User login
Incidence and Functional Outcomes of Malunion of Nonoperatively Treated Humeral Shaft Fractures
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
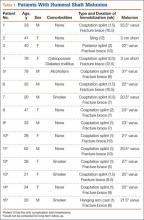
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Total Shoulder Arthroplasty Outcome for Treatment of Osteoarthritis: A Multicenter Study Using a Contemporary Implant
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
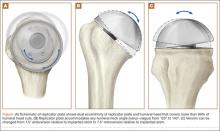
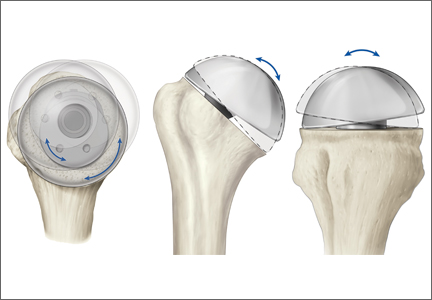
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Tension Pneumothorax After Ultrasound-Guided Interscalene Block and Shoulder Arthroscopy
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Isolated Avulsion of Extensor Carpi Radialis Longus and Brachioradialis Origins: A Case Report and Surgical Repair Technique
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
The literature includes only 2 case reports of bony avulsion fracture of the origin of the brachioradialis1,2 and, up until now, no case reports of isolated avulsion of the extensor carpi radialis longus and brachioradialis origins from the lateral epicondyle and lateral supracondylar ridge. In this article, we report the case of a 31-year-old man who sustained this injury during a fall onto his outstretched right hand, and we present our surgical repair technique. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 31-year-old right hand–dominant garbage truck worker sustained a right elbow injury and presented 2 months later. He described slipping and falling onto his outstretched right hand while doing his work. He could not describe the exact mechanism or action or position of the arm at time of impact but thought he tried to catch himself on the truck during the fall. At time of injury, he had immediate pain and swelling to the lateral aspect of the right elbow and difficulty when he attempted lifting. He denied antecedent elbow symptoms before the injury. After evaluation by an outside occupational medicine physician, he engaged in treatment consisting of activity modification and physical therapy, including range-of-motion (ROM) exercises and iontophoresis. This course of management failed to completely relieve his symptoms, and he was unable to return to work.
The patient presented to our institution 9 weeks after injury with complaints of pain along the lateral aspect of the elbow, painful flexion-extension, and continued swelling. The pain had been unrelieved with anti-inflammatory medications and opioids. Physical examination revealed tenderness and swelling along the lateral epicondyle and extensor mass of the right elbow. The patient had tenderness, marked weakness, and a palpable soft-tissue defect at the origin of the extensor mass with resisted extension of the wrist (Figure 1). Elbow ROM was from 20° to 120° of flexion, 60° of pronation, and 60° of supination. No varus or valgus instability was present about the elbow. Radiographs did not show any fracture or dislocation. Magnetic resonance imaging (MRI) did not definitively show extensor tendon avulsion but did identify signal change of the common extensor tendon (Figures 2A, 2B). Advanced imaging was inconclusive, but, given the patient’s history and physical examination findings, he was diagnosed with an avulsion injury of the origin of the extensor mass of the right elbow.
The patient was brought to the operating room, administered general anesthesia, and placed supine on the operating table with a tourniquet on the upper arm. A lateral 4.5-cm incision was made centered over the lateral epicondyle. The origin of the extensor mass was exposed, and isolated avulsions of the extensor carpi radialis longus and the brachioradialis were identified (Figures 3, 4). Underlying the avulsed sleeve of tissue, the origin of the extensor carpi radialis brevis was found intact. The lateral supracondylar ridge and the lateral epicondyle of the humerus were débrided, and 3 transosseous holes were drilled (using a 2.3-mm bit) through the lateral epicondyle. Four Mason-Allen sutures were placed into the tendon of the common extensor origin using No. 2 braided polyester suture (Ethibond Excel, Ethicon) (Figure 5). The tendon was reduced down to the native footprint, and the sutures were passed through the drill holes and tied down securely (Figure 6). The skin was then closed using layered 4-0 absorbable monofilament suture (Monocryl, Ethicon). The patient was placed in a posterior mold plaster splint with 90° of elbow flexion and with the wrist in 30° of extension.
On postoperative day 3, the patient was seen for a wound check and was placed in a long-arm fiberglass cast (90° of elbow flexion, forearm in neutral, 25° of wrist extension) for immobilization. One week after surgery, he was transitioned to a removable thermoplastic splint, and physical therapy for ROM was initiated. He was allowed therapist-guided active extension of the elbow and flexion of the wrist but was restricted to passive flexion of the elbow and extension of the wrist. Seven weeks after surgery, passive ROM about the elbow was measured, and he was found to have 120° of flexion, 0° extension, 80° pronation, and 80° supination. At 12 weeks, the physical therapy regimen was advanced to include muscle strengthening and active wrist extension and elbow flexion. At 16 weeks, the wrist extensors demonstrated 5/5 strength (Medical Research Council grading system), and the patient was cleared for full activity and weight-bearing without restriction. He returned to work pain-free and without restrictions 18 weeks after surgery. At 2-year follow-up, he had a Mayo performance elbow score of 100 and an Oxford elbow score of 48.3,4 He had full active ROM, full strength, and no subjective pain and was back doing heavy lifting at his job.
Discussion
The brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis originate from the anterolateral aspect of the lateral column of the distal humeral metaphysis and form the dorsal mobile wad. The origin of the brachioradialis is about 7 cm in length and begins about 10 to 11 cm above the elbow.5 The origin and insertions of the mobile wad, specifically the brachioradialis, provide a tremendous mechanical advantage with respect to elbow flexion against resistance, particularly with the forearm in the pronated and semipronated positions.6 With the elbow in 30° of flexion, a force 3 times the body weight can be encountered during strenuous lifting.6,7 We hypothesized these large forces likely led to this injury pattern in the patient we have described.
The literature includes 2 case reports of avulsion fracture of the brachioradialis muscle from its origin on the lateral supracondylar humeral ridge.1,2 To our knowledge, however, there have been no reports of pure avulsion. In our patient’s case, there was no bony fracture, but rather avulsion of the extensor carpi radialis longus and brachioradialis at their origin, with the underlying fibers of the extensor carpi radialis brevis remaining in continuity. Because of the rarity of this injury pattern, there was a significant delay in diagnosis. On initial presentation, the differential diagnosis for lateral elbow pain and tenderness included occult fracture, intracapsular plica, osteochondritis dissecans lesion, radial tunnel syndrome, lateral or posterolateral instability, and lateral epicondylitis. Given the absence of antecedent elbow symptoms before the injury, the dynamic soft-tissue asymmetry of the mobile wad with wrist extension, and the palpable soft-tissue defect, we thought the presentation was inconsistent with a simple inflammatory or overuse syndrome, such as lateral epicondylitis. In addition, the physical examination findings were inconsistent with radial tunnel syndrome or disruption of the lateral collateral ligament complex. Elbow MRI did not show an occult fracture, plica, or osteochondritis dissecans lesion but did reveal joint effusion and signal change in the common extensor tendon origin. Interestingly, MRI did not definitively show a tear of the mobile wad. This may be explained by the fact that the fibers of the underlying extensor carpi radialis brevis remained intact. Also potentially involved are the static nature of MRI and potentially suboptimal sequencing and axis of acquisition resulting from the relative infrequency of imaging this joint at certain health care institutions. Our case demonstrates the limitations of MRI in this setting and highlights the need for a detailed history and thorough physical examination for diagnosis.
Funk and colleagues8 used electromyography (EMG) to study the activity of the elbow musculature in uninjured subjects. EMG data were obtained with the elbow joint subjected to resisted flexion, extension, abduction, and adduction. During resisted elbow flexion, there was an increasing amount of activity in the extensor carpi radialis with larger angles of elbow flexion. In addition, the brachioradialis demonstrated the most muscle activity of any of the elbow flexors with 90° or more of elbow flexion and forearm pronation, as opposed to other positions in which the brachialis was the primary flexor. For this reason, we hypothesized that our patient’s forearm was pronated and his elbow flexed to 90° or more when he braced for impact. The ensuing injury resulted from a violent eccentric contraction that caused extensive rupture of the lateral elbow musculature from its broad origin. With the forearm in supination or neutral position, we would have expected a possible injury to the distal biceps as opposed to the brachioradialis and extensor carpi radialis.
In our patient, this injury caused much functional disability, especially with elbow flexion and wrist extension. We hypothesized that, for the muscles to function properly, anatomical restoration would have to be achieved at their known footprint to maintain their mechanical advantage. Therefore, surgical intervention was indicated in our patient, an active laborer. Given the absence of an osseous fracture fragment in this injury pattern, healing must occur at the bone–tendon interface. As tendinous healing is more tenuous and protracted than osseous healing, we preferred transosseous repair. We believed that better tendon-to-bone healing would be possible with drilled osseous tunnels rather than with suture anchors. New studies describing alternative successful methods of treatment would add to our limited body of knowledge regarding this rare injury.
Conclusion
This is the first report of avulsion of the extensor carpi radialis longus and brachioradialis from their origins. Given the biomechanics and anatomy of the dorsal mobile wad, we posit that our patient’s injury occurred when he fell onto his outstretched hand secondary to overwhelming eccentric muscle contracture at time of impact. This injury caused significant upper extremity dysfunction, and surgical intervention was required.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
1. Guettler JH, Mayo DB. Avulsion fracture of the origin of the brachioradialis muscle. Am J Orthop. 2001;30(9):693-694.
2. Marchant MH Jr, Gambardella RA, Podesta L. Superficial radial nerve injury after avulsion fracture of the brachioradialis muscle origin in a professional lacrosse player: a case report. J Shoulder Elbow Surg. 2009;18(6):e9-e12.
3. Dawson J, Doll H, Boller I, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br. 2008;90(4):466-473.
4. Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology. 2004;43(11):1434-1440.
5. Freehafer AA, Peckham PH, Keith MW, Mendelson LS. The brachioradialis: anatomy, properties, and value for tendon transfer in the tetraplegic. J Hand Surg Am. 1988;13(1):99-104.
6. Morrey BF, Sanchez-Sotelo J. The Elbow and Its Disorders. 4th ed. Philadelphia, PA: Saunders/Elsevier; 2009.
7. Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1993;66(3):214-220.
8. Funk DA, An KN, Morrey BF, Daube JR. Electromyographic analysis of muscles across the elbow joint. J Orthop Res. 1987;5(4):529-538.
Avascular Necrosis of Trochlea After Supracondylar Humerus Fractures in Children
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
The Supination-Pronation Test for Distal Biceps Tendon Rupture
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Tommy John Surgeries On the Rise In Youth Athletes
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Commentary to "Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty"
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
In this month’s issue of The American Journal of Orthopedics, Tannenbaum and colleagues present a “5 Points” article on “Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty.” This is an excellent article that summarizes the authors’ methodology of determining not only the overall cost of hospital care for shoulder replacement but a detailed analysis of many components contributing to that cost.
The steps are fairly straightforward: identify the various components of the cost, gather the data contributing to those costs, and then analyze what are the major expenditures that contribute to the overall cost. Sounds simple, but, in practice, it is anything but!
As health care expenditures in the United States continue to increase and approach 20% of the gross domestic product, every sector of the health care industry is searching for ways to curtail and eventually decrease the cost of health care. However, one cannot control costs without accurate data that defines those costs. In this article, Tannenbaum and colleagues have provided a methodology to help both hospital administrators and surgeons determine the overall cost of shoulder arthroplasty, but their principles of analysis can be applied to all aspects of hospital care.
Such efforts are gaining the attention of many leaders of the health care industry. For example, in the September 8, 2015, edition of The New York Times, I was very interested to read the article “What are a Hospital’s Costs? Utah System Is Trying to Learn.”1 The article reviewed the efforts of Dr. Vivian Lee, chief executive at University of Utah Health Care, to determine the actual cost of all care provided by the university hospital, the same goal as the present 5 Points article on shoulder arthroplasty but on a vastly greater scale. Analyzing those costs guided Dr. Lee and her colleagues to alter clinical programs, which led to a decrease of 30% in hospital expenditures and fewer complications.1
We are all indebted to Mr. Tannenbaum and his coauthors for providing the journal’s readers with a clear map that we can use to both understand and navigate the current maze of hospital costs. Using such a guide, we will be able to gather information that not only saves money, but will improve care by directing resources to services that actually benefit our patients.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.
Reference
1. Kolata G. What are a hospital’s costs? Utah system is trying to learn. New York Times. September 8, 2015:A1. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html. Accessed September 17, 2015.
Imaging Evaluation of Superior Labral Anteroposterior (SLAP) Tears
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
Superior labral anteroposterior (SLAP) tears are common labral injuries. They occur at the attachment of the long head of the biceps tendon on the superior glenoid and extend anterior and/or posterior to the biceps anchor. The mechanism of action for SLAP tears is traction on the superior labrum by the long head of the biceps tendon, resulting in “peeling” of the labrum off the glenoid. Such forces may result from repetitive overhead arm motion (pitching) or from direct trauma.
Clinical diagnosis is challenging with SLAP tears, as they often present with nonspecific shoulder pain and may not be associated with an acute injury. A further complication is that they are often associated with other shoulder pathology, such as rotator cuff tears.1 As physical examination is typically nonspecific, proper diagnostic imaging is essential for diagnosis.
We prefer to assess potential SLAP tears with magnetic resonance arthrography (MRA).2 Dilute (1:200) gadolinium contrast material (12-15 mL) is introduced into the glenohumeral joint under sonographic or fluoroscopic guidance. Capsular distention by dilute intra-articular contrast enables superior imaging resolution of the labroligamentous complex. We think the increase in diagnostic confidence enabled by direct arthrography outweighs the additional invasiveness and cost associated with MRA relative to noncontrast magnetic resonance imaging (MRI).
The MRA protocol differs from our routine noncontrast shoulder imaging. We perform a fat-saturated coronal oblique T1 sequence that maximizes the conspicuity of intra-articular contrast in the plane that optimally visualizes the superior labrum. Three planes of intermediate-weighted fast spin echo not only contrast the high-signal intra-articular fluid with the low-signal fibrocartilaginous labrum and the stratified intermediate signal of glenoid articular cartilage, but they also allow optimal assessment of the rotator cuff. In addition, we perform a fat-saturated coronal T2 sequence that highlights all fluid signal structures as well as edema.
SLAP tears appear on MRA as the insinuation of intra-articular contrast between the articular cartilage and the attachment of the superior labrum,3 within the substance of the labrum, or as detachment of the labrum from the glenoid rim4 (Figure 1). Findings can range from labral fraying to complete detachment with displacement. Tears can extend into other quadrants of the labrum, extend from a Bankart lesion, or involve the biceps tendon and/or the glenohumeral ligaments (Figures 2–4). Up to 10 types of SLAP tears have been described on arthroscopy. This classification scheme, however, is seldom helpful in the interpretation of SLAP tears on MRI. More important in guiding treatment is having a detailed description of the tear, including location, extent, and morphology, along with associated abnormalities.
Several findings can aid in the diagnosis of SLAP tears. Normal anatomical variants of the anterior-superior labrum do not extend posterior to the biceps anchor—an important finding for discerning normal morphologic variants from tears. Therefore, high signal within the posterior third of the superior labrum or extension of high signal laterally within the labrum and away from the glenoid suggests a SLAP tear.5 A paralabral cyst is almost always associated with a labral tear,1 so signal abnormality of the superior labrum with a paralabral cyst suggests a SLAP tear (Figure 5).
MRA is not the only method for diagnosing SLAP tears. Standard 3-Tesla MRI had 83% sensitivity and 99% specificity for diagnosing SLAP tears in a recent study, though MRA had 98% sensitivity and 99% specificity—a statistically significant sensitivity difference.6 In another study, computed tomography arthrography (CTA) had 95% sensitivity and 88% specificity for diagnosing recurrent SLAP tears after surgery.7 CTA is associated with ionizing radiation and is limited in its assessment of other structures that may show concomitant abnormalities, such as the articular cartilage and the rotator cuff. Indirect MRA, wherein magnetic resonance sequences are obtained after intravenous injection of gadolinium contrast and exercise of the affected shoulder, had a high sensitivity of detection of labral tears of all types.8
MRA is most sensitive and specific for diagnosing SLAP tears; 3-Tesla MRI, indirect MRA, and CTA are useful alternative modalities for cases in which MRA cannot be performed.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.
1. Chang D, Mohana-Borges A, Borso M, Chung CB. SLAP lesions: anatomy, clinical presentation, MR imaging diagnosis and characterization. Eur J Radiol. 2008;68(1):72-87.
2. Jee WH, McCauley TR, Katz LD, Matheny JM, Ruwe PA, Daigneault JP. Superior labral anterior posterior (SLAP) lesions of the glenoid labrum: reliability and accuracy of MR arthrography for diagnosis. Radiology. 2001;218(1):127-132.
3. Fitzpatrick D, Walz DM. Shoulder MR imaging normal variants and imaging artifacts. Magn Reson Imaging Clin N Am. 2010;18(4):615-632.
4. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000;214(1):267-271.
5. Tuite MJ, Cirillo RL, De Smet AA, Orwin JF. Superior labrum anterior-posterior (SLAP) tears: evaluation of three MR signs on T2-weighted images. Radiology. 2000;215(3):841-845.
6. Magee T. 3-T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192(1):86-92.
7. De Filippo M, Araoz PA, Pogliacomi F, et al. Recurrent superior labral anterior-to-posterior tears after surgery: detection and grading with CT arthrography. Radiology. 2009;252(3):781-788.
8. Fallahi F, Green N, Gadde S, Jeavons L, Armstrong P, Jonker L. Indirect magnetic resonance arthrography of the shoulder; a reliable diagnostic tool for investigation of suspected labral pathology. Skeletal Radiol. 2013;42(9):1225-1233.