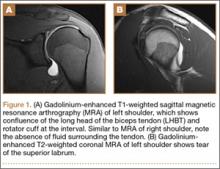
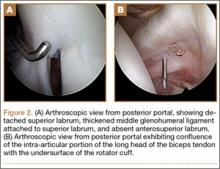
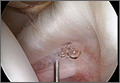
User login
Measurement of Resource Utilization for Total and Reverse Shoulder Arthroplasty
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
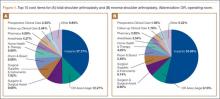
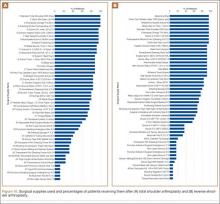
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
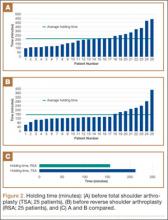
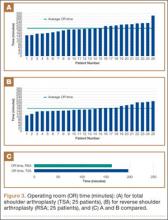
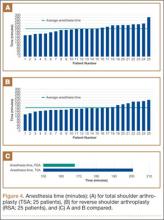
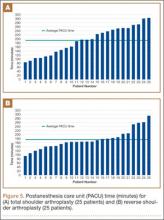
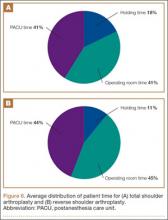
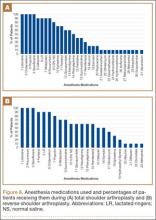
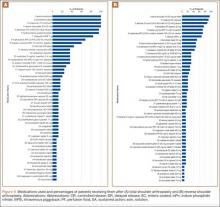
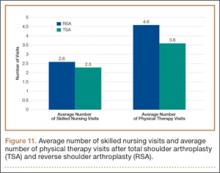
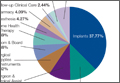
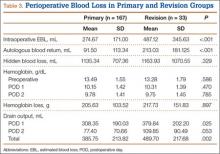
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
As total health care costs reach almost $3 trillion per year—capturing more than 17% of the total US gross domestic product—payers are searching for more effective ways to limit health care spending.1,2 One increasingly discussed plan is payment bundling.3 This one-lump-sum payment model arose as a result of rapid year-on-year increases in total reimbursements under the current, fee-for-service model. The Centers for Medicare & Medicaid Services hypothesized that a single all-inclusive payment for a procedure or set of services would incentivize improvements in patient-centered care and disincentivize cost-shifting behaviors.4 Bundled reimbursement is becoming increasingly common in orthopedic practice. With the recent introduction of the Bundled Payment for Care Improvement Initiative, several orthopedic practices around the United States are already actively engaged in creating models for bundled payment for common elective procedures and for associated services provided up to 90 days after surgery.3,5
Bundled payment increases the burden on the provider to understand the cost of care provided during a care cycle. However, not only has the current system blinded physicians to the cost of care, but current antitrust legislation has made discussions of pricing with colleagues (so-called price collusion) illegal and subject to fines of up to $1 million per person and $100 million per organization,6 therefore limiting orthopedic physician involvement.
Given these legal constraints, instead of measuring direct costs of goods, we developed a “grocery list” approach in which direct comparisons are made of resources (goods and services) used and delivered during the entire 90-day cycle of care for patients who undergo anatomical total shoulder arthroplasty (TSA) or reverse shoulder arthroplasty (RSA). We used one surgeon’s practice experience as a model for measuring other orthopedic surgeons’ resource utilization, based on their electronic medical records (EMR) system data. By capturing the costs of the components of resource utilization rather than just the final cost of care, we can assess, compare, understand, endorse, and address these driving factors.
1. The significance of resource utilization
To maximize the efficiency of their practices, high-volume shoulder surgeons have introduced standardization to health care delivery.7 Identifying specific efficiencies makes uniform acceptance of beneficial practice patterns possible.
To facilitate comparison of goods and services used during an episode of surgical care, Virani and colleagues8,9 studied the costs of TSA and RSA and calculated the top 10 driving cost factors for these procedures (Figure 1). Their systematic analysis provided a framework for a common method of communication, allowing an orthopedic surgeon to gain a more complete understanding of the resources used during a particular operative procedure in his or her practice, and allowing several physicians to compare and contrast the resources collectively used for a single procedure, facilitating an understanding of different practice patterns within a local community. At a societal level, these data can be collected to help guide overall recommendations.
2. How we defined utilization
To define the resources used, we had to decide which procedure components cost the most. Virani and colleagues8,9 found that the top 10 cost drivers accounted for 93.11% and 94.77% of the total cost of the TSA and RSA care cycles, respectively (Figure 1). For each cost driver, information on resources used (goods, services, overhead) was collected on 2 forms, the Hospital Utilization Form (7 hospital-based items) and the Clinical Utilization Form (3 non-hospital-based items). To make hospital data easy to compile, we piloted use of a “smart form” in the EpicCare EMR system to isolate and auto-populate specific data fields.
3. EMR data collection
With EMR becoming mandatory for all public and private health care providers starting in 2014, utilization data are now included in a single unified system. Working with our in-house information technology department, we developed an algorithm to populate this information in a separate, easy-to-follow hospital utilization form. This form can be adopted by other institutions. Although EpicCare EMR is used by 52% of hospitals and at our institution, the data points required to make the same measurements are generalizable and exist in other EMRs.
Smartlinks, a tool in this EMR, allows utilization data to be quickly retrieved from different locations in a medical record and allows a form to be electronically completed in seconds. Data can be retrieved for any patient in the EMR system, regardless of when that patient’s hospital stay occurred. We populated data from surgeries performed 2 years before the start of this project.
4. What we can learn from these data
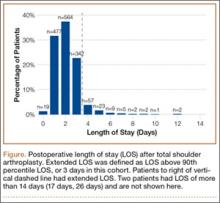
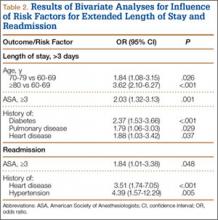
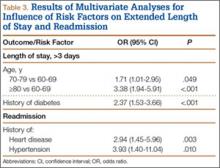
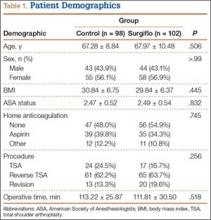
Data from a pilot study of 25 patients who underwent primary anatomical TSA for osteoarthritis and 25 patients who underwent primary RSA for massive rotator cuff tear allowed us to generate graphical representations of a single surgeon’s practice patterns that most affected the cost of care. Time in holding, time in the operating room, time in the postanesthesia care unit, and percentage of patients receiving different medications were recorded for each procedure (Figures 2–11). The study did not capture the wide variances in practice patterns in shoulder arthroplasty, and therefore other surgeons’ resource utilization may differ from ours. However, replicating this methodology at other institutions will produce a more robust data set from which conclusions about resource utilization and, indirectly, cost of care can be made.
5. Future possibilities
By using existing EMR tools to better understand resource utilization, orthopedic surgeons can play a constructive role in the dialogue on health care costs and new reimbursement models. The data presented here are not meant to be interpreted as hard and fast numbers on global resource utilization, but instead we intend to establish a model for collecting data on resource utilization. Resource utilization begins the dialogue that allows orthopedic surgeons and specialty societies to speak a common language without discussing actual cost numbers, which is discouraged under antitrust regulation. The data presented will allow comparisons to be made between surgeons in all practice settings to highlight areas of inconsistency in order to further improve patient care. Although this work involved only 50 patients undergoing only 2 types of surgeries, the resource-capturing methodology can be expanded to include more procedures and orthopedic practices. As all hospitals are now required to have EMRs, the metrics tracked in this work can be found on any patient medical record and auto-populated using our open-source utilization forms. Starting this data collection at your hospital may require no more than a conversation with the informatics department, as the metrics can for the most part be populated into a database on surgeon request.
As orthopedic surgeons return to the economic health care discussion, this information could prove essential in helping the individual surgeon and the orthopedic community justify the cost of care as well as fully understand the cost drivers for musculoskeletal care.
Click here to read the commentary on this article by Peter D. McCann, MD
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
1. National health expenditures 2013 highlights. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/highlights.pdf. Accessed September 14, 2015.
2. Wilson KB. Health care costs 101: slow growth persists. California HealthCare Foundation website. http://www.chcf.org/publications/2014/07/health-care-costs-101. Published July 2014. Accessed August 24, 2015.
3. Froimson MI, Rana A, White RE Jr, et al. Bundled Payments for Care Improvement Initiative: the next evolution of payment formulations: AAHKS Bundled Payment Task Force. J Arthroplasty. 2013;28(8 suppl):157-165.
4. Morley M, Bogasky S, Gage B, Flood S, Ingber MJ. Medicare post-acute care episodes and payment bundling. Medicare Medicaid Res Rev. 2014;4(1).
5. Teusink MJ, Virani NA, Polikandriotis JA, Frankle MA. Cost analysis in shoulder arthroplasty surgery. Adv Orthop. 2012;2012:692869.
6. Fassbender E, Pandya S. Legislation focuses on AAOS priorities. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/news/aaosnow/may14/advocacy2.asp. AAOS Now. Published May 2014. Accessed August 24, 2015.
7. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
8. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of reverse shoulder arthroplasty for the surgical treatment of advanced rotator cuff deficiency at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1612-1622.
9. Virani NA, Williams CD, Clark R, Polikandriotis J, Downes KL, Frankle MA. Preparing for the bundled-payment initiative: the cost and clinical outcomes of total shoulder arthroplasty for the surgical treatment of glenohumeral arthritis at an average 4-year follow-up. J Shoulder Elbow Surg. 2013;22(12):1601-1611.
Technique of Open Reduction and Internal Fixation of Comminuted Proximal Humerus Fractures With Allograft Femoral Head Metaphyseal Reconstruction
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
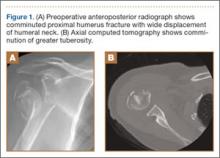
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
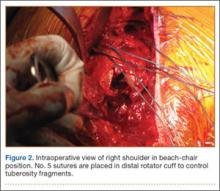
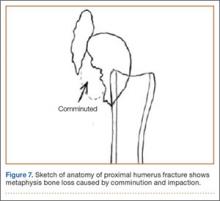
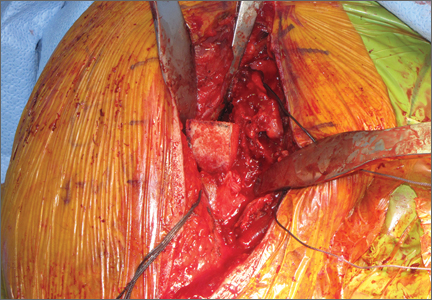
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
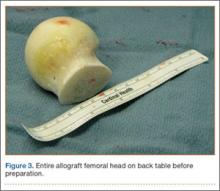
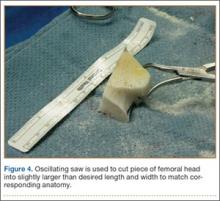
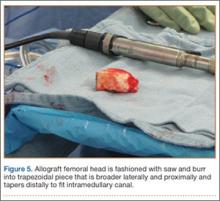
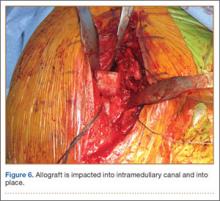
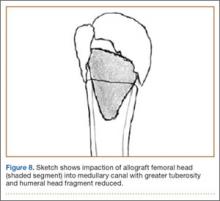
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
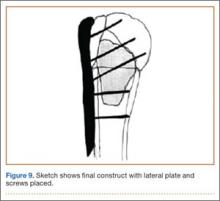
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
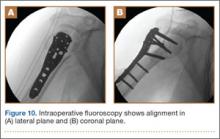
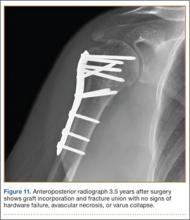
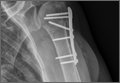
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
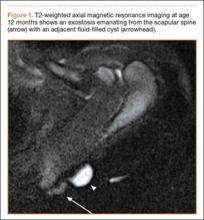
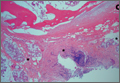
Osteochondroma With Contiguous Bronchogenic Cyst of the Scapula
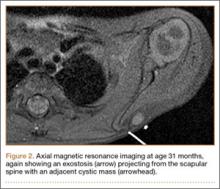
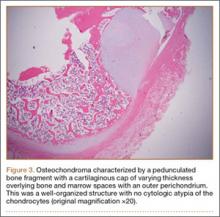
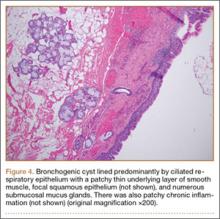
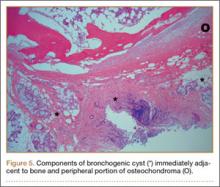
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
Midterm Follow-Up of Metal-Backed Glenoid Components in Anatomical Total Shoulder Arthroplasties
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
Factors Affecting Perceptions of Open, Mini-Open, and Arthroscopic Rotator Cuff Repair Techniques Among Medical Professionals
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Bilateral Superior Labrum Anterior to Posterior (SLAP) Tears With Abnormal Anatomy of Biceps Tendon
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
Length of Stay and Readmission After Total Shoulder Arthroplasty: An Analysis of 1505 Cases
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
Use of total shoulder arthroplasty (TSA) and reverse TSA for shoulder conditions has increased dramatically in recent years.1 Approximately 27,000 standard TSAs were performed in the United States in 2008, and this number is expected to double by 2015.2 TSA provides excellent pain relief, restoration of function, and patient satisfaction.3 The evolution of implant design over the past 25 years has contributed to excellent long-term implant survival, with rates comparable to those of total knee and hip arthroplasty.4 Similarly, compared with previous designs, contemporary designs and techniques have resulted in fewer complications.5
Several studies have investigated the long-term complications of TSA. These complications include prosthetic loosening, instability, periprosthetic fracture, rotator cuff tears, nerve injury, and deltoid dysfunction.6-11 In addition, Waterman and colleagues11 very recently assessed the influence of risk factors on short-term postoperative complications of TSA. However, none of these studies has assessed the influence of multiple risk factors on postoperative length of stay (LOS) after TSA. Only 1 study, using data from 2005 and earlier, has analyzed the potential effect of multiple patient characteristics on readmission after TSA12; other studies have been only descriptive.13-16
We conducted a retrospective cohort study to characterize the risk factors for extended LOS and readmission after TSA in a large sample of patients drawn from a national database. We hypothesized that patient factors, including age, sex, and obesity, would be significantly associated with postoperative LOS and readmission after TSA. National databases have been increasingly used in orthopedic research, as they offer particular advantages. Large sample sizes allow for powerful analyses of associations—analyses previously not possible in single-surgeon and single-institution studies. In addition, use of a large, national patient sample allows us to draw generalizable conclusions to better define patients’ and physicians’ postoperative expectations.
Methods
We conducted a retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP collects 150 patient variables from 374 participating US hospitals.17 Patients are prospectively identified, and information is collected from operative reports, medical records, and patient interviews by trained clinical reviewers.17,18 Routine auditing by the program ensures high-quality data, with reported interrater disagreement below 2% for all variables. Data are collected through the 30th postoperative day, including after discharge.
This study was granted an exemption from our institutional review board, as we used a deidentified and publicly available database. Patients who were 60 years or older and underwent TSA between 2011 and 2012 were identified in the ACS-NSQIP database. TSA patients were identified using Current Procedural Terminology (CPT) code 23472, which includes TSA and reverse TSA procedures.
Patients were divided into groups based on surgical indications, which were available as International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Patients with postoperative ICD-9 codes 714.0 (rheumatoid arthritis), 715.0-9 (osteoarthritis), 716.61/716.81/716.91 (unspecified arthropathy), 718.01 (articular cartilage disorder), 718.31 (recurrent dislocation of shoulder), 718.81 (other joint derangement of shoulder), 719.41/719.91 (unspecified shoulder pain/disorder), 726.0-2 (disorder of shoulder tendons and bursa), 727.61 (rotator cuff rupture), and 840.3-9 (rotator cuff sprain) were classified as having a nonfracture indication. Patients with postoperative ICD-9 codes 716.11 (traumatic arthropathy), 833.80-89 (malunion/nonunion of fracture), and 812.00-20 (fracture of proximal humerus) were classified as having a fracture-associated indication. Patients with incomplete perioperative data were excluded from the study, leaving 1505 patients for the study (out of an initial 1726).
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Information about medical comorbidities was also collected from the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) class 3 or higher indicates severe systemic disease. Steroid use was defined as regular administration of corticosteroid medications within 30 days before surgery. Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery, with the patient’s best functional status during this period recorded. Similar to how other variables were collected from the database, this information was obtained through medical record abstraction and patient interviews by trained personnel. ADLs are defined in the ACS-NSQIP as “activities usually performed in the course of a normal day in a person’s life” and include bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs, a partially dependent patient requires assistance for some ADLs, and a totally dependent patient requires assistance in completing all ADLs. Partially and totally dependent patients were grouped for analysis. Information about a patient’s discharge destination (to home or a facility) was also available in the database.17
Extended Length of Stay
Extended LOS was defined as a binary variable that was positive when the postoperative LOS exceeded the 90th percentile LOS. The 90th percentile LOS was chosen as a cutoff to account for normal variations in LOS and differing practices of surgeons while still capturing patients with abnormally extended LOS.
Readmission
Readmission was defined as a binary variable that was positive when a patient had an unplanned readmission 1 or more times after the initial postoperative discharge.
Patient Demographics
Table 1 summarizes the demographics and comorbidities of the 1505 TSA patients who met our study inclusion criteria. Mean age was 72.8 years (range, 60-90 years). Mean BMI was 30.3 kg/m2 (range, 15.7-63.9 kg/m2); 46.7% of patients were classified as obese (BMI, ≥30 kg/m2). The cohort was 58.9% female. Four percent of patients underwent TSA for a fracture-associated indication.
Statistical Analyses
Statistical analyses were performed with Stata 11.2 (StataCorp). Bivariate and multivariate analyses were used to test patient characteristics for association with extended LOS and readmission. Discharge destination and LOS were included in the readmission analysis because this information would be available at time of discharge and would be useful to include in a model that predicts odds of readmission.
Final multivariate models were constructed using a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .20 remained. Variables with .05 < P < .20 were left in the model to control for potential confounding but were not considered significantly associated with the outcome. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
Results
Median LOS after TSA was 2 days (interquartile range, 1-3 days), and extended LOS was defined as LOS of more than 3 days (90th percentile LOS). The distribution of LOS is depicted in the Figure. Results of the bivariate and multivariate analyses are reported in Table 2 and Table 3, respectively. Bivariate analysis revealed an association between extended LOS and increased age, ASA class 3 or higher, and history of diabetes, pulmonary disease, and heart disease. On multivariate analysis, extended LOS was associated with age 70 to 79 years (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.01-2.95; P = .049), age 80 years or older (OR, 3.38; 95% CI, 1.94-5.91; P < .001), and history of diabetes (OR, 2.37; 95% CI, 1.53-3.66; P < .001).
Forty-nine patients (3.3%) were readmitted within the first 30 postoperative days. Bivariate analysis revealed an association between readmission and ASA class 3 or higher, history of heart disease, and history of hypertension. On multivariate analysis, readmission was associated only with history of heart disease (OR, 2.94; 95% CI, 1.45-5.96; P = .003) and history of hypertension (OR, 3.93; 95% CI, 1.40-11.04; P = .010).
Discussion
In the United States, TSA has become increasingly popular because of its favorable outcomes and continued implant development.1-5 However, there is a shortage of information about risk factors for short-term outcomes after TSA. In this study, we used multivariate analyses to identify patient-related factors associated with extended LOS and readmission after discharge. By identifying these factors, we can improve the preoperative discussion and postoperative planning for this procedure.
In the present study, extended LOS (>3 days) was found to be associated with older age and history of diabetes. The TSA literature has little information that can be used to compare these results, though age over 80 years was previously described as a risk factor for extended LOS after TSA.19 Uncontrolled diabetes has been identified as a risk factor for extended LOS in hip and knee arthroplasty,20 and management of diabetes may similarly complicate postoperative care, leading to extended LOS and increased costs in TSA patients. Patients with the identified risk factors for extended LOS should be counseled before surgery. In addition, this is important information for health care organizations and providers.
Readmission within 30 days after TSA was found to be independently associated with history of heart disease and history of hypertension. Similar to factors affecting LOS, patient-related risk factors for readmission are also poorly defined in the TSA literature. In total hip arthroplasty patients, heart disease has been found to be associated with readmission.21,22 Hypertension has also been associated with readmission for other orthopedic procedures.23 Results of the present study indicate these comorbidities may increase the risk for complications after discharge. It is important to note, however, that LOS did not correlate with readmission rates, indicating patients are likely being discharged at the most clinically appropriate time.
Waterman and colleagues11 very recently identified (in the ACS-NSQIP database) a patient population that underwent TSA between 2006 and 2011 to describe risk factors for postoperative complications within 30 days. They found that comorbid cardiac disease and older age were independently associated with mortality. Interestingly, the present study identified older age as associated with extended LOS, and cardiac disease as associated with readmission. Together with the results from the previous study, age and cardiac disease seem to be important patient factors to consider when planning TSA, as they are associated with a significantly worse postoperative course.
This study had several limitations. First, given the nature of the ACS-NSQIP database, readmissions are recorded only up to 30 days after surgery, including after discharge. Second, though the ACS-NSQIP tries to collect as many patient variables as possible, some information is not captured. Additional variables that could potentially affect LOS and readmission (eg, insurance status, hospital volume) were not available for analysis. However, we think the high-quality data collection process used by the ACS-NSQIP outweighs the lack of certain variables. Third, original operative notes are not available in the ACS-NSQIP database, and the only way to identify operative procedures is to check CPT codes. Unfortunately, CPT code 23472 is used for both TSA and reverse TSA, so these procedures could not be separated for analysis, and the results of this study can be used to comment only on the risks of both procedures. Another limitation is that there were not enough patients to further analyze the data by each indication.
Conclusion
With the increasing popularity of TSA for an expanding set of indications, it is important to understand the factors that can affect the postoperative course. In this study, we found several patient-related risk factors for extended LOS and readmission. Although the identified factors are generally not modifiable, this information can be used to better define the expectations of patients, providers, and organizations for this increasingly common procedure.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
3. Adams JE, Sperling JW, Hoskin TL, Melton LJ 3rd, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976–2000: a population-based study. J Shoulder Elbow Surg. 2006;15(1):50-55.
4. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;(455):183-189.
5. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
8. Sneppen O, Fruensgaard S, Johannsen HV, Olsen BS, Søjbjerg JO, Andersen NH. Total shoulder replacement in rheumatoid arthritis: proximal migration and loosening. J Shoulder Elbow Surg. 1996;5(1):47-52.
9. Søjbjerg JO, Frich LH, Johannsen HV, Sneppen O. Late results of total shoulder replacement in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;(366):39-45.
10. Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am. 2014;96(3):198-205.
11. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
12. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
13. Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Gay DM, Lyman S, Do H, Hotchkiss RN, Marx RG, Daluiski A. Indications and reoperation rates for total elbow arthroplasty: an analysis of trends in New York state. J Bone Joint Surg Am. 2012;94(2):110-117.
16. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
17. American College of Surgeons. User Guide for the 2012 ACS NSQIP Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug12.ashx. Published October 2013. Accessed June 21, 2015.
18. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
19. Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR Jr. Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469(4):1042-1049.
20. Marchant MH Jr, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
21. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
22. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
23. Lovecchio F, Hsu WK, Smith TR, Cybulski G, Kim B, Kim JY. Predictors of thirty-day readmission after anterior cervical fusion. Spine. 2014;39(2):127-133.
Use of a Topical Thrombin-Based Hemostatic Agent in Shoulder Arthroplasty
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.
Using Plate Osteosynthesis to Treat Isolated Greater Tuberosity Fractures
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Commentary to "The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going"
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.