User login
Fewer transplants for MM with quadruplet therapy?
“It is not a big leap of faith to imagine that, in the near future, with the availability of quadruplets and T-cell therapies, the role of high-dose melphalan and autologous stem cell transplant will be diminished,” said Dickran Kazandjian, MD, and Ola Landgren, MD, PhD, of the myeloma division, Sylvester Comprehensive Cancer Center, University of Miami.
They commented in a editorial in JAMA Oncology, prompted by a paper describing new results with a novel quadruple combination of therapies. These treatments included the monoclonal antibody elotuzumab (Empliciti) added onto the established backbone of carfilzomib (Kyprolis), lenalidomide (Revlimid), and dexamethasone (known as KRd).
“Regardless of what the future holds for elotuzumab-based combinations, it is clear that the new treatment paradigm of newly diagnosed MM will incorporate antibody-based quadruplet regimens,” the editorialists commented.
“Novel immunotherapies are here to stay,” they added, “as they are already transforming the lives of patients with multiple MM and bringing a bright horizon to the treatment landscape.”
Study details
The trial of the novel quadruplet regimen was a multicenter, single-arm, phase 2 study that involved 46 patients with newly diagnosed multiple myeloma, explain first author Benjamin A. Derman, MD, of the University of Chicago Medical Center, and colleagues.
These patients had a median age of 62; more than two-thirds were male (72%) and White (70%). About half (48%) had high-risk cytogenetic abnormalities.
All patients were treated with 12 cycles of the quadruple therapy Elo-KRd regimen. They underwent bone marrow assessment of measurable residual disease (MRD; with 10-5 sensitivity) after cycle 8 and cycle 12.
“An MRD-adapted treatment approach is rational because it may identify which patients can be administered shorter courses of intensive therapy without compromising efficacy,” the authors explained.
Patients who had MRD negativity at both time points did not receive further Elo-KRd, while patients who converted from MRD positivity to negativity in between cycles 8 and 12 received 6 additional cycles of Elo-KRd. Those who remained MRD positive or converted to positivity after 12 cycles received an additional 12 cycles of Elo-KRd.
Following Elo-KRd treatment, all patients transitioned to triple therapy with Elo-Rd (with no carfilzomib), for indefinite maintenance therapy or until disease progression.
For the primary endpoint, the rate of stringent complete response and/or MRD-negativity after cycle 8 was 58% (26 of 45), meeting the predefined definition of efficacy.
Importantly, 26% of patients converted from MRD positivity after cycle 8 to negativity at a later time point, while 50% of patients reached 1-year sustained MRD negativity.
Overall, the estimated 3-year, progression-free survival was 72%, and the rate was 92% for patients with MRD-negativity at cycle 8. The overall survival rate was 78%.
The most common grade 3 or 4 adverse events were lung and nonpulmonary infections (13% and 11%, respectively), and one patient had a grade 5 MI. Three patients discontinued the treatment because of intolerance.
“An MRD-adapted design using elotuzumab and weekly KRd without autologous stem cell transplantation showed a high rate of stringent complete response (sCR) and/or MRD-negativity and durable responses,” the authors wrote.
“This approach provides support for further evaluation of MRD-guided de-escalation of therapy to decrease treatment exposure while sustaining deep responses.”
To better assess the difference of the therapy versus treatment including stem cell transplantation, a phase 3, randomized trial is currently underway to compare the Elo-KRd regimen against KRd with autologous stem cell transplant in newly diagnosed MM.
“If Elo-KRd proves superior, a randomized comparison of Elo versus anti-CD38 mAb-based quadruplets would help determine the optimal combination of therapies in the frontline setting,” the authors noted.
Randomized trial anticipated to clarify benefit
In their editorial, Dr. Kazandjian and Dr. Landgren agreed with the authors that the role of elotuzumab needs to be better clarified in a randomized trial setting.
Elotuzumab received FDA approval in 2015 based on results from the ELOQUENT-2 study, which showed improved progression-free survival and overall survival with the addition of elotuzumab to lenalidomide and dexamethasone in patients with multiple myeloma who have previously received one to three other therapies.
However, the editorialists pointed out that recently published results from the randomized ELOQUENT-1 trial of lenalidomide and dexamethasone with and without elotuzumab showed the addition of elotuzumab was not associated with a statistically significant difference in progression-free survival.
The editorialists also pointed out that, in the setting of newly diagnosed multiple myeloma, another recent, similarly designed study found that the backbone regimen of carfilzomib, lenalidomide, and dexamethasone – on its own – was also associated with a favorable MRD-negative rate of 62%.
In addition, several studies involving novel quadruple treatments with the monoclonal antibody daratumumab (Darzalex) instead of elotuzumab, have also shown benefit in newly diagnosed multiple myeloma, resulting in high rates of MRD negativity.
Collectively, the findings bode well for the quadruple regimens in the treatment of MM, the editorialists emphasized.
“Importantly, with the rate of deep remissions observed with antibody-based quadruplet therapies, one may question the role of using early high-dose melphalan and autologous stem cell transplant in every patient, especially in those who have achieved MRD negativity with the quadruplet alone,” they added.
The study was sponsored in part by Amgen, Bristol-Myers Squibb, and the Multiple Myeloma Research Consortium. Dr. Derman reported advisory board fees from Sanofi, Janssen, and COTA Healthcare; honoraria from PleXus Communications and MJH Life Sciences. Dr. Kazandjian declares receiving advisory board or consulting fees from Bristol-Myers Squibb, Sanofi, and Arcellx outside the submitted work. Dr. Landgren has received grant support from numerous organizations and pharmaceutical companies. Dr. Landgren has also received honoraria for scientific talks/participated in advisory boards for Adaptive Biotech, Amgen, Binding Site, Bristol-Myers Squibb, Celgene, Cellectis, Glenmark, Janssen, Juno, and Pfizer, and served on independent data monitoring committees for international randomized trials by Takeda, Merck, Janssen, and Theradex.
A version of this article first appeared on Medscape.com.
“It is not a big leap of faith to imagine that, in the near future, with the availability of quadruplets and T-cell therapies, the role of high-dose melphalan and autologous stem cell transplant will be diminished,” said Dickran Kazandjian, MD, and Ola Landgren, MD, PhD, of the myeloma division, Sylvester Comprehensive Cancer Center, University of Miami.
They commented in a editorial in JAMA Oncology, prompted by a paper describing new results with a novel quadruple combination of therapies. These treatments included the monoclonal antibody elotuzumab (Empliciti) added onto the established backbone of carfilzomib (Kyprolis), lenalidomide (Revlimid), and dexamethasone (known as KRd).
“Regardless of what the future holds for elotuzumab-based combinations, it is clear that the new treatment paradigm of newly diagnosed MM will incorporate antibody-based quadruplet regimens,” the editorialists commented.
“Novel immunotherapies are here to stay,” they added, “as they are already transforming the lives of patients with multiple MM and bringing a bright horizon to the treatment landscape.”
Study details
The trial of the novel quadruplet regimen was a multicenter, single-arm, phase 2 study that involved 46 patients with newly diagnosed multiple myeloma, explain first author Benjamin A. Derman, MD, of the University of Chicago Medical Center, and colleagues.
These patients had a median age of 62; more than two-thirds were male (72%) and White (70%). About half (48%) had high-risk cytogenetic abnormalities.
All patients were treated with 12 cycles of the quadruple therapy Elo-KRd regimen. They underwent bone marrow assessment of measurable residual disease (MRD; with 10-5 sensitivity) after cycle 8 and cycle 12.
“An MRD-adapted treatment approach is rational because it may identify which patients can be administered shorter courses of intensive therapy without compromising efficacy,” the authors explained.
Patients who had MRD negativity at both time points did not receive further Elo-KRd, while patients who converted from MRD positivity to negativity in between cycles 8 and 12 received 6 additional cycles of Elo-KRd. Those who remained MRD positive or converted to positivity after 12 cycles received an additional 12 cycles of Elo-KRd.
Following Elo-KRd treatment, all patients transitioned to triple therapy with Elo-Rd (with no carfilzomib), for indefinite maintenance therapy or until disease progression.
For the primary endpoint, the rate of stringent complete response and/or MRD-negativity after cycle 8 was 58% (26 of 45), meeting the predefined definition of efficacy.
Importantly, 26% of patients converted from MRD positivity after cycle 8 to negativity at a later time point, while 50% of patients reached 1-year sustained MRD negativity.
Overall, the estimated 3-year, progression-free survival was 72%, and the rate was 92% for patients with MRD-negativity at cycle 8. The overall survival rate was 78%.
The most common grade 3 or 4 adverse events were lung and nonpulmonary infections (13% and 11%, respectively), and one patient had a grade 5 MI. Three patients discontinued the treatment because of intolerance.
“An MRD-adapted design using elotuzumab and weekly KRd without autologous stem cell transplantation showed a high rate of stringent complete response (sCR) and/or MRD-negativity and durable responses,” the authors wrote.
“This approach provides support for further evaluation of MRD-guided de-escalation of therapy to decrease treatment exposure while sustaining deep responses.”
To better assess the difference of the therapy versus treatment including stem cell transplantation, a phase 3, randomized trial is currently underway to compare the Elo-KRd regimen against KRd with autologous stem cell transplant in newly diagnosed MM.
“If Elo-KRd proves superior, a randomized comparison of Elo versus anti-CD38 mAb-based quadruplets would help determine the optimal combination of therapies in the frontline setting,” the authors noted.
Randomized trial anticipated to clarify benefit
In their editorial, Dr. Kazandjian and Dr. Landgren agreed with the authors that the role of elotuzumab needs to be better clarified in a randomized trial setting.
Elotuzumab received FDA approval in 2015 based on results from the ELOQUENT-2 study, which showed improved progression-free survival and overall survival with the addition of elotuzumab to lenalidomide and dexamethasone in patients with multiple myeloma who have previously received one to three other therapies.
However, the editorialists pointed out that recently published results from the randomized ELOQUENT-1 trial of lenalidomide and dexamethasone with and without elotuzumab showed the addition of elotuzumab was not associated with a statistically significant difference in progression-free survival.
The editorialists also pointed out that, in the setting of newly diagnosed multiple myeloma, another recent, similarly designed study found that the backbone regimen of carfilzomib, lenalidomide, and dexamethasone – on its own – was also associated with a favorable MRD-negative rate of 62%.
In addition, several studies involving novel quadruple treatments with the monoclonal antibody daratumumab (Darzalex) instead of elotuzumab, have also shown benefit in newly diagnosed multiple myeloma, resulting in high rates of MRD negativity.
Collectively, the findings bode well for the quadruple regimens in the treatment of MM, the editorialists emphasized.
“Importantly, with the rate of deep remissions observed with antibody-based quadruplet therapies, one may question the role of using early high-dose melphalan and autologous stem cell transplant in every patient, especially in those who have achieved MRD negativity with the quadruplet alone,” they added.
The study was sponsored in part by Amgen, Bristol-Myers Squibb, and the Multiple Myeloma Research Consortium. Dr. Derman reported advisory board fees from Sanofi, Janssen, and COTA Healthcare; honoraria from PleXus Communications and MJH Life Sciences. Dr. Kazandjian declares receiving advisory board or consulting fees from Bristol-Myers Squibb, Sanofi, and Arcellx outside the submitted work. Dr. Landgren has received grant support from numerous organizations and pharmaceutical companies. Dr. Landgren has also received honoraria for scientific talks/participated in advisory boards for Adaptive Biotech, Amgen, Binding Site, Bristol-Myers Squibb, Celgene, Cellectis, Glenmark, Janssen, Juno, and Pfizer, and served on independent data monitoring committees for international randomized trials by Takeda, Merck, Janssen, and Theradex.
A version of this article first appeared on Medscape.com.
“It is not a big leap of faith to imagine that, in the near future, with the availability of quadruplets and T-cell therapies, the role of high-dose melphalan and autologous stem cell transplant will be diminished,” said Dickran Kazandjian, MD, and Ola Landgren, MD, PhD, of the myeloma division, Sylvester Comprehensive Cancer Center, University of Miami.
They commented in a editorial in JAMA Oncology, prompted by a paper describing new results with a novel quadruple combination of therapies. These treatments included the monoclonal antibody elotuzumab (Empliciti) added onto the established backbone of carfilzomib (Kyprolis), lenalidomide (Revlimid), and dexamethasone (known as KRd).
“Regardless of what the future holds for elotuzumab-based combinations, it is clear that the new treatment paradigm of newly diagnosed MM will incorporate antibody-based quadruplet regimens,” the editorialists commented.
“Novel immunotherapies are here to stay,” they added, “as they are already transforming the lives of patients with multiple MM and bringing a bright horizon to the treatment landscape.”
Study details
The trial of the novel quadruplet regimen was a multicenter, single-arm, phase 2 study that involved 46 patients with newly diagnosed multiple myeloma, explain first author Benjamin A. Derman, MD, of the University of Chicago Medical Center, and colleagues.
These patients had a median age of 62; more than two-thirds were male (72%) and White (70%). About half (48%) had high-risk cytogenetic abnormalities.
All patients were treated with 12 cycles of the quadruple therapy Elo-KRd regimen. They underwent bone marrow assessment of measurable residual disease (MRD; with 10-5 sensitivity) after cycle 8 and cycle 12.
“An MRD-adapted treatment approach is rational because it may identify which patients can be administered shorter courses of intensive therapy without compromising efficacy,” the authors explained.
Patients who had MRD negativity at both time points did not receive further Elo-KRd, while patients who converted from MRD positivity to negativity in between cycles 8 and 12 received 6 additional cycles of Elo-KRd. Those who remained MRD positive or converted to positivity after 12 cycles received an additional 12 cycles of Elo-KRd.
Following Elo-KRd treatment, all patients transitioned to triple therapy with Elo-Rd (with no carfilzomib), for indefinite maintenance therapy or until disease progression.
For the primary endpoint, the rate of stringent complete response and/or MRD-negativity after cycle 8 was 58% (26 of 45), meeting the predefined definition of efficacy.
Importantly, 26% of patients converted from MRD positivity after cycle 8 to negativity at a later time point, while 50% of patients reached 1-year sustained MRD negativity.
Overall, the estimated 3-year, progression-free survival was 72%, and the rate was 92% for patients with MRD-negativity at cycle 8. The overall survival rate was 78%.
The most common grade 3 or 4 adverse events were lung and nonpulmonary infections (13% and 11%, respectively), and one patient had a grade 5 MI. Three patients discontinued the treatment because of intolerance.
“An MRD-adapted design using elotuzumab and weekly KRd without autologous stem cell transplantation showed a high rate of stringent complete response (sCR) and/or MRD-negativity and durable responses,” the authors wrote.
“This approach provides support for further evaluation of MRD-guided de-escalation of therapy to decrease treatment exposure while sustaining deep responses.”
To better assess the difference of the therapy versus treatment including stem cell transplantation, a phase 3, randomized trial is currently underway to compare the Elo-KRd regimen against KRd with autologous stem cell transplant in newly diagnosed MM.
“If Elo-KRd proves superior, a randomized comparison of Elo versus anti-CD38 mAb-based quadruplets would help determine the optimal combination of therapies in the frontline setting,” the authors noted.
Randomized trial anticipated to clarify benefit
In their editorial, Dr. Kazandjian and Dr. Landgren agreed with the authors that the role of elotuzumab needs to be better clarified in a randomized trial setting.
Elotuzumab received FDA approval in 2015 based on results from the ELOQUENT-2 study, which showed improved progression-free survival and overall survival with the addition of elotuzumab to lenalidomide and dexamethasone in patients with multiple myeloma who have previously received one to three other therapies.
However, the editorialists pointed out that recently published results from the randomized ELOQUENT-1 trial of lenalidomide and dexamethasone with and without elotuzumab showed the addition of elotuzumab was not associated with a statistically significant difference in progression-free survival.
The editorialists also pointed out that, in the setting of newly diagnosed multiple myeloma, another recent, similarly designed study found that the backbone regimen of carfilzomib, lenalidomide, and dexamethasone – on its own – was also associated with a favorable MRD-negative rate of 62%.
In addition, several studies involving novel quadruple treatments with the monoclonal antibody daratumumab (Darzalex) instead of elotuzumab, have also shown benefit in newly diagnosed multiple myeloma, resulting in high rates of MRD negativity.
Collectively, the findings bode well for the quadruple regimens in the treatment of MM, the editorialists emphasized.
“Importantly, with the rate of deep remissions observed with antibody-based quadruplet therapies, one may question the role of using early high-dose melphalan and autologous stem cell transplant in every patient, especially in those who have achieved MRD negativity with the quadruplet alone,” they added.
The study was sponsored in part by Amgen, Bristol-Myers Squibb, and the Multiple Myeloma Research Consortium. Dr. Derman reported advisory board fees from Sanofi, Janssen, and COTA Healthcare; honoraria from PleXus Communications and MJH Life Sciences. Dr. Kazandjian declares receiving advisory board or consulting fees from Bristol-Myers Squibb, Sanofi, and Arcellx outside the submitted work. Dr. Landgren has received grant support from numerous organizations and pharmaceutical companies. Dr. Landgren has also received honoraria for scientific talks/participated in advisory boards for Adaptive Biotech, Amgen, Binding Site, Bristol-Myers Squibb, Celgene, Cellectis, Glenmark, Janssen, Juno, and Pfizer, and served on independent data monitoring committees for international randomized trials by Takeda, Merck, Janssen, and Theradex.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Evusheld for COVID-19: Lifesaving and free, but still few takers
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Pig heart transplants and the ethical challenges that lie ahead
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”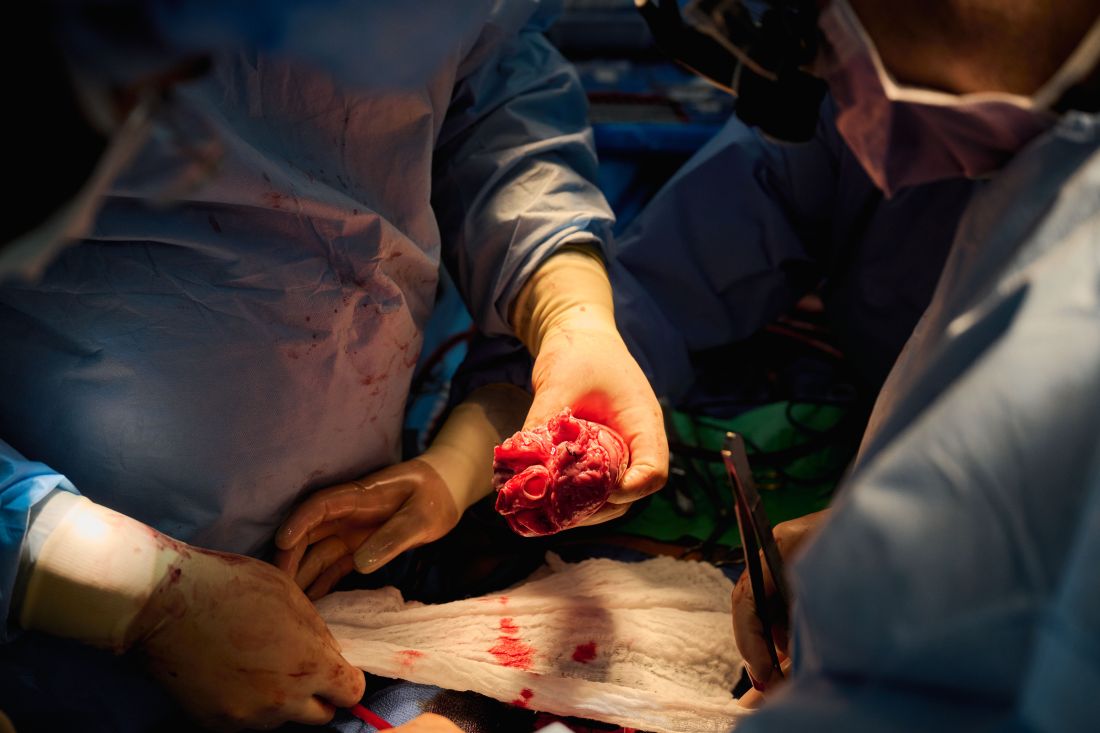
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
Transplanted pig hearts functioned normally in deceased persons on ventilator support
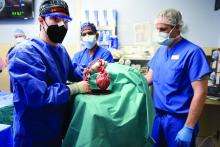
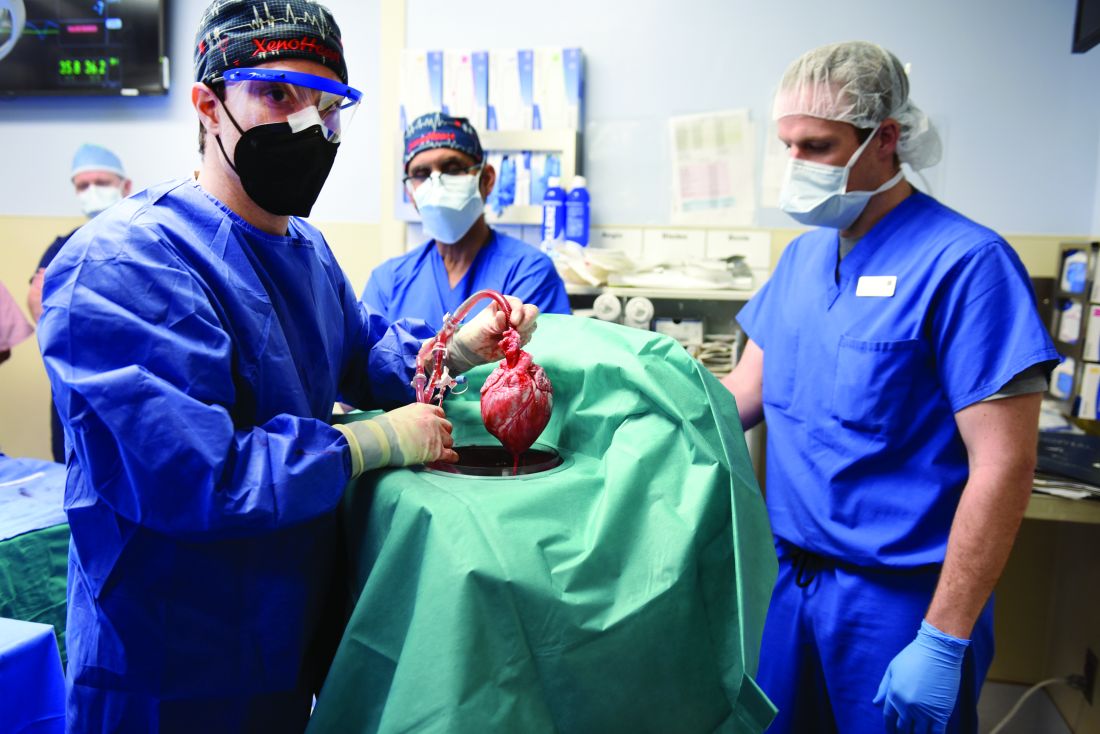
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
Pig-heart transplant case published with new details, insights
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
Novel gene therapy offers hope for some lymphomas
Not all patients with relapsed or refractory large B cell lymphoma (r/r LBCL) are candidates for high-dose chemotherapy or hematopoietic stem cell transplantation (HSCT), and options for second-line therapies for this population are limited, said Dr. Alison Sehgal of the University of Pittsburgh Medical Center in her presentation of the findings.
Lisocabtagene maraleucel (liso-cel) is a CD19-directed CAR T-cell product. In a previous phase 3 randomized trial (the TRANSFORM study), lisocabtagene showed superiority over salvage chemotherapy for LBCL patients who were fit candidates for stem cell transplant, but its use in older, frail patients who are not transplant candidates remains uncertain, wrote Dr. Sehgal and colleagues in their poster at the meeting.
In the study, the researchers identified 74 patients with r/r LBCL. Of these, 61 were treated with liso-cel. The patients ranged in age from 53 to 84 years, with a median age of 74 years, 61% were male, and 89% were white. Approximately half were refractory and half were relapsed.
For the therapy, patients underwent lymphodepletion with cyclophosphamide and fludarabine, followed 2-7 days later by an infusion of liso-cel at a target dose of 100 x 106 CAR+ T cells; all patients had at least 6 months of follow-up from their first response.
The primary endpoint of overall response rate occurred in 80% of the patients, and clinically meaningful complete response occurred in 54% over a median follow-up of 12.3 months.
“Clinically meaningful CRs were observed across all subgroups,” Dr. Sehgal said in her presentation.
The response lasted a median of 21.7 months, and the median follow-up for duration of response was 15.5 months. The median overall survival was not reached, but the median progression-free survival was 9.0 months, with a median follow-up period of 13.0 months.
Responses occurred across all prespecified subgroups, with no significant differences in either safety or efficacy based on hematopoietic cell transplantation–specific comorbidity index (HCT-CI) scores.
“Despite the advanced age and comorbidities of the population, the safety profile was consistent with previous reports,” and no new or increased safety signals appeared, Dr. Sehgal said.
The most common treatment-emergent adverse events of grade 3 or higher were neutropenia (48%), leukopenia (21%), thrombocytopenia (20%), and anemia (11%). Cytokine-release syndrome (CRS) occurred in 23 patients (38%); of these, 1 patient was grade 3 and none were grades 4 or 5.
Approximately one-third of the patients (31%) experienced neurological events during the study; three cases were grade 3, none were grades 4 or 5. Patients with CRS or NE were treated with tocilizumab (10%), corticosteroids (3%), or both (20%). Treatment-emergent adverse events of grade 3 or higher occurred in 79% of patients overall, including grade 5 events in two patients because of COVID-19.
The study findings were limited by the small sample size and lack of controls. However, the results support the potential use of liso-cel as a second-line therapy for r/r LBCL patients who are not candidates for HSCT, Dr. Sehgal concluded.
Addressing an ongoing unmet need
In an interview, study coauthor Dr. Leo I. Gordon of Northwestern University, Chicago, observed, “Patients with relapsed or refractory large B-cell lymphoma who are not considered candidates for stem cell transplant following first-line treatment, based on age, comorbidities, health status, or other prognostic factors, have more difficult-to-treat disease, poor prognosis, and more limited treatment options.”
Dr. Gordon noted that the PILOT study is the only trial to evaluate a CAR T-cell therapy as a second-line treatment for r/r LBCL patients who are not considered candidates for stem cell transplant.
“Data from the primary analysis of the PILOT study further demonstrate the potential value of using CAR T-cell therapies earlier in the treatment paradigm for relapsed or refractory LBCL to help improve clinical outcomes and address ongoing unmet need,” he said.
CAR T-cell therapies have shown benefits in later lines for r/r LBCL and as a second-line treatment for r/r LBCL patients who are deemed candidates for stem cell transplant, “so we were encouraged and not surprised by these data.”
However, Dr. Gordon noted, “There may be some patients with similar presentations that might have a transplant, so one limitation of the trial is how one defines patients where transplant is the intended therapy, and that assessment varies among institutions and clinicians.”
An application for liso-cel as a treatment for patients with r/r LBCL who have failed front-line therapy is currently under Priority Review with the FDA, with a Prescription Drug User Fee Act (PDUFA) goal date of June 24, 2022, he added.
Liso-cel may fill treatment gap as second-line therapy
The current study is important because “the long-term outcomes of patients with relapsed or refractory large B-cell lymphoma who are not candidates for stem cell transplantation is very poor,” said Dr. Brian Till of Fred Hutchinson Cancer Research Center, Seattle, in an interview.
“CAR T therapy leads to about a 40% cure rate, but is currently only available in this population after the failure of second-line therapy,” said Dr. Till, who was not involved in the study.
“Given that liso-cel was shown to improve outcomes in the second-line setting among transplant candidates, it is logical to consider it as second-line therapy in nontransplant candidates as well, who are otherwise fit enough to receive CAR T therapy,” Dr. Till explained.
“This study showed a rate of long-term progression-free survival similar to what has been observed in the third-line setting and was reasonably well tolerated in these older patients,” said Dr. Till. The results suggest “that second-line liso-cel may be an attractive treatment strategy for patients who are not candidates for stem cell transplantation due to advanced age or comorbidities,” he noted.
Dr. Till had no relevant financial conflicts to disclose.
The study was funded by Bristol Myers Squibb.
Not all patients with relapsed or refractory large B cell lymphoma (r/r LBCL) are candidates for high-dose chemotherapy or hematopoietic stem cell transplantation (HSCT), and options for second-line therapies for this population are limited, said Dr. Alison Sehgal of the University of Pittsburgh Medical Center in her presentation of the findings.
Lisocabtagene maraleucel (liso-cel) is a CD19-directed CAR T-cell product. In a previous phase 3 randomized trial (the TRANSFORM study), lisocabtagene showed superiority over salvage chemotherapy for LBCL patients who were fit candidates for stem cell transplant, but its use in older, frail patients who are not transplant candidates remains uncertain, wrote Dr. Sehgal and colleagues in their poster at the meeting.
In the study, the researchers identified 74 patients with r/r LBCL. Of these, 61 were treated with liso-cel. The patients ranged in age from 53 to 84 years, with a median age of 74 years, 61% were male, and 89% were white. Approximately half were refractory and half were relapsed.
For the therapy, patients underwent lymphodepletion with cyclophosphamide and fludarabine, followed 2-7 days later by an infusion of liso-cel at a target dose of 100 x 106 CAR+ T cells; all patients had at least 6 months of follow-up from their first response.
The primary endpoint of overall response rate occurred in 80% of the patients, and clinically meaningful complete response occurred in 54% over a median follow-up of 12.3 months.
“Clinically meaningful CRs were observed across all subgroups,” Dr. Sehgal said in her presentation.
The response lasted a median of 21.7 months, and the median follow-up for duration of response was 15.5 months. The median overall survival was not reached, but the median progression-free survival was 9.0 months, with a median follow-up period of 13.0 months.
Responses occurred across all prespecified subgroups, with no significant differences in either safety or efficacy based on hematopoietic cell transplantation–specific comorbidity index (HCT-CI) scores.
“Despite the advanced age and comorbidities of the population, the safety profile was consistent with previous reports,” and no new or increased safety signals appeared, Dr. Sehgal said.
The most common treatment-emergent adverse events of grade 3 or higher were neutropenia (48%), leukopenia (21%), thrombocytopenia (20%), and anemia (11%). Cytokine-release syndrome (CRS) occurred in 23 patients (38%); of these, 1 patient was grade 3 and none were grades 4 or 5.
Approximately one-third of the patients (31%) experienced neurological events during the study; three cases were grade 3, none were grades 4 or 5. Patients with CRS or NE were treated with tocilizumab (10%), corticosteroids (3%), or both (20%). Treatment-emergent adverse events of grade 3 or higher occurred in 79% of patients overall, including grade 5 events in two patients because of COVID-19.
The study findings were limited by the small sample size and lack of controls. However, the results support the potential use of liso-cel as a second-line therapy for r/r LBCL patients who are not candidates for HSCT, Dr. Sehgal concluded.
Addressing an ongoing unmet need
In an interview, study coauthor Dr. Leo I. Gordon of Northwestern University, Chicago, observed, “Patients with relapsed or refractory large B-cell lymphoma who are not considered candidates for stem cell transplant following first-line treatment, based on age, comorbidities, health status, or other prognostic factors, have more difficult-to-treat disease, poor prognosis, and more limited treatment options.”
Dr. Gordon noted that the PILOT study is the only trial to evaluate a CAR T-cell therapy as a second-line treatment for r/r LBCL patients who are not considered candidates for stem cell transplant.
“Data from the primary analysis of the PILOT study further demonstrate the potential value of using CAR T-cell therapies earlier in the treatment paradigm for relapsed or refractory LBCL to help improve clinical outcomes and address ongoing unmet need,” he said.
CAR T-cell therapies have shown benefits in later lines for r/r LBCL and as a second-line treatment for r/r LBCL patients who are deemed candidates for stem cell transplant, “so we were encouraged and not surprised by these data.”
However, Dr. Gordon noted, “There may be some patients with similar presentations that might have a transplant, so one limitation of the trial is how one defines patients where transplant is the intended therapy, and that assessment varies among institutions and clinicians.”
An application for liso-cel as a treatment for patients with r/r LBCL who have failed front-line therapy is currently under Priority Review with the FDA, with a Prescription Drug User Fee Act (PDUFA) goal date of June 24, 2022, he added.
Liso-cel may fill treatment gap as second-line therapy
The current study is important because “the long-term outcomes of patients with relapsed or refractory large B-cell lymphoma who are not candidates for stem cell transplantation is very poor,” said Dr. Brian Till of Fred Hutchinson Cancer Research Center, Seattle, in an interview.
“CAR T therapy leads to about a 40% cure rate, but is currently only available in this population after the failure of second-line therapy,” said Dr. Till, who was not involved in the study.
“Given that liso-cel was shown to improve outcomes in the second-line setting among transplant candidates, it is logical to consider it as second-line therapy in nontransplant candidates as well, who are otherwise fit enough to receive CAR T therapy,” Dr. Till explained.
“This study showed a rate of long-term progression-free survival similar to what has been observed in the third-line setting and was reasonably well tolerated in these older patients,” said Dr. Till. The results suggest “that second-line liso-cel may be an attractive treatment strategy for patients who are not candidates for stem cell transplantation due to advanced age or comorbidities,” he noted.
Dr. Till had no relevant financial conflicts to disclose.
The study was funded by Bristol Myers Squibb.
Not all patients with relapsed or refractory large B cell lymphoma (r/r LBCL) are candidates for high-dose chemotherapy or hematopoietic stem cell transplantation (HSCT), and options for second-line therapies for this population are limited, said Dr. Alison Sehgal of the University of Pittsburgh Medical Center in her presentation of the findings.
Lisocabtagene maraleucel (liso-cel) is a CD19-directed CAR T-cell product. In a previous phase 3 randomized trial (the TRANSFORM study), lisocabtagene showed superiority over salvage chemotherapy for LBCL patients who were fit candidates for stem cell transplant, but its use in older, frail patients who are not transplant candidates remains uncertain, wrote Dr. Sehgal and colleagues in their poster at the meeting.
In the study, the researchers identified 74 patients with r/r LBCL. Of these, 61 were treated with liso-cel. The patients ranged in age from 53 to 84 years, with a median age of 74 years, 61% were male, and 89% were white. Approximately half were refractory and half were relapsed.
For the therapy, patients underwent lymphodepletion with cyclophosphamide and fludarabine, followed 2-7 days later by an infusion of liso-cel at a target dose of 100 x 106 CAR+ T cells; all patients had at least 6 months of follow-up from their first response.
The primary endpoint of overall response rate occurred in 80% of the patients, and clinically meaningful complete response occurred in 54% over a median follow-up of 12.3 months.
“Clinically meaningful CRs were observed across all subgroups,” Dr. Sehgal said in her presentation.
The response lasted a median of 21.7 months, and the median follow-up for duration of response was 15.5 months. The median overall survival was not reached, but the median progression-free survival was 9.0 months, with a median follow-up period of 13.0 months.
Responses occurred across all prespecified subgroups, with no significant differences in either safety or efficacy based on hematopoietic cell transplantation–specific comorbidity index (HCT-CI) scores.
“Despite the advanced age and comorbidities of the population, the safety profile was consistent with previous reports,” and no new or increased safety signals appeared, Dr. Sehgal said.
The most common treatment-emergent adverse events of grade 3 or higher were neutropenia (48%), leukopenia (21%), thrombocytopenia (20%), and anemia (11%). Cytokine-release syndrome (CRS) occurred in 23 patients (38%); of these, 1 patient was grade 3 and none were grades 4 or 5.
Approximately one-third of the patients (31%) experienced neurological events during the study; three cases were grade 3, none were grades 4 or 5. Patients with CRS or NE were treated with tocilizumab (10%), corticosteroids (3%), or both (20%). Treatment-emergent adverse events of grade 3 or higher occurred in 79% of patients overall, including grade 5 events in two patients because of COVID-19.
The study findings were limited by the small sample size and lack of controls. However, the results support the potential use of liso-cel as a second-line therapy for r/r LBCL patients who are not candidates for HSCT, Dr. Sehgal concluded.
Addressing an ongoing unmet need
In an interview, study coauthor Dr. Leo I. Gordon of Northwestern University, Chicago, observed, “Patients with relapsed or refractory large B-cell lymphoma who are not considered candidates for stem cell transplant following first-line treatment, based on age, comorbidities, health status, or other prognostic factors, have more difficult-to-treat disease, poor prognosis, and more limited treatment options.”
Dr. Gordon noted that the PILOT study is the only trial to evaluate a CAR T-cell therapy as a second-line treatment for r/r LBCL patients who are not considered candidates for stem cell transplant.
“Data from the primary analysis of the PILOT study further demonstrate the potential value of using CAR T-cell therapies earlier in the treatment paradigm for relapsed or refractory LBCL to help improve clinical outcomes and address ongoing unmet need,” he said.
CAR T-cell therapies have shown benefits in later lines for r/r LBCL and as a second-line treatment for r/r LBCL patients who are deemed candidates for stem cell transplant, “so we were encouraged and not surprised by these data.”
However, Dr. Gordon noted, “There may be some patients with similar presentations that might have a transplant, so one limitation of the trial is how one defines patients where transplant is the intended therapy, and that assessment varies among institutions and clinicians.”
An application for liso-cel as a treatment for patients with r/r LBCL who have failed front-line therapy is currently under Priority Review with the FDA, with a Prescription Drug User Fee Act (PDUFA) goal date of June 24, 2022, he added.
Liso-cel may fill treatment gap as second-line therapy
The current study is important because “the long-term outcomes of patients with relapsed or refractory large B-cell lymphoma who are not candidates for stem cell transplantation is very poor,” said Dr. Brian Till of Fred Hutchinson Cancer Research Center, Seattle, in an interview.
“CAR T therapy leads to about a 40% cure rate, but is currently only available in this population after the failure of second-line therapy,” said Dr. Till, who was not involved in the study.
“Given that liso-cel was shown to improve outcomes in the second-line setting among transplant candidates, it is logical to consider it as second-line therapy in nontransplant candidates as well, who are otherwise fit enough to receive CAR T therapy,” Dr. Till explained.
“This study showed a rate of long-term progression-free survival similar to what has been observed in the third-line setting and was reasonably well tolerated in these older patients,” said Dr. Till. The results suggest “that second-line liso-cel may be an attractive treatment strategy for patients who are not candidates for stem cell transplantation due to advanced age or comorbidities,” he noted.
Dr. Till had no relevant financial conflicts to disclose.
The study was funded by Bristol Myers Squibb.
FROM ASCO 2022
Stem cell transplants could be ‘transformational’ in type 1 diabetes
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Two patients with type 1 diabetes have now experienced improved blood glucose control with Vertex Pharmaceutical’s investigational allogeneic stem cell–derived islets (VX-880), with the first person now completely insulin independent at 9 months post transplant.
Prior to the procedure, both patients had hypoglycemic unawareness and had experienced multiple episodes of severe hypoglycemia, conditions considered severe enough to justify the risk of immune suppression (which is required for such stem cell–derived islet transplants as they are “foreign” to the recipient).
The first patient, a 64-year-old man with type 1 diabetes for more than 40 years, now has a hemoglobin A1c in the normal range without taking any insulin more than 9 months after the procedure. The second, a 35-year-old woman with type 1 diabetes for 10.7 years, experienced a 30% reduction in insulin use and significant increased time spent in target glucose range, by 5 months post transplant. Both patients were given just half the targeted VX-880 dose.
Data for those two patients – the first in Vertex’s phase 1/2 multicenter, single-arm, open-label clinical trial of VX-880 – were reported at the annual scientific sessions of the American Diabetes Association, by James F. Markmann, MD, PhD.
He has been transplanting pancreatic islet cells from deceased donors into humans via infusion into the hepatic portal vein for over 20 years.
Transplantation of pancreatic islet cells obtained from cadavers have been shown to eliminate severe hypoglycemia and improve glycemic control in patients with type 1 diabetes, but they’re limited in quantity and are of variable quality. Islets that are manufactured via differentiation from human pluripotent stem cells represent an alternative, explained Dr. Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, Boston.
“This is a new area. ... We hope this will be the same or potentially better. With stem cell–derived islets the quality, consistency, and reliability might produce a better result than with cadaveric islets,” he commented during a press briefing here.
A third patient has recently received the full targeted VX-880 dose but was not part of the current report. The planned enrollment is 17 patients. The trial is currently on clinical hold per the Food and Drug Administration concerning the criteria around dose escalation, but Vertex is working with the FDA to sort that out. Meanwhile, enrollment remains open in Canada, Dr. Markmann said.
In answer to a question about how patient 1 is doing now, Dr. Markmann replied, “He’s doing great. He’s probably the most appreciative patient I’ve ever met. His life was being destroyed by diabetes. He couldn’t work. He crashed his motorcycle from [low blood sugar]. He really was tremendously appreciative that he could participate.”
When Dr. Markmann explained the potential uncertainties and risks to the patient prior to the procedure, the patient replied: “I want to participate. If I die from this and I help somebody else I’d be happy, but I can’t go on living the way I’m living.”
“These people really suffer and this, I think, brings hope to them,” Dr. Markmann said.
‘Beautiful data’ seen in two patients, with ‘transformational’ potential
Asked to comment, Marlon Pragnell, PhD, vice president for Research & Science at the ADA, told this news organization: “It’s beautiful data. People who have type 1 diabetes lack [pancreatic] beta cells ... it was impossible to get sufficient beta cells from cadaveric transplants. It’s just nowhere near enough. If this is safe and effective, if they continue to show safety and efficacy like patient 1, this will be transformational.”
Dr. Markmann presented data for the most recent study visit for each of the two patients, 270 days for patient 1 and 150 days for patient 2. Prior to the transplants, patient 1 had experienced five severe hypoglycemic events and patient 2 had experienced three.
Both had undetectable C-peptide levels at baseline. In response to a mixed-meal tolerance test, patient 1 showed a “robust” C-peptide response by day 90, which increased by day 180. Those levels had dropped but were still detectable by day 270, “possibly due to improved insulin sensitivity,” Dr. Markmann said.
Similarly, Patient 2 also had increased C-peptide that increased to detectable range by day 90 with improved glucose disposal.
Hemoglobin A1c dropped in patient 1 from 8.6% at baseline to 6.9% at day 180, to a “remarkable” 5.2% at day 270. For patient 2, the drop was from 7.5% to a nadir of 6.4% by day 57, then reversing back to 7.1% at day 150.
Both patients also had significant reductions in insulin dose. For patient 1, the dose reduction was more than 90% – from 34 units at baseline to 2.6 units by day 90. By day 210 he was able to stop insulin and by day 270 he met formal criteria for insulin independence.
Patient 2 also had a significant reduction in insulin dose, from 25.9 units to 18.7 by day 57 and remained stable thereafter, at 18.2 units by day 150.
Asked why Patient 2’s results weren’t quite as impressive as patient 1’s, Dr. Markmann replied “I think what’s important is that both patients did great. And since this was a half-dose, we might have expected that the outcome was going to be more like patient 2 rather than patient 1. So, I think we’re just going to have to [study this in] more patients to understand where it falls.”
Although patient 1 experienced a cluster of six severe hypoglycemic events early in the posttransplant period, he had no further events after day 35. Patient 2 had no severe hypoglycemic events.
Other safety events were generally consistent with that seen with the immunosuppressive regimen in the perioperative period. Patient 1 had a “mild and self-limited” rise in liver function test and also experienced two severe adverse events: A rash from the immune suppression that resolved spontaneously, and dehydration requiring hospitalization on day 186. Patient 2’s adverse events were all mild to moderate, most commonly headache and hypomagnesemia and not related to VX-880.
Immunosuppression: Work is ongoing
The immunosuppression regimen used comprises a depletion of lymphocytes at induction, followed by a maintenance regimen of two standard agents used in kidney transplant patients and found to be well tolerated, Dr. Markmann said.
Still, the risk of immunosuppression generally outweighs the potential benefit for most people with type 1 diabetes who are managing reasonably well with insulin treatment.
“This is part one of a two-part problem. One is to have a reliable, consistent, effective cell therapy. The second is to develop an approach that doesn’t require immunosuppression. ... But if we had a way of transplanting the cells without the need for immunosuppression, then it could be really widely available. That’s an opportunity for the future since these cells can be made in unlimited quantities,” Dr. Markmann commented during the press briefing.
Asked for his thoughts about the immunosuppression aspect, Dr. Pragnell told this news organization: “Immune suppression is a concern, but I feel that this is just the start of so much research in this area. They’re going to take this step by step. This is just the start. My understanding is they have additional strategies around immune suppression, and in the future they might not even need immunosuppression. But even at this stage right now, it’s amazing.”
He added: “The ‘artificial pancreas’ is a huge step forward, but it’s just a bridge to a cure, whereas if they’re able to show safety and efficacy, this is potentially a cure. ... I’m very excited about it.”
Dr. Markmann serves on advisory boards for iTolerance, eGenesis, and QihanBio. He is a consultant to Vertex Pharmaceuticals. Dr. Pragnell is an ADA employee and has no further disclosures.
A version of this article first appeared on Medscape.com.
AT ADA 2022