User login
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
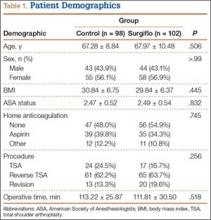
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
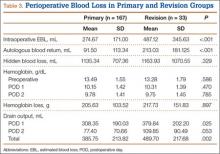
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
Shoulder arthroplasty can be associated with significant perioperative blood loss, with the overall rate of postoperative allogeneic blood transfusion ranging from 7.4% to 43%.1-6 Blood transfusions are associated with a range of health risks.7 Soft-tissue dissection and cutting and reaming of bone surfaces can be sources of significant blood loss. Directly visualized sources of bleeding can be addressed using standard surgical hemostasis, including electrocautery, suture ligation, compression, and careful avoidance of vascular structures. However, difficult-to-visualize areas and bony sources of bleeding are more difficult to manage.
Numerous products for mitigating perioperative blood loss are commercially available. Topical hemostatic agents have been used in many surgical specialties, including orthopedic surgery, cardiothoracic surgery, neurosurgery, vascular surgery, and general surgery.8-10 In orthopedic surgery, use of topical thrombin- and fibrin-based products as hemostatic agents has been studied in knee and hip arthroplasty, with varying results.11-14 Early studies have shown reduced blood loss and postoperative transfusion rates with use of a fibrin sealant or fibrin tissue adhesive,11,12,15 whereas others have shown no significant benefit of using these hemostatic agents. Massin and colleagues14 found no difference in blood loss in the setting of total knee arthroplasty (TKA) with use of a fibrin sealant. In a 2012 prospective study, Kim and colleagues13 also showed no significant reduction in blood loss in patients treated with a topical thrombin-based hemostatic agent in TKA.
Surgiflo (Ethicon) is a hemostatic matrix that is combined with a topical human thrombin solution before sterile application. The matrix consists of an absorbable porcine gelatin powder that provides a structure for platelet adhesion and aggregation.16 When used in combination with thrombin, it aids in fibrin clot formation, leading to hemostasis of oozing blood and minor bleeding from small capillaries and venules. According to the manufacturer’s data, it can halt bleeding in less than 2 minutes and retains its efficacy for up to 8 hours.
To our knowledge, there are no reports of studies on use of topical fibrin- or thrombin-based hemostatic agents in shoulder arthroplasty. We conducted a study to investigate perioperative blood loss, transfusion rates, and complications during the hospital stays of patients who underwent shoulder arthroplasty and were treated with or without the Surgiflo topical hemostatic agent. Our hypothesis was that patients intraoperatively treated with this agent would have significantly less perioperative blood loss and lower transfusion rates without increased rates of in-hospital complications.
Patients and Methods
We retrospectively reviewed data from 211 consecutive shoulder arthroplasties performed by Dr. J. Michael Wiater between December 2012 and August 2013. All primary and revision anatomical and reverse total shoulder arthroplasty (TSA) procedures were included. Patients with a preoperative diagnosis of acute fracture, and patients with a diagnosis of any type of blood diathesis, including anemia and platelet disorders that lead to excessive clotting or bleeding, were excluded. Patients treated between May 2013 and August 2013 had the hemostatic matrix applied to the soft tissues before final wound closure. Chart review for any exclusion criteria left 102 patients in the experimental (hemostatic agent) group and 98 patients in the control group.
For all patients, any anticoagulation or anti-inflammatory medication was discontinued 1 week before the elective arthroplasty. An interscalene regional block combined with general anesthesia was used in all cases. All procedures were performed through a standard anterior deltopectoral approach. Patients in the experimental group had 10 mL of the hemostatic agent topically applied to the soft tissues of the wound before closure. Half the mixture (5 mL) was applied to the deep tissues of the axillary recess, subacromial, and joint spaces, and the other half was applied superficially after closure of the deltopectoral interval. A medium Hemovac (Zimmer) drain was used in all cases, with 1 tubing placed in the deep space and another between the deltoid and the skin, both draining to a single drain evacuator.
After surgery, all patients received deep venous thrombosis (DVT) prophylaxis consisting of 5000 units of subcutaneous unfractionated heparin every 8 hours until discharge, and then aspirin 325 mg twice daily for 2 weeks after discharge unless contraindicated. Any long-term anticoagulation therapy discontinued before surgery was resumed on postoperative day 2 (POD 2). All drains were removed on POD 2 unless they had more than 50 mL of output over an 8-hour period. Complete blood cell counts were collected for all patients before surgery and on PODs 1 and 2. Whether to transfuse blood was based on clinical judgment of severe or symptomatic acute blood loss anemia; however, no strict predetermined criteria were followed.
Patient electronic medical records were reviewed for demographic information, including age, sex, height, weight, comorbidities, American Society of Anesthesiologists (ASA) physical status, and preoperative anticoagulation use. Anesthesia records were reviewed for intraoperative estimated blood loss (EBL) and intraoperative autologous blood return (Cell Saver, Haemonetics). Patient laboratory results were reviewed for preoperative and postoperative hemoglobin (Hb) and hematocrit levels. Electronic medical records were also reviewed for incidence of transfusion and any major or minor complications occurring within 90 days of the procedure. All data were collected and reviewed under the approval of the human investigations committee at our institution.
Hemoglobin loss and hidden blood loss (HBL) were calculated as described by Good and colleagues.17 Total Hb loss was estimated using the total blood volume formula described by Nadler and colleagues.18 Difference between preoperative Hb level and final Hb level recorded during hospital stay was corrected for units of blood transfused (estimate, 52 g of Hb per unit). Hemoglobin loss was then used to calculate total blood loss, and total drain output was added to total blood loss to determine HBL. These formulas were used:
Hbloss = Blood Volume (L) × [Hbinitial (g/L) – Hbfinal (g/L)] + Hbtransfused
Total Blood Loss (mL) = 1000 × Hbloss/Hbinitial
HBL (mL) = Total Blood Loss (mL) + Total Drain Output (mL)
All statistical analyses were performed using SPSS Statistics Version 20 (IBM). A Shapiro-Wilk test was used to test for normality. All variables collected were compared between the experimental and control cohorts. For continuous variables, independent t test was used to compare normal data, and the Mann-Whitney rank sum test was used for non-normal data. Categorical variables were compared with the Fisher exact test for 2×2 tables and with the χ2 test for larger tables. In all tests, P < .05 was considered statistically significant.
Results
The experimental and control cohorts were demographically similar with respect to age, sex, body mass index (BMI), ASA status, and home anticoagulation treatment (Table 1). Patients who received preoperative anticoagulation therapy were evenly distributed between the 2 patient groups (P = .745). Thirty-five patients in the experimental group and 39 in the control group were taking aspirin. In addition, in the experimental group, 5 patients were taking warfarin, 4 clopidogrel, 1 dabigatran, and 1 prasugrel. In the control group, 6 patients were taking warfarin, 3 clopidogrel, 2 dabigatran, and 1 rivaroxaban. Type of arthroplasty (primary anatomical, primary reverse, revision shoulder arthroplasty) was also evenly distributed (P = .256), and operative time did not vary significantly between cohorts (P = .518).
Markers of operative blood loss were also compared between patient groups (Table 2). There was no significant difference in intraoperative EBL or cell saver volume between cohorts (Ps = .301 and .800). Drain output on PODs 1 and 2 did not differ between cohorts (Ps = .789 and .777); the same was true for total postoperative drain output (P = .906). Hemoglobin levels did vary significantly between groups before surgery (P = .002) and on PODs 1 and 2 (Ps = .027 and .005), with the experimental group having a lower mean Hb level at each time point. Mean Hb loss, however, did not vary significantly (P = .253). There was also no difference in HBL between cohorts (P = .601), the calculation of which accounts for patient height and weight, Hb loss, and transfusions. The incidence of transfusion was 25% in the experimental group and 20% in the control group—not a statistically significant difference (P = .407). Mean (SD) number of transfused units of packed red blood cells was 0.54 (1.05) in the experimental group and 0.40 (0.91) in the control group—again, not a statistically significant difference (P = .377).
Preoperative Hb level under 13 g/dL has been reported as a risk factor for transfusion after surgery.19 To account for the significantly lower Hb level in the experimental group, we examined the incidence of transfusion in patients with preoperative Hb levels above and below this cutoff. Among patients with preoperative Hb levels under 13 g/dL, transfusion incidence was 45.8% (experimental group) and 42.9% (control group) (P > .99); among those with preoperative Hb levels above 13 g/dL, transfusion incidence was 7.7% (experimental) and 11.1% (control) (P = .760).
To account for reportedly higher blood loss and transfusion rates in revision cases,1,2,20 we stratified our data by primary and revision cases, comparing them within the entire patient cohort and comparing the experimental and control groups within these subsets. Tables 3 and 4 list the results. Revision cases had more EBL (P < .001), autologous blood return (P < .001), drain output on POD 1 (P = .025), and total drain output (P = .002). There was no significant difference in transfusion rate between primary (22.2%) and revision (27.3%) cases (P = .505) or when the experimental and control groups were compared within primary and revision subsets. Among primary cases, transfusion rates were 23% (experimental) and 21.2% (control) (P = .853); among revision cases, rates were 35% (experimental) and 15% (control) (P = .263). Revisions showed a significant (P = .043) difference in HBL between the experimental and control groups, with more blood loss in the experimental group. EBL and autologous blood return were equivocal. Hb levels and drain outputs were statistically different only for POD 2, but there was no difference between overall Hb loss or total drain outputs. Among primary cases, no parameters of blood loss were statistically significantly different. The significantly lower preoperative and postoperative Hb levels were again seen in the experimental group.
The groups’ complication rates were comparable, and there was no significant risk associated with use of the hemostatic agent (P = .764). In each group, there were no complications that would be of particular concern with use of this agent. These complications included wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). Nine patients (5 control, 4 experimental) had minor medical complications, and 2 (1 control, 1 experimental) had major medical complications. The control group’s 5 minor medical complications were acute kidney infection treated with antibiotics (1 patient), persistent urinary retention requiring Foley catheter for short period after discharge (1), minor upper gastrointestinal bleed treated medically (1), recalcitrant tachycardia in setting of chronic atrial fibrillation (1), and vasovagal syncope with no identified cardiovascular cause or periprosthetic complication (1); the control patient with the major medical complication died 2 weeks after surgery, after discharge to the inpatient rehabilitation unit. This death was secondary to pneumonia, sepsis, and eventual multisystem organ failure. The experimental group’s 4 minor medical complications were urinary retention requiring catheterization for short period (1 patient), urinary tract infections diagnosed 2 weeks after surgery and treated with antibiotics (2), and new-onset atrial fibrillation treated medically (1); the experimental patient with the major medical complication developed Takotsubo cardiomyopathy, a nonischemic stress-induced weakening of the myocardium requiring medical management. An experimental patient also had reverse TSA shoulder dislocation 12 days after surgery—thought to be caused by inadequate soft-tissue tension and unrelated to hemostatic agent use. The patient was returned to the operating room for polyethylene liner exchange and metallic spacer implantation.
Discussion
Reported rates of transfusion after shoulder arthroplasty have ranged from 7.4% to 43%, when including revision and reverse TSAs.2,3 In the present study, the overall transfusion rate was 23% (includes patients who underwent primary or revision shoulder arthroplasties with anatomical or reverse prostheses). Although the risk for complications is low, serious issues may arise with blood transfusions. Allogeneic blood transfusions can cause fluid overload, allergic reactions, fever, acute immune hemolytic reaction, transfusion-related acute lung injury (TRALI), bloodborne infections, and formation of antibodies complicating any future need for transfusions.7 According to the National Heart, Lung, and Blood Institute, the chances of becoming infected from transfusion are 1 in 2 million for the hepatitis C and human immunodeficiency viruses and 1 in 205,000 for the hepatitis B virus.7 Some studies have also found higher rates of infection after hip or knee arthroplasty in patients who received allogeneic blood transfusions.21,22 In addition, for hospitals, transfusion costs are significant. One study showed that direct and indirect overhead costs amounted to $522 to $1183 per red blood cell unit.23 Given the risks and costs associated with blood transfusions, use of an effective intraoperative blood loss management agent could be beneficial in the setting of shoulder arthroplasty.
The use and efficacy of intraoperative blood management agents remain controversial. Numerous agents for managing perioperative blood loss are commercially available. Previous clinical studies have shown variable results with use of topical hemostatic agents, but not in the setting of shoulder arthroplasty.24 In 1999, Levy and colleagues11 showed that use of fibrin tissue adhesive reduced blood loss and postoperative transfusion rates in patients who underwent TKA. In 2001, Wang and colleagues15 showed that using a fibrin sealant in TKA reduced bloody drainage and maintained higher Hb levels. In 2003, the same group showed that use of fibrin sealant also reduced perioperative blood loss in total hip arthroplasty.12 More recent studies have had contradicting results,13,14 similar to ours. A 2012 prospective study failed to show any significant difference in blood loss after TKA in patients treated with a topical thrombin-based hemostatic agent.13 The authors did find significantly higher Hb values in the treated group on PODs 1 and 2, though the drain outputs and transfusion rates did not differ.
To our knowledge, the present study is the first to evaluate use of a topical hemostatic agent during shoulder arthroplasty. We did not find a significant difference in perioperative blood loss with application of Surgiflo, a topical thrombin-based hemostatic agent. Interestingly, we found that Hb levels both before surgery and on PODs 1 and 2 were significantly lower in the experimental group. However, the difference was about 0.7 g/dL, which would not be clinically significant. The lower Hb levels on PODs 1 and 2 likely resulted from lower preoperative levels.
Other studies have found higher transfusion rates for revision versus primary shoulder arthroplasty.1,2,20 In our series, EBL, autologous blood return, and drain output were higher overall for revision versus primary cases. When we stratified by primary and revision cases, we could not detect a difference in transfusion rates between the experimental and control groups. The lack of significant difference in the revision group could be caused by low statistical power, as the control group had only 13 revision cases. Having more patients in the study may have revealed a larger difference in blood loss with use of the hemostatic agent in revision cases.
We also found no significant increase in adverse events related to use of the hemostatic agent. Complications of particular concern would include wound complications, deep prosthesis infection, and systemic thromboembolic disease (eg, myocardial infarction, stroke, DVT, pulmonary embolus). There were no statistical differences in major and minor complications between the groups and no identifiable complications related to the hemostatic agent used.
Our results should be viewed in light of study limitations. First, with this retrospective study, we relied heavily on the accuracy of computer-based patient documentation. In addition, blood loss estimates are imperfect regardless of measurement technique. Intraoperative EBL is often determined by the surgeon and is highly variable, and autologous blood collection does not account for blood lost in operative sponges, instruments, and irrigation. To minimize this issue, we tried to assess perioperative blood loss through multiple data points, including intraoperative EBL, autologous blood returned during surgery, drain output, transfusion rates, and HBL calculations. Also, blood transfusion criteria depend on the physician’s clinical assessment and decision making, as well as patient condition, which could certainly add variability to the transfusion rate between groups. Another limitation is that the procedures studied were not homogeneous, and including primary and revision anatomical and reverse shoulder arthroplasties may have added variability to the results. In this single-surgeon study, however, we were able to ensure that the same standard techniques and hemostasis were applied in all procedures. Last, given the relatively small sample used, more patients may be needed to reveal a significant and clinically relevant difference in blood loss.
Conclusion
Perioperative blood loss poses serious risks to patient health. In light of the varying findings in the literature and the cost of transfusions and blood loss management products, use of these hemostatic agents remains controversial. In the present study, we found no significant difference in perioperative blood loss or transfusion rates with use of a hemostatic agent during shoulder arthroplasty. Therefore, we cannot conclude that this agent is effective for blood loss management in shoulder arthroplasty. Highly powered prospective studies are needed to confirm our findings.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.
1. Millett PJ, Porramatikul M, Chen N, Zurakowski D, Warner JJ. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(6):1223-1230.
2. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
3. Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18(2):225-230.
4. Schumer RA, Chae JS, Markert RJ, Sprott D, Crosby LA. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):91-96.
5. Sperling JW, Duncan SF, Cofield RH, Schleck CD, Harmsen WS. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(6):599-601.
6. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43-48.
7. National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion? http://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks.html. Published January 30, 2012. Accessed June 24, 2015.
8. Bracale U, Rovani M, Picardo A, et al. Beneficial effects of fibrin glue (Quixil) versus Lichtenstein conventional technique in inguinal hernia repair: a randomized clinical trial. Hernia. 2014;18(2):185-192.
9. Gazzeri R, Galarza M, Alfier A. Safety biocompatibility of gelatin hemostatic matrix (Floseal and Surgiflo) in neurosurgical procedures. Surg Technol Int. 2012;22:49-54.
10. Krishnan S, Conner TM, Leslie R, Stemkowski S, Shander A. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2009;13(4):225-230.
11. Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81(11):1580-1588.
12. Wang GJ, Goldthwaite CA Jr, Burks S, Crawford R, Spotnitz WD; Orthopaedic Investigators Group. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long Term Eff Med Implants. 2003;13(5):399-411.
13. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
14. Massin P, Scemama C, Jeanrot C, Boyer P. Does fibrin sealant use in total knee replacement reduce transfusion rates? A non-randomised comparative study. Orthop Traumatol Surg Res. 2012;98(2):180-185.
15. Wang GJ, Hungerford DS, Savory CG, et al. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83(10):1503-1505.
16. Surgiflo Hemostatic Matrix Kit [package insert]. Somerville, NJ: Ethicon; 2012.
17. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
18. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
19. Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 suppl):s135-s140.
20. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
21. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
22. Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86(5):669-673.
23. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753-765.
24. Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17(12):727-736.