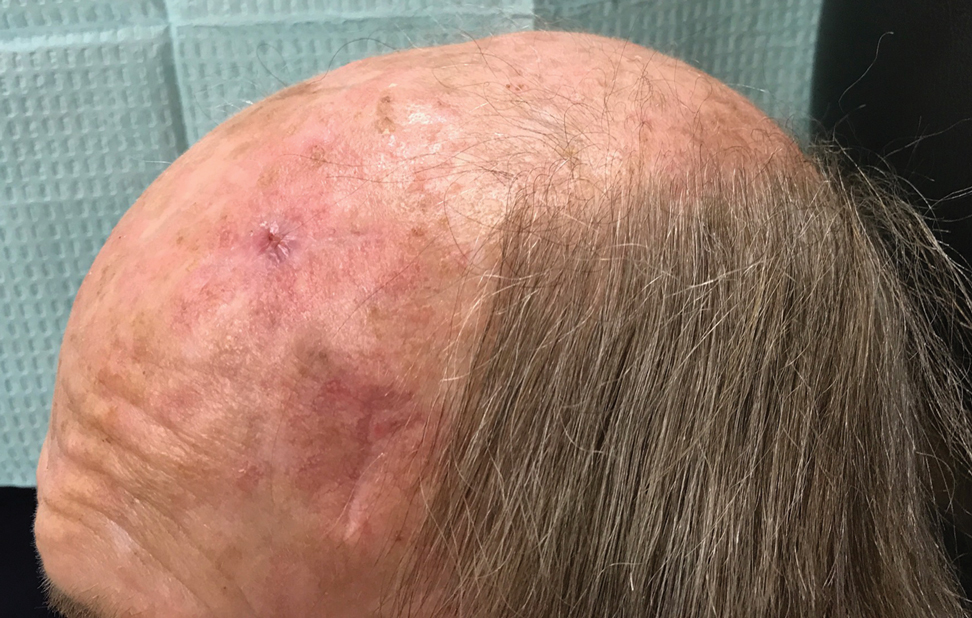
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
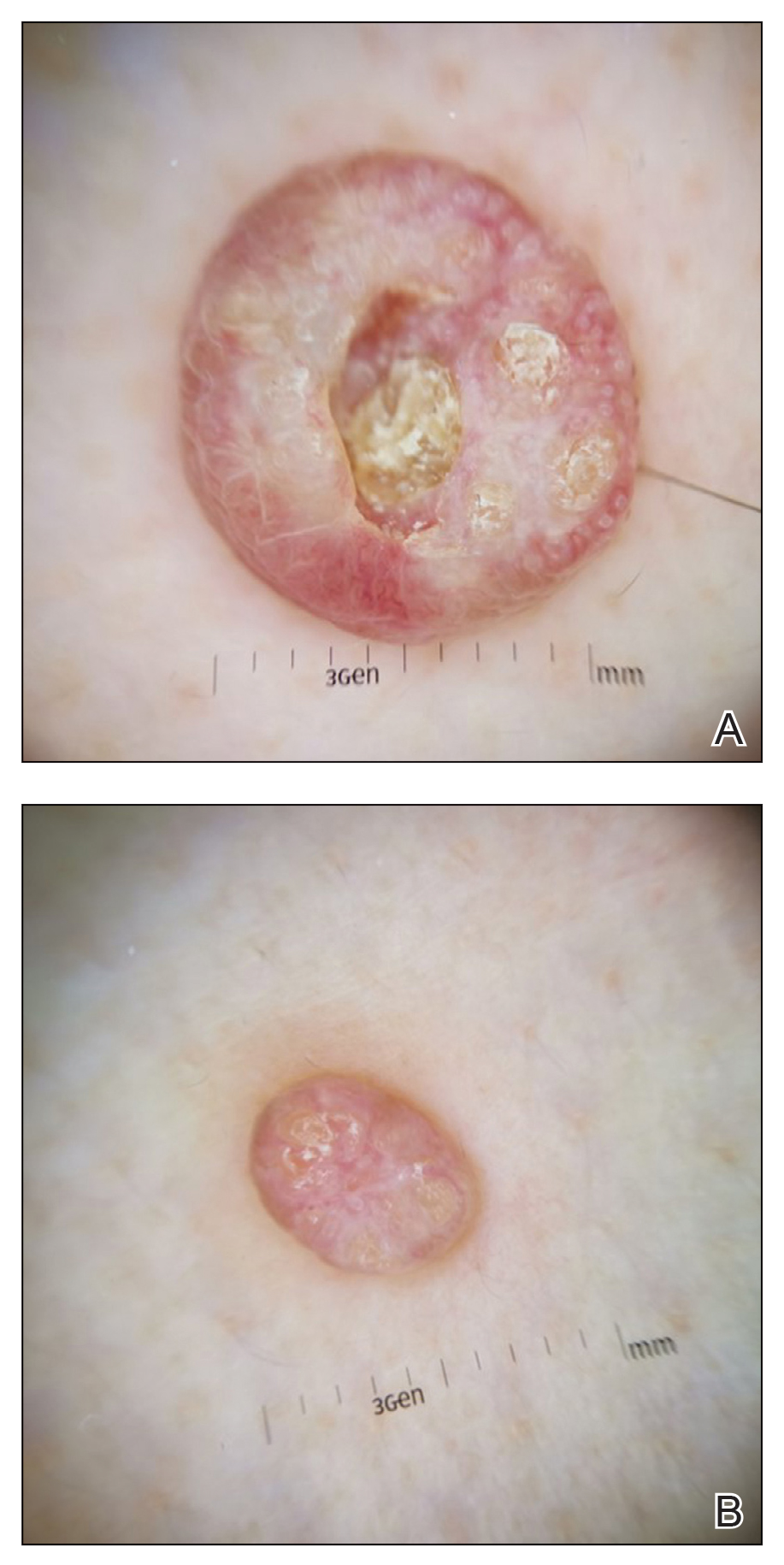
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.
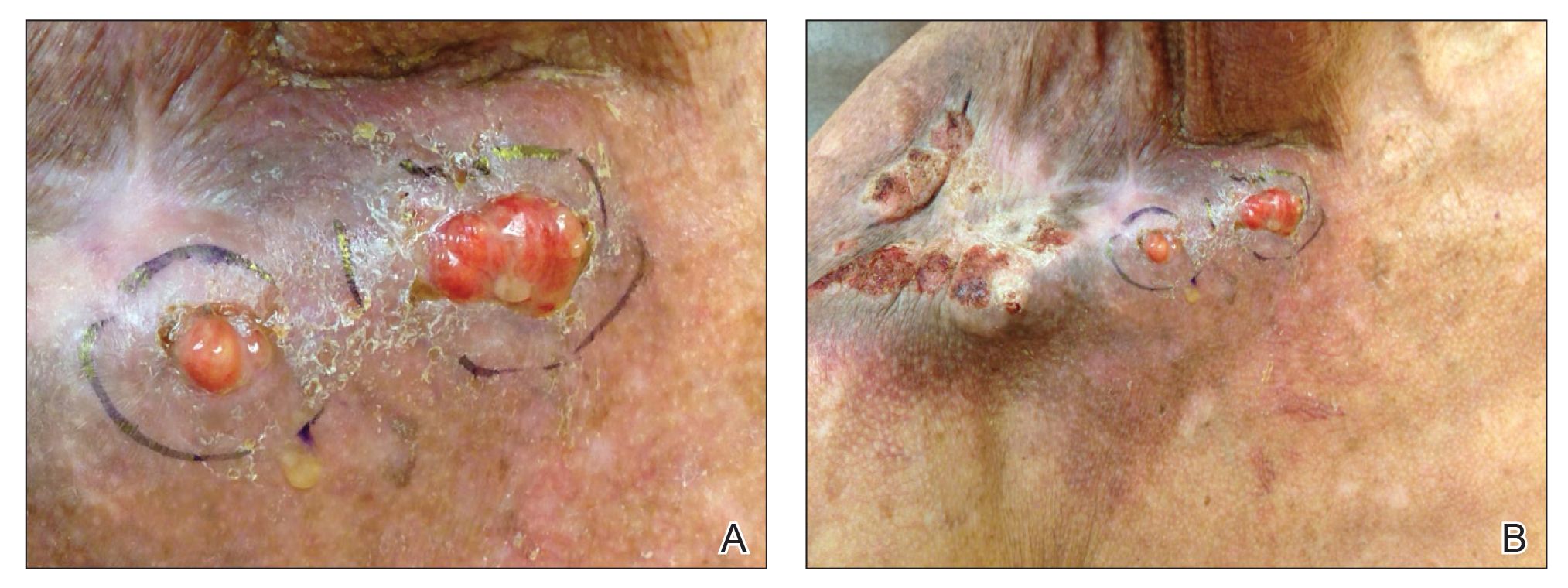
The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.
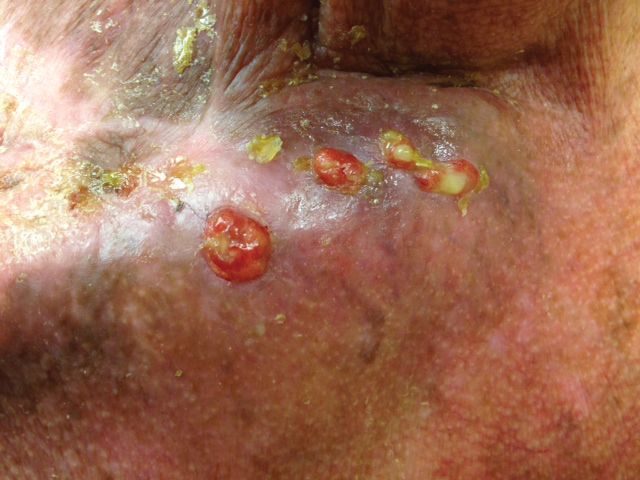
Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.
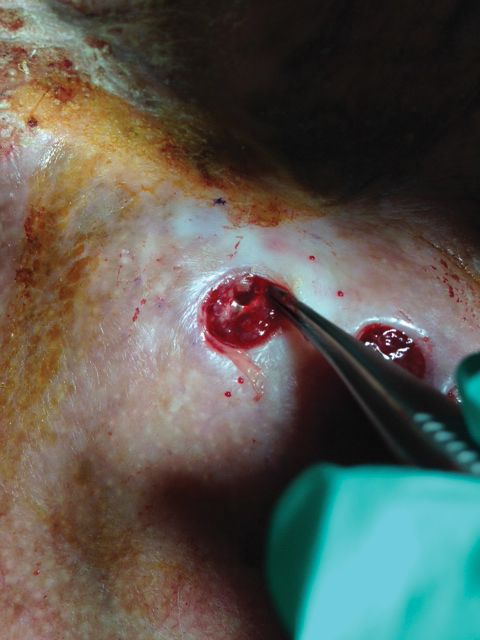
Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.

The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.

Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.

Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.

The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.

Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.

Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
Practice Points
- Cutaneous reactions to implantable devices occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions.
- Most reactions typically occur within the first weeks or months after implantation; however, a reaction rarely may present several years after implantation.
Umbilicated Neoplasm on the Chest
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
A 49-year-old man presented with a slow-growing mass on the chest of 1 year’s duration. The neoplasm started as a small papule that gradually increased in size. The patient denied pain, itching, bleeding, or discharge. He had a history of end-stage renal disease with a kidney transplant 8 years prior. His medication history included long-term use of oral tacrolimus, mycophenolate mofetil, and prednisone. Physical examination revealed a yellowish red, exogenous, pedunculated neoplasm on the right side of the chest measuring 1 cm in diameter with an umbilicated center and keratotic material (top). There were 2 more yellowish red papules on the left side of the chest measuring 0.5 cm in diameter without an umbilicated center (bottom). Dermoscopy and a biopsy were performed.
Intraoperative Tissue Expansion to Allow Primary Linear Closure of 2 Large Adjacent Surgical Defects
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
The Gips Procedure for Pilonidal Disease: A Retrospective Review of Adolescent Patients
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Practice Points
- The Gips procedure is an easy-to-use outpatient procedure for adolescents with pilonidal disease.
- This procedure has a high success rate and does not restrict school or social activities.
Birch bark derivative gel found effective for EB, in phase 3 study
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
A . The results come from the largest double-blind, randomized trial performed in this patient population.
More than 41% of EB target wounds that were treated with Oleogel-S10 healed within 45 days, compared with about 29% of target wounds treated with placebo, in the EASE phase 3 trial, conducted at 58 sites in 28 countries.
A group of rare genetic disorders, EB “is described as the worst disease you’ve never heard of,” explained lead investigator Dedee Murrell, MD, director of dermatology, St. George Hospital at the University of New South Wales, Sydney. “It starts in children and is like having burns that heal with scars, and no treatment has been approved for it” by the Food and Drug Administration.
“This is the first large clinical trial with placebo of a topical treatment that’s worked for this terrible disease,” Dr. Murrell said in an interview. She noted that standard EB treatment currently consists of applying nonstick dressings to wounds to protect skin from trauma and infection.
Dr. Murrell, who has focused her work on EB patients since 1990, presented the findings at the virtual annual Congress of the European Academy of Dermatology and Venereology.
The trial enrolled 223 patients (average age, 12 years, but ages ranged to 81 years) with three types of EB, including dystrophic and junctional EB and Kindler syndrome. For each participant, a target wound was selected for use as the primary efficacy endpoint. Those wounds had a partial thickness of between 10 cm2 and 50 cm2 and lasted between 21 days and 9 months. Patients were stratified into groups depending on type of EB and size of target wound.
Participants were randomly assigned to receive either Oleogel-S10 (n = 109) or placebo (n = 114). All applied the blinded-study gel to all their wounds at least every 4 days at the time dressings were changed.
The primary endpoint was the percentage of patients whose target wounds completely closed within 45 days. Key secondary endpoints included time to wound healing and percentage of target wounds that healed within 90 days of treatment; incidence and severity of target wound infection; change in total body wound burden, as measured by the Epidermolysis Bullosa Disease Activity and Scarring Index skin activity subscore; change in itching, as measured by the Itch Man Scale and the Leuven Itch Scale; and adverse events.
Nearly 92% of patients who were treated with Oleogel-S10 completed the double-blind phase of the trial, compared with nearly 87% who received placebo. As noted, the primary endpoint was met, with 41.3% of Oleogel-S10 patients achieving target wound closure within 45 days, compared with 28.9% of the patients who received placebo (P = .013).
But the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of Oleogel-S10 patients achieving wound closure vs. 43.9% of control patients.
Target-wound infection occurred in eight participants, including three who used Oleogel-S10 and five who received placebo; all moderate or severe infections occurred in patients who received placebo. Total wound burden was reduced to a greater extent among Oleogel-S10 patients by day 60, but there was no apparent difference at day 90.
Both treatment groups reported qualitative improvements in itch, with no significant differences between groups. The prevalence of adverse events was also similar between groups (Oleogel-S10, 81.7%; placebo, 80.7%). The most frequently reported adverse events among Oleogel-S10 patients, compared with patients who received placebo, were wound complications, pyrexia, wound infection, pruritus, and anemia; only 4.5% of adverse events were deemed severe.
Dr. Murrell said that, on the basis of the trial results, she expects the FDA to fast-track approval of Oleogel-S10, which contains triterpene extract and sunflower oil.
The gel is “a treatment patients will be able to put under their dressings, added to normal treatment, which will accelerate their wound healing, with no significant increase in any side effects,” she added.
Jemima Mellerio, MD, of St. Thomas’ Hospital in London who sees about 400 EB patients each year, agreed with Dr. Murrell that the results are “very exciting.” Dr. Mellerio was not involved in the study.
“Practicing dermatologists seeing people with EB will have something to offer that appears to speed up wound healing in chronic wounds,” Dr. Mellerio said in an interview. “It’s a positive option rather than just supportive treatment, something that makes a difference to the natural history of wounds.”
She said the trial’s biggest strength was including “such a large cohort of patients.
“It’s extremely difficult to do that kind of study, especially with a placebo-controlled arm and especially in a rare disease,” Dr. Mellerio said. “If you think about the product itself, it’s easy to apply, so it’s not particularly onerous for people to add to their daily regimen of dressings.”
The study was funded by Amryt Pharma. Dr. Murrell is an advisory board member for Amryt Pharma. Dr. Mellerio is a consultant for Amryt Pharma.
A version of this article originally appeared on Medscape.com.
Paronychia and Target Lesions After Hematopoietic Cell Transplant
The Diagnosis: Fusariosis
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.
Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
The Diagnosis: Fusariosis
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.

Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
The Diagnosis: Fusariosis
A periodic acid-Schiff stain of the seropurulent drainage from a skin nodule revealed neutrophils and scarce branching hyaline hyphae. Skin and blood cultures grew a white cottony colony. Microscopic examination showed sickle-shaped macroconidia and septate hyaline hyphae with branching acute angles (Figure). Molecular analysis by polymerase chain reaction yielded Fusarium solani species complex. Histopathology as well as culture and molecular findings were consistent with a diagnosis of disseminated fusariosis. Amphotericin B was started with rapid clinical improvement. The patient was asymptomatic upon discharge with voriconazole 200 mg twice daily.

Fusariosis is an emerging, opportunistic, and life-threatening mycosis. In immunocompetent patients it may cause onychomycosis and keratitis.1 Invasive fusariosis predominantly is caused by the F solani species complex and affects immunocompromised patients, especially those with neutropenia or acute leukemia or hematopoietic stem cell transplant recipients.2
Before invasion, the infection frequently may begin by affecting the nail apparatus as onychomycosis or paronychia of the skin. As in our case, trauma or manipulation of the nail favors dissemination.3 Skin manifestations include erythematous to violaceous papules, macules, and nodules with central necrosis or crust; some may exhibit target morphology. Other organs may be affected, including the sinuses, lungs, liver, spleen, and kidneys. A comprehensive clinical examination before hematopoietic cell transplant and during fever and neutropenia may opportunely identify these potential infective foci.3,4
The differential diagnosis of disseminated fusariosis includes bacterial infections, especially Staphylococcus aureus and Pseudomonas aeruginosa, and other invasive fungal infections, particularly aspergillosis, mucormycosis, and candidiasis.5 Symptom persistence after broad-spectrum antibiotic initiation should raise diagnostic suspicion of systemic mycosis or mycobacterial infection. Mucormycosis and candidiasis have histopathologic profiles that differ from fusariosis, presenting with broad ribbonlike hyphae with 90° angulation and pseudohyphae with budding yeast cells, respectively. Differentiation of disseminated fusariosis and aspergillosis in neutropenic patients is difficult. Hyphae cannot be differentiated from those of Aspergillus species on histology.6 Furthermore, serologic assays, such as galactomannan and (1,3)-β-D-glucan, cross-react with both genera. Clinically, Fusarium species exhibit metastatic skin lesions, cellulitis, and positive blood cultures due to adventitious sporulation more frequently than Aspergillus species. Patients with aspergillosis more commonly present with sinusitis, pneumonia, and pulmonary macronodules with the halo sign.6 Although nocardiosis presents with disseminated subcutaneous nodules with pulmonary affection in immunocompromised patients, its morphology is very different from fusariosis. Nocardia presents with a gram-positive bacillus with the microscopic appearance of branching filaments. Yeastlike microorganisms with morphology ranging from oval to sausagelike are found in talaromycosis, an uncommon fungal infection predominantly caused by Talaromyces marneffei. Fusarium species culture reveals white cottony colonies with characteristic hyaline, canoe-shaped or sickle-shaped (banana-shaped), multicellular macroconidia, and microconidia. Precise species identification requires molecular analyses such as polymerase chain reaction.
Mortality is high, ranging from 50% to 70% of cases.5 Voriconazole or lipid-based amphotericin B are considered first-line treatments. Posaconazole may be employed as a second-line alternative. Surgical debridement of infected tissues and removal of colonized venous catheters is recommended. Secondary prophylaxis should be considered with agents such as voriconazole, posaconazole, or amphotericin B.5 Resolution of immunosuppression and neutropenia is an important factor to reduce the mortality rate.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
- Ranawaka RR, Nagahawatte A, Gunasekara TA. Fusarium onychomycosis: prevalence, clinical presentations, response toitraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int J Dermatol. 2015;54:1275-1282.
- Nucci F, Nouer SA, Capone D, et al. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714.
- Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68:85-89.
- Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20:115-117.
- Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- Nucci F, Nouer SA, Capone D, et al. Invasive mould disease in haematologic patients: comparison between fusariosis and aspergillosis. Clin Microbiol Infect. 2018;24:1105.e1-1105.e4.
A 19-year-old man with acute lymphoblastic leukemia was admitted for an allogeneic hematopoietic cell transplant. On the 11th day of hospitalization, he experienced a right toe trauma in his hospital room and subsequently developed edema, erythema, and pain on the right hallux (top). The next day, a general surgeon performed a minor incision and drainage of the affected area. After 2 days, the patient developed a fever and a disseminated dermatosis located on the arms and legs characterized by target lesions with a necrotic center and erythematous papules and macules (bottom). On day 3, he developed severe neutropenia (0.042×109 cells/L [reference range, 2.0–6.9×109 cells/L]). Broad-spectrum antibiotics were initiated without clinical improvement. The patient developed dyspnea on day 5. Skin, nail, and blood cultures were obtained. High-resolution computed tomography of the chest displayed multiple small pulmonary nodules, ground-glass opacities, and the tree-in-bud sign.
Management of Classic Ulcerative Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).
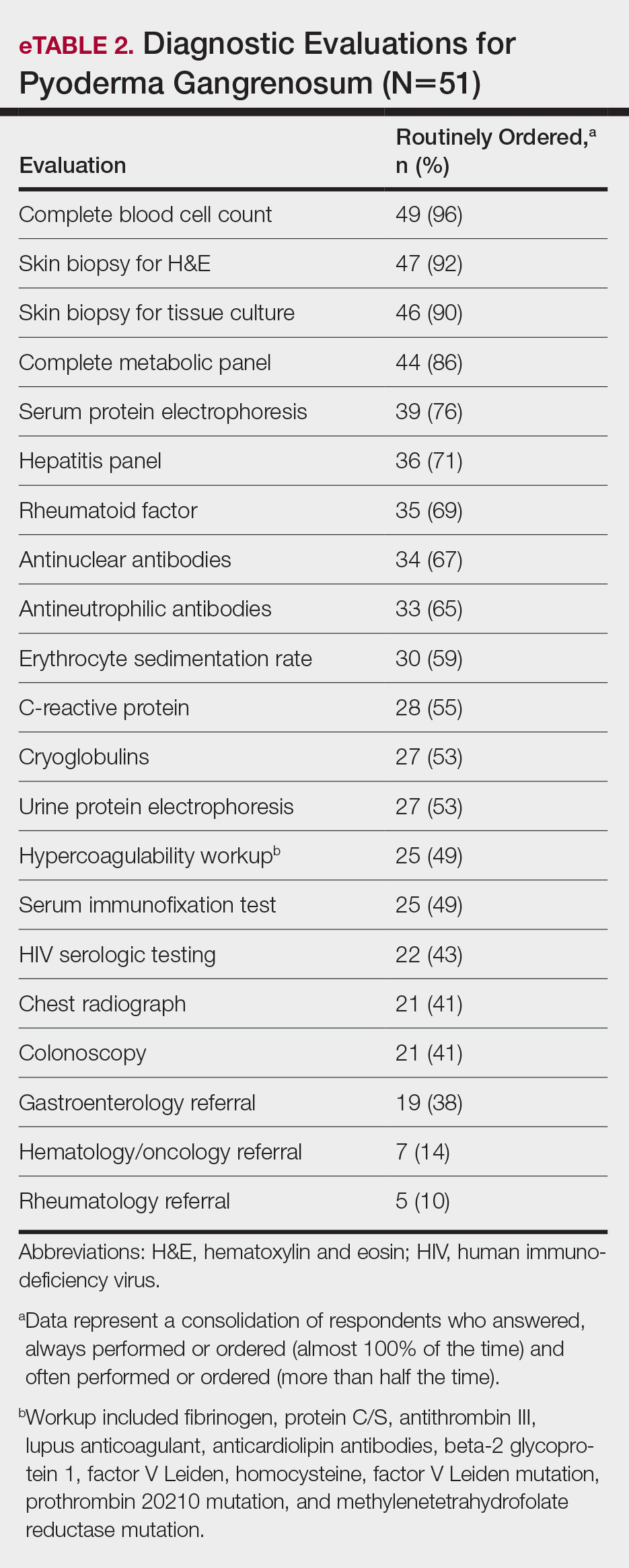
Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).
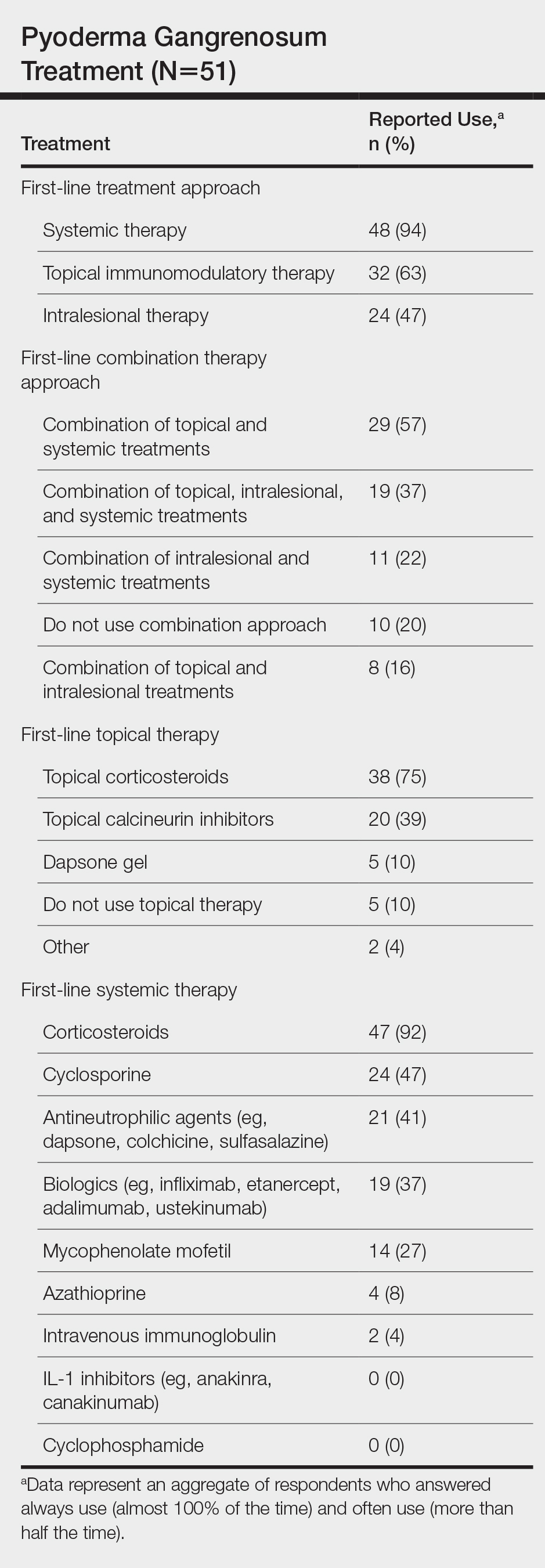
A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).
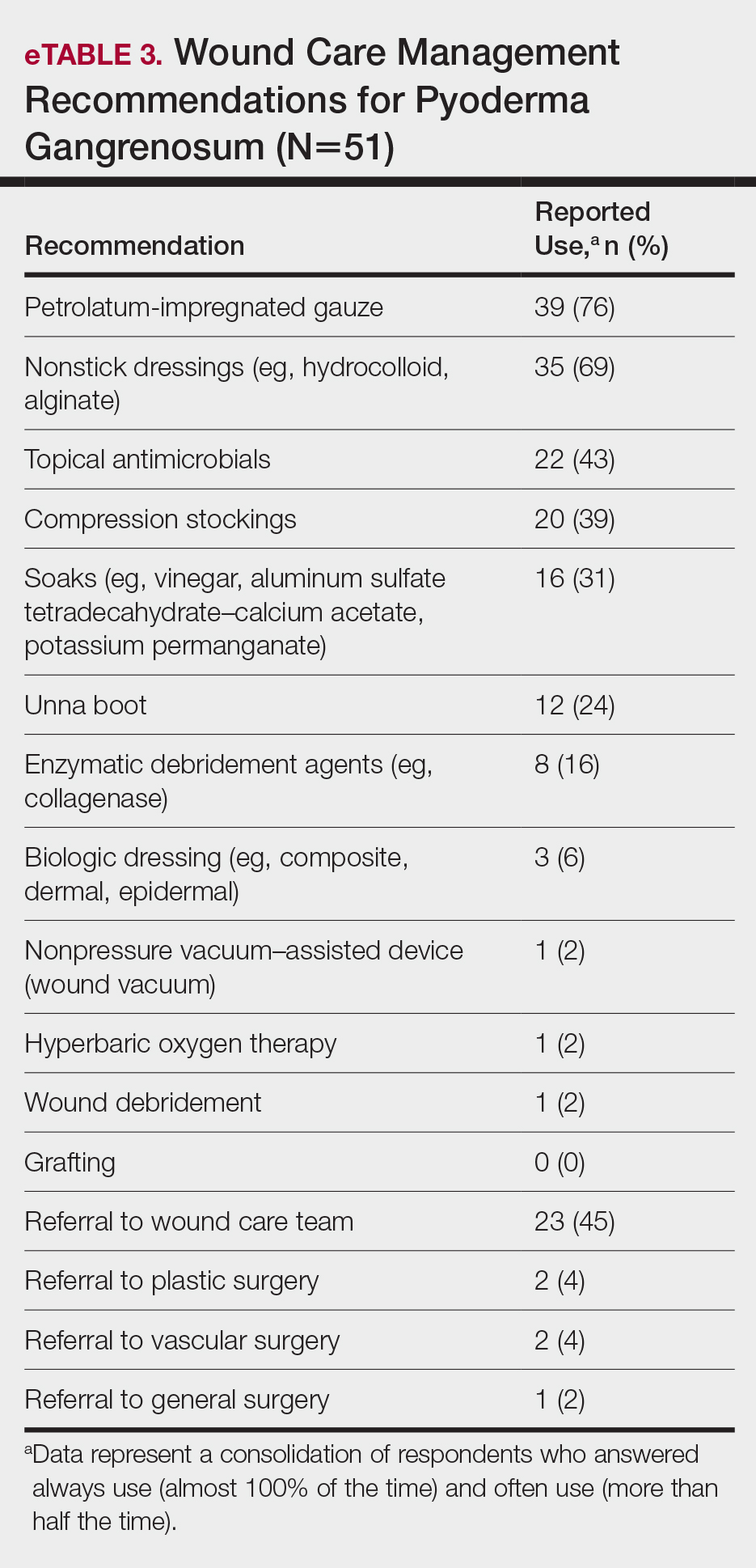
Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9
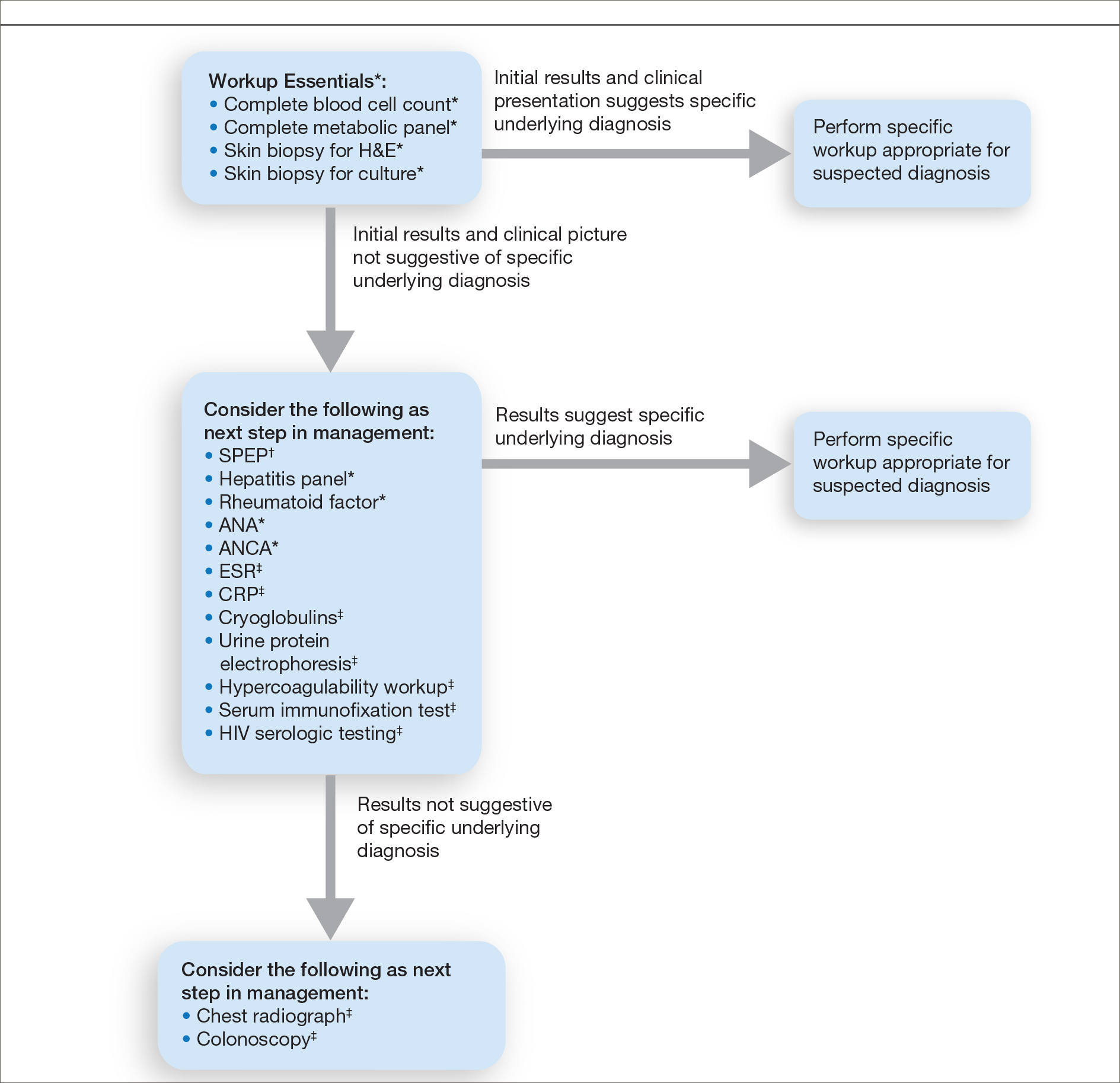
Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1
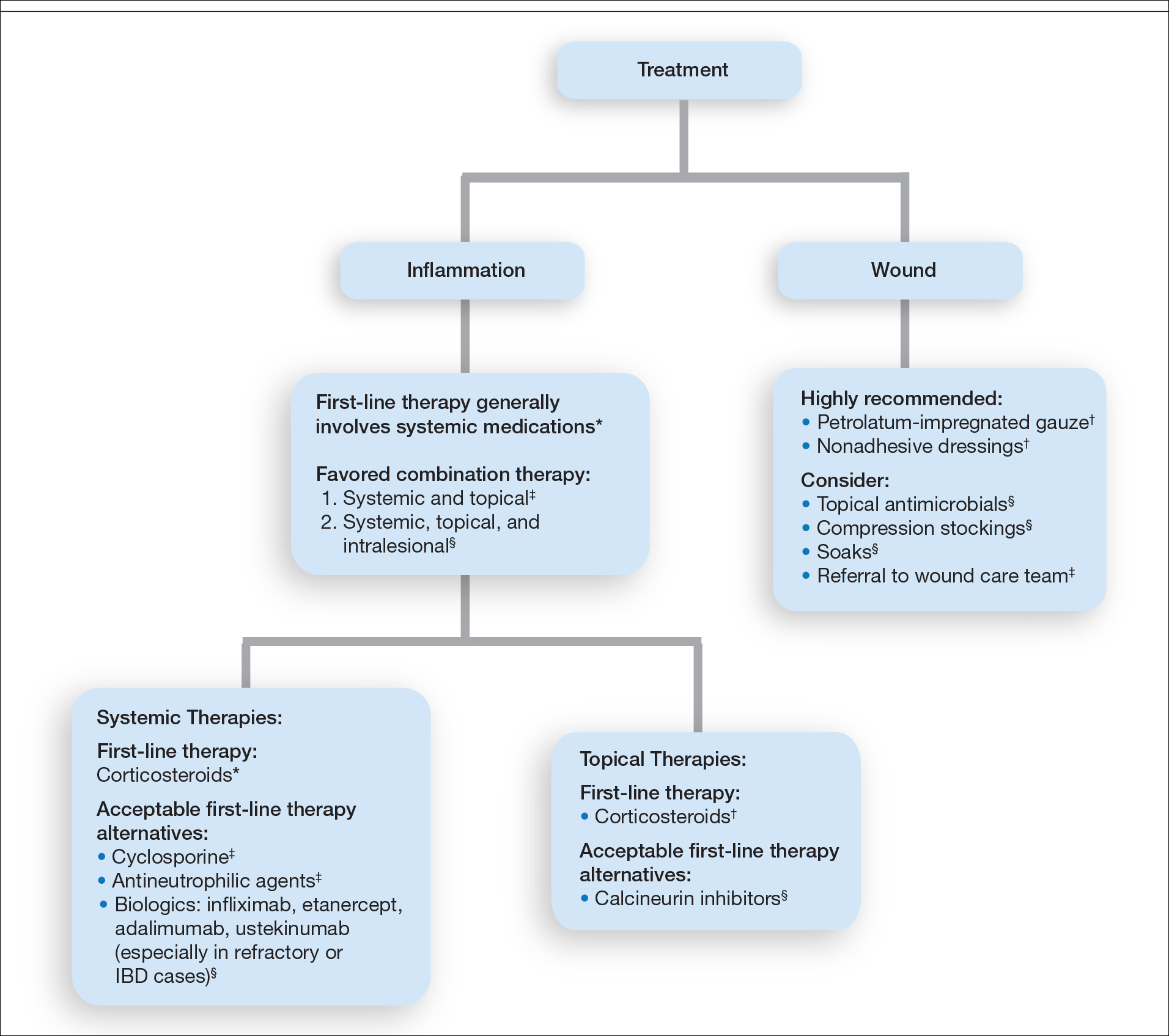
Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Practice Points
- The diagnosis of pyoderma gangrenosum (PG) poses a challenge in clinical practice that could be minimized by following a stepwise algorithm based on initial test results (including skin biopsies) and features of the patient’s clinical presentation.
- As there is no US Food and Drug Administration–approved treatment for PG, a stepwise algorithm approach in combination with the clinical experience addressing inflammation and wound care is essential to reach control and remission of PG.