User login
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
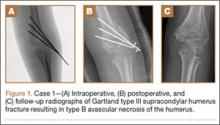
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
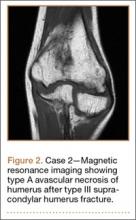
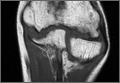
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
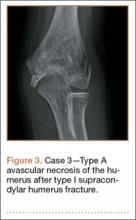
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
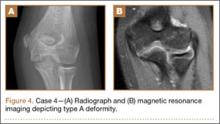
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
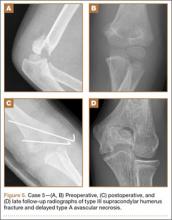
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
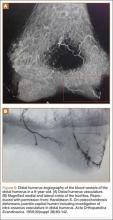
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
Supracondylar humerus fractures, which are the most common elbow fractures in the pediatric population, account for approximately 3% of all pediatric fractures.1 Complications of the injury or surgery include pin migration (2%), pin-site infection (1%), malunion, loss of reduction, compartment syndrome, nerve injury, and cubitus varus.1 A less frequently reported complication is avascular necrosis (AVN) of the trochlea.
First reported in 1948, posttraumatic deformity of the trochlea has appeared sparingly throughout the literature.2 This complication has been reported in varying fracture patterns and degrees of injury. The exact incidence is unknown because AVN of the humerus can occur without known trauma. The etiology of the deformity is thought to be interruption of the blood supply of the trochlea. Patterns include type A (AVN of the lateral ossification center) and type B (AVN of the entire medial crista along with a metaphyseal portion). Type A necrosis leads to early degenerative joint disease and loss of range of motion (ROM); angular deformities are uncommon. Type B AVN results in a progressive varus deformity of the trochlea.3 The deformities typically worsen as the child ages. Late-onset ulnar neuropathy can be seen, as medial condyle hypoplasia allows the ulnar nerve to move anterior with the medial head of the triceps. Treatment options address the sequelae and include observation, muscle strengthening, supracondylar osteotomy, and ulnar nerve transposition. Arthroscopic joint débridement has been shown, in short-term follow-up, to relieve pain and restore motion.4
We present 5 cases of AVN of the trochlea after supracondylar humerus fractures to highlight this unusual complication. Unlike more common complications of supracondylar humerus fractures, AVN of the trochlea can be a late clinical finding. We speculate that, in cases resulting from nondisplaced fractures, tamponade from fracture hematoma may play a role. It is important to keep this complication in the differential diagnosis of patients with a history of a supracondylar humerus fracture and unexplained elbow motion loss or pain.
Case Reports
Retrospective data were collected for all patients after approval by the institutional review board at our institution. Patients were identified by a computerized search using the Current Procedural Terminology code for closed reduction percutaneous pinning of supracondylar humerus fracture. The search was limited to patients treated at our institution from 2000 to 2012; 1159 patients were initially identified. Three patients were found to have postoperative AVN of the trochlea; 2 other patients were treated at an outside hospital and were identified by surgeon recall. These 5 cases are presented here.
Case 1
A girl aged 5 years, 3 months sustained a Gartland type III supracondylar humerus fracture. She was originally seen at an outside facility and transferred to our tertiary care facility for definitive management. She underwent closed reduction and fixation with 3 lateral-based pins 1 day after her injury. Her pins and cast were removed 22 days postoperatively. She returned to full elbow function after her fracture care; 6 months later, she returned to the clinic with painless, decreased flexion of her elbow to 95º. Radiographs showed a lucency of the trochlea extending into the metaphysis (Figure 1). Thirteen months postoperatively, her examination was unchanged with motion at 0º to 95º; her radiographs showed a persistent lateral and medial lucency of the trochlea consistent with type B AVN involving the medial crista.
Case 2
An 8-year-old girl sustained a Gartland type III supracondylar humerus fracture that was treated at an outside facility with closed reduction and fixation with lateral pins. She had an uneventful postoperative course with painless return of motion. She presented 6 months after her surgery with progressive decreased ROM. She underwent conservative treatment with therapy and stretching without much improvement. She presented to our institution 4 years postoperatively with painless decreased motion from 40º to 110º. Radiographs showed dissolution of the lateral ossification center of the trochlea with a fishtail deformity consistent with type A AVN. Magnetic resonance imaging (MRI) confirmed AVN of the trochlea (Figure 2).
Case 3
A girl aged 5 years, 6 months sustained a Gartland type I supracondylar humerus fracture that was treated uneventfully by casting. She did not have a reduction or manipulation and healed without complications. She returned to the clinic 3 years after the injury complaining of intermittent elbow pain, neglect, and loss of motion. Her ROM was 0º to 110º. Radiographs showed dissolution of the lateral trochlea with sclerosis of the metaphysis consistent with a type A deformity (Figure 3). Contralateral radiographs were not obtained. MRI confirmed AVN of the trochlea.
Case 4
A 10-year-old girl sustained a Gartland type III supracondylar humerus fracture treated with closed reduction and pinning at an outside facility. She experienced full return to function postoperatively until developing stiffness and popping 1 year after surgery. She was evaluated at our institution 5 years postoperatively with elbow popping in full extension. Radiographs showed a type A deformity; MRI confirmed the diagnosis of AVN of the humerus (Figure 4). She underwent elbow arthroscopy with débridement of a posterior cartilage flap and synovial band. After elbow arthroscopy and débridement, she had resolution of symptoms with full elbow ROM.
Case 5
A 5-year-old boy sustained a Gartland III supracondylar humerus fracture that was treated with closed reduction and pinning at our institution. He had full return of painless motion postoperatively. Seven years after surgery, he presented with popping sensation in his elbow. Examination showed a 5º lack of full extension without effusion or crepitus. Radiographs showed a type A deformity with dissolution of the lateral ossification center (Figure 5).
Discussion
Avascular necrosis of the trochlea after supracondylar humerus fractures was first reported by McDonnell and Wilson in 1948.2 Four of 53 patients (7.5%) developed AVN of the trochlea. Clinical presentation happened at 2 to 7 years after injury. No causative effect was given; however, 2 cases of AVN were associated with narrowing of joint space and thinning of articular cartilage. One incident was associated with multiple reduction attempts.2 The etiology and exact incidence remain unclear, but both vascular insult and idiopathic growth disturbance have been proposed.4
Morrissy and Wilkins5 in 1984 reported 3 cases of dissolution of the trochlea after supracondylar humerus fractures: 1 fracture was casted, 1 was splinted, and 1 underwent closed reduction and pinning. Radiographic abnormality was noted at 5 years, 1 year, and 9 months, respectively. These authors explained the dissolution as a vascular phenomenon. Interruption of the medial or lateral vessels supplying the cartilage of the trochlea would lead to the central necrosis pattern seen in their 3 cases. In addition, the rapid onset in Morrissy and Wilkin’s second and third cases (both 7 years old) supports a vascular etiology.5
A more recent study of 6 cases found dissolution of the trochlea occurred as a result of severe displaced supracondylar fractures.6 Four of the 6 cases involved nerve injuries. Evidence of fishtail deformity was delayed from fracture time until 7 to 8 years of age, consistent with the ossification of the trochlea. Additionally, MRI findings, as well as loose body formation, added to the plausibility of AVN.6
Haraldsson7 demonstrated the 2 main sources of blood supply to the medial crista of the trochlea. The lateral vessels are intra-articular and supply the apex and lateral aspect of the trochlea. The medial vessels supply the medial aspect of the medial crista of the trochlea and are extra-articular. The lateral and medial vessels do not have an anastomosis between them (Figure 6).7 Disruption of the lateral vessels results in a type A deformity; disruption of the lateral and medial vessels results in a type B deformity. Displaced supracondylar humerus fractures disrupt the periosteum and can result in disruption of the medial and/or lateral vessels, resulting in AVN and deformity.
Another case of AVN of the trochlea after a Gartland type I fracture was reported by Schulte and Ramseier.8 Similar to our case 3, the patient developed type A AVN of the distal humerus,9 illustrating an interruption of the lateral, intra-articular vessels. The etiology of vascular disruption in these nondisplaced supracondylar humerus fractures is less clear, but we propose that tamponade may play a role. Nondisplaced fractures result in a fracture hematoma contained in an intact capsule, having the potential to increase pressures and lead to occlusion of the lateral, intra-articular vessels. This would result in a type A deformity. Nondisplaced supracondylar humerus fractures are common, and this complication is very rare. Typically, they would be expected to generate modest fracture hematoma. However, patient factors, such as bleeding disorders or anatomic variants, including a constricted capsule, could predispose patients to development of increased intracapsular pressure. In contrast, Gartland type II and III fractures, although higher-energy, presumably tear the surrounding capsule leading to release of the fracture hematoma. We do not have direct evidence to support this theory, but measurement of intracapsular pressures could help support or refute the occurrence of tamponade. Similar studies have been reported in hip fracture and slipped capital femoral epiphysis, in which hematoma has been shown to increase intracapsular pressure.8,10 This pressure increase can theoretically cause a tamponade of the femoral head blood supply leading to AVN. Additional alternate explanations for AVN of the trochlea after type I fractures may include a rare occurrence of direct trauma to the vessels at the moment of fracture, increased intracapsular pressure from cast positioning, or that they are unrelated events that occurred in the same elbow (because atraumatic AVN has also been reported).
Conclusion
Avascular necrosis of the trochlea is a rare but important complication of supracondylar humerus fractures. Generally, this complication has a late clinical presentation, and its cause is interruption of the trochlea blood supply. In displaced fractures, the medial and/or lateral vessels are injured, leading to Gartland type A or type B deformity. In nondisplaced fractures, the lateral vessels are affected. We propose that the lateral vessels may be interrupted by tamponade caused by encased fracture hematoma; this presents as a type A deformity. Both type A and type B deformities can be clinically significant. Avascular necrosis of the trochlea should be considered in patients with late presentation of pain or loss of motion after treatment of supracondylar humerus fractures.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.
1. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20(2):69-77.
2. McDonnell DP, Wilson JC. Fractures of the lower end of the humerus in children. J Bone Joint Surg Am. 1948;30(2):347-358.
3. Toniolo R, Renato M, Wilkins KE. Avascular necrosis of the humeral trochlea. In: Rockwood C, Beaty J, Green D, eds. Fractures in Children. Vol. 3. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1996:821-830.
4. Glotzbecker MP, Bae DS, Links AC, Waters PM. Fishtail deformity of the distal humerus: a report of 15 cases. J Pediatr Orthop. 2013;33(6):592-597.
5. Morrissy RT, Wilkins KE. Deformity following distal humeral fracture in childhood. J Bone Joint Surg Am. 1984;66(4):557-562.
6. Bronfen CE, Gefford B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop. 2007;27(5):547-550.
7. Haraldsson S. On osteochondrosis deformans juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand. 1959;30(suppl 38):83-142.
8. Schulte DW, Ramseier LE. Fishtail deformity as a result of a non-displaced supracondylar fracture of the humerus. Acta Orthop Belg. 2009;75(3):408-410.
9. Herrera-Soto JA, Duffy MF, Birnbaum MA, Vander Have KL. Increased intracapsular pressures after unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2008;28(7):723-728.
10. Bonnaire F, Schaefer DJ, Kuner EH. Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res. 1998;(353):148-155.