User login
High-Volume Burn Resuscitation Increases Neurologic Risk
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Key Updates in Resuscitation Procedure After Drowning
New recommendations on rescuing adults and children who have drowned include an important update for healthcare professionals, trained rescuers, and untrained lay rescuers.
The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) have issued recommendations that highlight delivering rescue breaths as well as calling 911 and performing chest compressions in cardiopulmonary resuscitation (CPR) as first steps when a person pulled from the water is in cardiac arrest.
This is the first collaboration between the two organizations on resuscitation after drowning. The recommendations were published simultaneously in Circulation and Pediatrics.
Included in the recommendations are two key principles:
- Anyone pulled from the water who has no signs of normal breathing or consciousness should be presumed to be in cardiac arrest.
- Rescuers should immediately start CPR that includes rescue breathing in addition to chest compressions. Multiple large studies show more people with cardiac arrest from noncardiac causes such as drowning survive when CPR includes rescue breaths, compared with hands-only CPR (calling 911 and pushing hard and fast in the center of the chest).
If someone is untrained, unwilling, or unable to give breaths, they can perform chest compressions until help arrives, the recommendations advise.
Reasoning Behind the Update
The authors, led by writing group cochair Tracy E. McCallin, MD, associate professor in the division of pediatric emergency medicine at Rainbow Babies and Children’s Hospital in Cleveland , Ohio, explained that drowning generally advances from initial respiratory arrest from submersion-related hypoxia to cardiac arrest, and therefore it can be difficult to distinguish respiratory arrest from cardiac arrest because pulses are difficult to accurately palpate within the recommended 10-second window.
“Therefore, resuscitation from cardiac arrest due to this specific circumstance must focus on restoring breathing as much as it does circulation,” the authors wrote.
Resuscitation after drowning may begin in the water with rescue breathing when safely provided by trained rescuers and should continue with chest compressions, once the drowned person and the rescuer are on land or in a boat, the report authors wrote.
“The focused update on drowning contains the most up-to-date, evidence-based recommendations on how to resuscitate someone who has drowned,” McCallin states in a press release.
In addition to the new guidance on rescue breaths, the update includes new topics that the AHA has not previously addressed with treatment recommendations, such as oxygen administration after drowning; automated external defibrillator use in cardiac arrest after drowning and public-access defibrillation programs.
Pediatricians Can Help Spread the Word
Alexandra Stern, MD, assistant professor in the Department of Pediatrics at University of Florida, Gainesville, who was not part of the update, said pediatricians can help disseminate this new information.
“Water safety is a topic frequently discussed as a pediatrician, with focus often being on primary prevention of drowning,” she said. “We stress the importance of the multiple layers of protection against drowning, such as touch supervision (staying within arm’s length); secure fencing, access to appropriate life jackets, and teaching our children to swim. Learning CPR is a large part of these measures and continuing these discussions with our patients and families is important.”
She added that updating the recommended procedures will likely require changes to all forms of education and community outreach regarding drowning from basic life support classes to more advanced lifeguard training. She noted that the update provides practical guidance not just for trained rescuers and healthcare professionals, but also for family members.
The paper notes that drowning is the third leading cause of death from unintentional injury globally, accounting for 7% of all injury-related deaths. In the United States, drowning is the leading cause of death in children aged 1-4 years and the second leading cause of death from unintentional injury in children aged 5-14 years.
The update is based on systematic reviews from 2021 to 2023 performed by the International Liaison Committee on Resuscitation related to the resuscitation of drowning.
The authors and Stern reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
New recommendations on rescuing adults and children who have drowned include an important update for healthcare professionals, trained rescuers, and untrained lay rescuers.
The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) have issued recommendations that highlight delivering rescue breaths as well as calling 911 and performing chest compressions in cardiopulmonary resuscitation (CPR) as first steps when a person pulled from the water is in cardiac arrest.
This is the first collaboration between the two organizations on resuscitation after drowning. The recommendations were published simultaneously in Circulation and Pediatrics.
Included in the recommendations are two key principles:
- Anyone pulled from the water who has no signs of normal breathing or consciousness should be presumed to be in cardiac arrest.
- Rescuers should immediately start CPR that includes rescue breathing in addition to chest compressions. Multiple large studies show more people with cardiac arrest from noncardiac causes such as drowning survive when CPR includes rescue breaths, compared with hands-only CPR (calling 911 and pushing hard and fast in the center of the chest).
If someone is untrained, unwilling, or unable to give breaths, they can perform chest compressions until help arrives, the recommendations advise.
Reasoning Behind the Update
The authors, led by writing group cochair Tracy E. McCallin, MD, associate professor in the division of pediatric emergency medicine at Rainbow Babies and Children’s Hospital in Cleveland , Ohio, explained that drowning generally advances from initial respiratory arrest from submersion-related hypoxia to cardiac arrest, and therefore it can be difficult to distinguish respiratory arrest from cardiac arrest because pulses are difficult to accurately palpate within the recommended 10-second window.
“Therefore, resuscitation from cardiac arrest due to this specific circumstance must focus on restoring breathing as much as it does circulation,” the authors wrote.
Resuscitation after drowning may begin in the water with rescue breathing when safely provided by trained rescuers and should continue with chest compressions, once the drowned person and the rescuer are on land or in a boat, the report authors wrote.
“The focused update on drowning contains the most up-to-date, evidence-based recommendations on how to resuscitate someone who has drowned,” McCallin states in a press release.
In addition to the new guidance on rescue breaths, the update includes new topics that the AHA has not previously addressed with treatment recommendations, such as oxygen administration after drowning; automated external defibrillator use in cardiac arrest after drowning and public-access defibrillation programs.
Pediatricians Can Help Spread the Word
Alexandra Stern, MD, assistant professor in the Department of Pediatrics at University of Florida, Gainesville, who was not part of the update, said pediatricians can help disseminate this new information.
“Water safety is a topic frequently discussed as a pediatrician, with focus often being on primary prevention of drowning,” she said. “We stress the importance of the multiple layers of protection against drowning, such as touch supervision (staying within arm’s length); secure fencing, access to appropriate life jackets, and teaching our children to swim. Learning CPR is a large part of these measures and continuing these discussions with our patients and families is important.”
She added that updating the recommended procedures will likely require changes to all forms of education and community outreach regarding drowning from basic life support classes to more advanced lifeguard training. She noted that the update provides practical guidance not just for trained rescuers and healthcare professionals, but also for family members.
The paper notes that drowning is the third leading cause of death from unintentional injury globally, accounting for 7% of all injury-related deaths. In the United States, drowning is the leading cause of death in children aged 1-4 years and the second leading cause of death from unintentional injury in children aged 5-14 years.
The update is based on systematic reviews from 2021 to 2023 performed by the International Liaison Committee on Resuscitation related to the resuscitation of drowning.
The authors and Stern reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
New recommendations on rescuing adults and children who have drowned include an important update for healthcare professionals, trained rescuers, and untrained lay rescuers.
The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) have issued recommendations that highlight delivering rescue breaths as well as calling 911 and performing chest compressions in cardiopulmonary resuscitation (CPR) as first steps when a person pulled from the water is in cardiac arrest.
This is the first collaboration between the two organizations on resuscitation after drowning. The recommendations were published simultaneously in Circulation and Pediatrics.
Included in the recommendations are two key principles:
- Anyone pulled from the water who has no signs of normal breathing or consciousness should be presumed to be in cardiac arrest.
- Rescuers should immediately start CPR that includes rescue breathing in addition to chest compressions. Multiple large studies show more people with cardiac arrest from noncardiac causes such as drowning survive when CPR includes rescue breaths, compared with hands-only CPR (calling 911 and pushing hard and fast in the center of the chest).
If someone is untrained, unwilling, or unable to give breaths, they can perform chest compressions until help arrives, the recommendations advise.
Reasoning Behind the Update
The authors, led by writing group cochair Tracy E. McCallin, MD, associate professor in the division of pediatric emergency medicine at Rainbow Babies and Children’s Hospital in Cleveland , Ohio, explained that drowning generally advances from initial respiratory arrest from submersion-related hypoxia to cardiac arrest, and therefore it can be difficult to distinguish respiratory arrest from cardiac arrest because pulses are difficult to accurately palpate within the recommended 10-second window.
“Therefore, resuscitation from cardiac arrest due to this specific circumstance must focus on restoring breathing as much as it does circulation,” the authors wrote.
Resuscitation after drowning may begin in the water with rescue breathing when safely provided by trained rescuers and should continue with chest compressions, once the drowned person and the rescuer are on land or in a boat, the report authors wrote.
“The focused update on drowning contains the most up-to-date, evidence-based recommendations on how to resuscitate someone who has drowned,” McCallin states in a press release.
In addition to the new guidance on rescue breaths, the update includes new topics that the AHA has not previously addressed with treatment recommendations, such as oxygen administration after drowning; automated external defibrillator use in cardiac arrest after drowning and public-access defibrillation programs.
Pediatricians Can Help Spread the Word
Alexandra Stern, MD, assistant professor in the Department of Pediatrics at University of Florida, Gainesville, who was not part of the update, said pediatricians can help disseminate this new information.
“Water safety is a topic frequently discussed as a pediatrician, with focus often being on primary prevention of drowning,” she said. “We stress the importance of the multiple layers of protection against drowning, such as touch supervision (staying within arm’s length); secure fencing, access to appropriate life jackets, and teaching our children to swim. Learning CPR is a large part of these measures and continuing these discussions with our patients and families is important.”
She added that updating the recommended procedures will likely require changes to all forms of education and community outreach regarding drowning from basic life support classes to more advanced lifeguard training. She noted that the update provides practical guidance not just for trained rescuers and healthcare professionals, but also for family members.
The paper notes that drowning is the third leading cause of death from unintentional injury globally, accounting for 7% of all injury-related deaths. In the United States, drowning is the leading cause of death in children aged 1-4 years and the second leading cause of death from unintentional injury in children aged 5-14 years.
The update is based on systematic reviews from 2021 to 2023 performed by the International Liaison Committee on Resuscitation related to the resuscitation of drowning.
The authors and Stern reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
From Pediatrics
Heat Waves Pose Significant Health Risks for Dually Eligible Older Individuals
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Heat waves are associated with an increase in heat-related emergency department visits, hospitalizations, and deaths among dually eligible individuals older than 65 years.
METHODOLOGY:
- The researchers conducted a retrospective time-series study using national Medicare and Medicaid data from 2016 to 2019 to assess the link between heat waves during warm months and adverse health events.
- A total of 5,448,499 dually eligible individuals (66% women; 20% aged ≥ 85 years) were included from 28,404 zip code areas across 50 states and Washington, DC.
- Heat waves were defined as three or more consecutive days of extreme heat with a maximum temperature of at least 90 °F and within the 97th percentile of daily maximum temperatures for each zip code.
- Primary outcomes were daily counts of heat-related emergency department visits and hospitalizations.
- Secondary outcomes were all-cause and heat-specific emergency department visits, all-cause and heat-specific hospitalizations, deaths, and long-term nursing facility placements within 3 months after a heat wave.
TAKEAWAY:
- Heat waves were associated with a 10% increase in heat-related emergency department visits (incidence rate ratio [IRR], 1.10; 95% CI, 1.08-1.12) and a 7% increase in heat-related hospitalizations (IRR, 1.07; 95% CI, 1.04-1.09).
- Mortality rates were 4% higher during heat wave days than during non–heat wave days (IRR, 1.04; 95% CI, 1.01-1.07).
- No significant difference was found in rates of long-term nursing facility placements or heat-related emergency department visits for nursing facility residents.
- All racial and ethnic groups showed higher incidence rates of heat-related emergency department visits during heat waves, especially among beneficiaries identified as Asian (IRR, 1.21; 95% CI, 1.12-1.29). Rates were higher among individuals residing in the Northwest, Ohio Valley, and the West.
IN PRACTICE:
“In healthcare settings, clinicians should incorporate routine heat wave risk assessments into clinical practice, especially in regions more susceptible to extreme heat, for all dual-eligible beneficiaries and other at-risk patients,” wrote Jose F. Figueroa, MD, MPH, of the Harvard T.H. Chan School of Public Health in Boston, in an invited commentary. “Beyond offering preventive advice, clinicians can adjust medications that may increase their patients’ susceptibility during heat waves, or they can refer patients to social workers and social service organizations to ensure that they are protected at home.”
SOURCE:
This study was led by Hyunjee Kim, PhD, of the Center for Health Systems Effectiveness at Oregon Health & Science University, Portland. It was published online in JAMA Health Forum.
LIMITATIONS:
This study relied on a claims database to identify adverse events, which may have led to omissions in coding, particularly for heat-related conditions if the diagnostic codes for heat-related symptoms had not been adopted. This study did not adjust for variations in air quality or green space, which could have confounded the association of interest. Indoor heat exposures or adaptive behaviors, such as air conditioning use, were not considered. The analysis could not compare the association of heat waves with adverse events between those with dual eligibility and those without dual eligibility.
DISCLOSURES:
This study was supported by the National Institute on Aging. One author reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Alert System Could Warn of Impact of Severe Weather on Health
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
US 911 System Is Nearing Its Own Emergency
Just after lunchtime on June 18, Massachusetts’ leaders discovered that the statewide 911 system was down.
A scramble to handle the crisis was on.
Police texted out administrative numbers that callers could use, Boston Mayor Michelle Wu gave outage updates at a press conference outlining plans for the Celtics’ championship parade, and local officials urged people to summon help by pulling red fire alarm boxes.
About 7 million people went roughly 2 hours with no 911 service. Such crashes have become more of a feature than a bug in the nation’s fragmented emergency response system.
While some states, cities, and counties have already modernized their systems or have made plans to upgrade, many others are lagging.
911 is typically supported by fees tacked on to phone bills, but state and local governments also tap general funds or other resources.
“Now there are haves and have-nots,” said Jonathan Gilad, vice president of government affairs at the National Emergency Number Association (NENA), which represents 911 first responders. “Next-generation 911 shouldn’t be for people who happen to have an emergency in a good location.”
Meanwhile, federal legislation that could steer billions of dollars into modernizing the patchwork 911 system remains waylaid in Congress.
“This is a national security imperative,” said George Kelemen, executive director of the Industry Council for Emergency Response Technologies, a trade association that represents companies that provide hardware and software to the emergency response industry.
“In a crisis — a school shooting or a house fire or, God forbid, a terrorist attack — people call 911 first,” he said. “The system can’t go down.”
The United States debuted a single, universal 911 emergency number in February 1968 to simplify crisis response. But instead of a seamless national program, the 911 response network has evolved into a massive puzzle of many interlocking pieces. There are more than 6,000 911 call centers to handle an estimated 240 million emergency calls each year, according to federal data. More than three-quarters of call centers experienced outages in the prior 12 months, according to a survey in February by NENA, which sets standards and advocates for 911, and Carbyne, a provider of public safety technology solutions.
In April, widespread 911 outages affected millions in Nebraska, Nevada, South Dakota, and Texas. The shutdown was blamed on workers’ severing a fiber line while installing a light pole.
In February, tens of thousands of people in areas of California, Georgia, Illinois, Texas, and other states lost cellphone service, including some 911 services, from an outage.
And in June, Verizon agreed to pay a $1.05 million fine to settle a Federal Communications Commission (FCC) probe into a December 2022 outage that affected 911 calls in Alabama, Florida, Georgia, North Carolina, South Carolina, and Tennessee.
The fires that raced across the Hawaiian island of Maui in August 2023 highlighted the critical importance of 911 systems. Dispatchers there fielded more than 4,500 contacts, meaning calls and texts, on Aug. 8, the day the fires broke out, compared with about 400 on a typical day, said Davlynn Racadio, emergency services dispatch coordinator in Maui County.
“We’re dying out here,” one caller told 911 operators.
But some cell towers faltered because of widespread service outages, according to county officials. Maui County in May filed a lawsuit against four telecommunications companies, saying they failed to inform dispatchers about the outages.
“If 911 calls came in with no voice, we would send text messages,” Ms. Racadio said. “The state is looking at upgrading our system. Next-generation 911 would take us even further into the future.”
Florida, Illinois, Montana, and Oklahoma passed legislation in 2023 to advance or fund modernized 911 systems, according to the National Conference of State Legislatures. The upgrades include replacing analog 911 infrastructure with digital, Internet-based systems.
Instead of just fielding calls, next-generation systems can pinpoint a caller’s location, accept texts, and enable residents in a crisis to send videos and images to dispatchers. While outages can still occur, modernized systems often include more redundancy to minimize the odds of a shutdown, Mr. Gilad said.
Lawmakers have looked at modernizing 911 systems by tapping revenue the FCC gets from auctioning off the rights to transmit signals over specific bands of the electromagnetic spectrum.
But the U.S. Senate, in March 2023, for the first time allowed a lapse of the FCC’s authority to auction spectrum bands.
Legislation that would allocate almost $15 billion in grants from auction proceeds to speed deployment of next-generation 911 in every state unanimously passed the House Energy and Commerce Committee in May 2023. The bill, HR 3565, sponsored by Rep. Cathy McMorris Rodgers (R-Wash.), would also extend the FCC’s auction authority.
Other bills have been introduced by various lawmakers, including one in March from Sen. Ted Cruz (R-Texas) and legislation from Sen. Maria Cantwell (D-Wash.) to extend the auction authority. For now, neither effort has advanced. Nine former FCC chairs wrote lawmakers in February, urging them to make 911 upgrades a national priority. They suggested Congress tap unspent federal COVID-19 money.
“Whatever the funding source, the need is urgent and the time to act is now,” they wrote.
Ajit Pai, who served as chair of the FCC from 2017 to 2021, said outages often occur in older, legacy systems.
“The fact that the FCC doesn’t have authority to auction spectrum is a real hindrance now,” Mr. Pai said in an interview. “You may never need to call 911, but it can make the difference between life and death. We need more of an organized effort at the federal level because 911 is so decentralized.”
Meanwhile, some safety leaders are making backup plans for 911 outages or conducting investigations into their causes. In Massachusetts, a firewall designed to prevent hacking led to the recent 2-hour outage, according to the state 911 department.
“Outages bring to everyone’s attention that we rely on 911 and we don’t think about how we really rely on it until something happens,” said April Heinze, chief of 911 operations at NENA.
Mass General Brigham, a health system in the Boston area, sent out emergency alerts when the outage happened letting clinics and smaller practices know how to find their 10-digit emergency numbers. In the wake of the outage, it plans to keep the backup numbers next to phones at those facilities.
“Two hours can be a long time,” said Paul Biddinger, chief preparedness and continuity officer at the health system.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Just after lunchtime on June 18, Massachusetts’ leaders discovered that the statewide 911 system was down.
A scramble to handle the crisis was on.
Police texted out administrative numbers that callers could use, Boston Mayor Michelle Wu gave outage updates at a press conference outlining plans for the Celtics’ championship parade, and local officials urged people to summon help by pulling red fire alarm boxes.
About 7 million people went roughly 2 hours with no 911 service. Such crashes have become more of a feature than a bug in the nation’s fragmented emergency response system.
While some states, cities, and counties have already modernized their systems or have made plans to upgrade, many others are lagging.
911 is typically supported by fees tacked on to phone bills, but state and local governments also tap general funds or other resources.
“Now there are haves and have-nots,” said Jonathan Gilad, vice president of government affairs at the National Emergency Number Association (NENA), which represents 911 first responders. “Next-generation 911 shouldn’t be for people who happen to have an emergency in a good location.”
Meanwhile, federal legislation that could steer billions of dollars into modernizing the patchwork 911 system remains waylaid in Congress.
“This is a national security imperative,” said George Kelemen, executive director of the Industry Council for Emergency Response Technologies, a trade association that represents companies that provide hardware and software to the emergency response industry.
“In a crisis — a school shooting or a house fire or, God forbid, a terrorist attack — people call 911 first,” he said. “The system can’t go down.”
The United States debuted a single, universal 911 emergency number in February 1968 to simplify crisis response. But instead of a seamless national program, the 911 response network has evolved into a massive puzzle of many interlocking pieces. There are more than 6,000 911 call centers to handle an estimated 240 million emergency calls each year, according to federal data. More than three-quarters of call centers experienced outages in the prior 12 months, according to a survey in February by NENA, which sets standards and advocates for 911, and Carbyne, a provider of public safety technology solutions.
In April, widespread 911 outages affected millions in Nebraska, Nevada, South Dakota, and Texas. The shutdown was blamed on workers’ severing a fiber line while installing a light pole.
In February, tens of thousands of people in areas of California, Georgia, Illinois, Texas, and other states lost cellphone service, including some 911 services, from an outage.
And in June, Verizon agreed to pay a $1.05 million fine to settle a Federal Communications Commission (FCC) probe into a December 2022 outage that affected 911 calls in Alabama, Florida, Georgia, North Carolina, South Carolina, and Tennessee.
The fires that raced across the Hawaiian island of Maui in August 2023 highlighted the critical importance of 911 systems. Dispatchers there fielded more than 4,500 contacts, meaning calls and texts, on Aug. 8, the day the fires broke out, compared with about 400 on a typical day, said Davlynn Racadio, emergency services dispatch coordinator in Maui County.
“We’re dying out here,” one caller told 911 operators.
But some cell towers faltered because of widespread service outages, according to county officials. Maui County in May filed a lawsuit against four telecommunications companies, saying they failed to inform dispatchers about the outages.
“If 911 calls came in with no voice, we would send text messages,” Ms. Racadio said. “The state is looking at upgrading our system. Next-generation 911 would take us even further into the future.”
Florida, Illinois, Montana, and Oklahoma passed legislation in 2023 to advance or fund modernized 911 systems, according to the National Conference of State Legislatures. The upgrades include replacing analog 911 infrastructure with digital, Internet-based systems.
Instead of just fielding calls, next-generation systems can pinpoint a caller’s location, accept texts, and enable residents in a crisis to send videos and images to dispatchers. While outages can still occur, modernized systems often include more redundancy to minimize the odds of a shutdown, Mr. Gilad said.
Lawmakers have looked at modernizing 911 systems by tapping revenue the FCC gets from auctioning off the rights to transmit signals over specific bands of the electromagnetic spectrum.
But the U.S. Senate, in March 2023, for the first time allowed a lapse of the FCC’s authority to auction spectrum bands.
Legislation that would allocate almost $15 billion in grants from auction proceeds to speed deployment of next-generation 911 in every state unanimously passed the House Energy and Commerce Committee in May 2023. The bill, HR 3565, sponsored by Rep. Cathy McMorris Rodgers (R-Wash.), would also extend the FCC’s auction authority.
Other bills have been introduced by various lawmakers, including one in March from Sen. Ted Cruz (R-Texas) and legislation from Sen. Maria Cantwell (D-Wash.) to extend the auction authority. For now, neither effort has advanced. Nine former FCC chairs wrote lawmakers in February, urging them to make 911 upgrades a national priority. They suggested Congress tap unspent federal COVID-19 money.
“Whatever the funding source, the need is urgent and the time to act is now,” they wrote.
Ajit Pai, who served as chair of the FCC from 2017 to 2021, said outages often occur in older, legacy systems.
“The fact that the FCC doesn’t have authority to auction spectrum is a real hindrance now,” Mr. Pai said in an interview. “You may never need to call 911, but it can make the difference between life and death. We need more of an organized effort at the federal level because 911 is so decentralized.”
Meanwhile, some safety leaders are making backup plans for 911 outages or conducting investigations into their causes. In Massachusetts, a firewall designed to prevent hacking led to the recent 2-hour outage, according to the state 911 department.
“Outages bring to everyone’s attention that we rely on 911 and we don’t think about how we really rely on it until something happens,” said April Heinze, chief of 911 operations at NENA.
Mass General Brigham, a health system in the Boston area, sent out emergency alerts when the outage happened letting clinics and smaller practices know how to find their 10-digit emergency numbers. In the wake of the outage, it plans to keep the backup numbers next to phones at those facilities.
“Two hours can be a long time,” said Paul Biddinger, chief preparedness and continuity officer at the health system.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Just after lunchtime on June 18, Massachusetts’ leaders discovered that the statewide 911 system was down.
A scramble to handle the crisis was on.
Police texted out administrative numbers that callers could use, Boston Mayor Michelle Wu gave outage updates at a press conference outlining plans for the Celtics’ championship parade, and local officials urged people to summon help by pulling red fire alarm boxes.
About 7 million people went roughly 2 hours with no 911 service. Such crashes have become more of a feature than a bug in the nation’s fragmented emergency response system.
While some states, cities, and counties have already modernized their systems or have made plans to upgrade, many others are lagging.
911 is typically supported by fees tacked on to phone bills, but state and local governments also tap general funds or other resources.
“Now there are haves and have-nots,” said Jonathan Gilad, vice president of government affairs at the National Emergency Number Association (NENA), which represents 911 first responders. “Next-generation 911 shouldn’t be for people who happen to have an emergency in a good location.”
Meanwhile, federal legislation that could steer billions of dollars into modernizing the patchwork 911 system remains waylaid in Congress.
“This is a national security imperative,” said George Kelemen, executive director of the Industry Council for Emergency Response Technologies, a trade association that represents companies that provide hardware and software to the emergency response industry.
“In a crisis — a school shooting or a house fire or, God forbid, a terrorist attack — people call 911 first,” he said. “The system can’t go down.”
The United States debuted a single, universal 911 emergency number in February 1968 to simplify crisis response. But instead of a seamless national program, the 911 response network has evolved into a massive puzzle of many interlocking pieces. There are more than 6,000 911 call centers to handle an estimated 240 million emergency calls each year, according to federal data. More than three-quarters of call centers experienced outages in the prior 12 months, according to a survey in February by NENA, which sets standards and advocates for 911, and Carbyne, a provider of public safety technology solutions.
In April, widespread 911 outages affected millions in Nebraska, Nevada, South Dakota, and Texas. The shutdown was blamed on workers’ severing a fiber line while installing a light pole.
In February, tens of thousands of people in areas of California, Georgia, Illinois, Texas, and other states lost cellphone service, including some 911 services, from an outage.
And in June, Verizon agreed to pay a $1.05 million fine to settle a Federal Communications Commission (FCC) probe into a December 2022 outage that affected 911 calls in Alabama, Florida, Georgia, North Carolina, South Carolina, and Tennessee.
The fires that raced across the Hawaiian island of Maui in August 2023 highlighted the critical importance of 911 systems. Dispatchers there fielded more than 4,500 contacts, meaning calls and texts, on Aug. 8, the day the fires broke out, compared with about 400 on a typical day, said Davlynn Racadio, emergency services dispatch coordinator in Maui County.
“We’re dying out here,” one caller told 911 operators.
But some cell towers faltered because of widespread service outages, according to county officials. Maui County in May filed a lawsuit against four telecommunications companies, saying they failed to inform dispatchers about the outages.
“If 911 calls came in with no voice, we would send text messages,” Ms. Racadio said. “The state is looking at upgrading our system. Next-generation 911 would take us even further into the future.”
Florida, Illinois, Montana, and Oklahoma passed legislation in 2023 to advance or fund modernized 911 systems, according to the National Conference of State Legislatures. The upgrades include replacing analog 911 infrastructure with digital, Internet-based systems.
Instead of just fielding calls, next-generation systems can pinpoint a caller’s location, accept texts, and enable residents in a crisis to send videos and images to dispatchers. While outages can still occur, modernized systems often include more redundancy to minimize the odds of a shutdown, Mr. Gilad said.
Lawmakers have looked at modernizing 911 systems by tapping revenue the FCC gets from auctioning off the rights to transmit signals over specific bands of the electromagnetic spectrum.
But the U.S. Senate, in March 2023, for the first time allowed a lapse of the FCC’s authority to auction spectrum bands.
Legislation that would allocate almost $15 billion in grants from auction proceeds to speed deployment of next-generation 911 in every state unanimously passed the House Energy and Commerce Committee in May 2023. The bill, HR 3565, sponsored by Rep. Cathy McMorris Rodgers (R-Wash.), would also extend the FCC’s auction authority.
Other bills have been introduced by various lawmakers, including one in March from Sen. Ted Cruz (R-Texas) and legislation from Sen. Maria Cantwell (D-Wash.) to extend the auction authority. For now, neither effort has advanced. Nine former FCC chairs wrote lawmakers in February, urging them to make 911 upgrades a national priority. They suggested Congress tap unspent federal COVID-19 money.
“Whatever the funding source, the need is urgent and the time to act is now,” they wrote.
Ajit Pai, who served as chair of the FCC from 2017 to 2021, said outages often occur in older, legacy systems.
“The fact that the FCC doesn’t have authority to auction spectrum is a real hindrance now,” Mr. Pai said in an interview. “You may never need to call 911, but it can make the difference between life and death. We need more of an organized effort at the federal level because 911 is so decentralized.”
Meanwhile, some safety leaders are making backup plans for 911 outages or conducting investigations into their causes. In Massachusetts, a firewall designed to prevent hacking led to the recent 2-hour outage, according to the state 911 department.
“Outages bring to everyone’s attention that we rely on 911 and we don’t think about how we really rely on it until something happens,” said April Heinze, chief of 911 operations at NENA.
Mass General Brigham, a health system in the Boston area, sent out emergency alerts when the outage happened letting clinics and smaller practices know how to find their 10-digit emergency numbers. In the wake of the outage, it plans to keep the backup numbers next to phones at those facilities.
“Two hours can be a long time,” said Paul Biddinger, chief preparedness and continuity officer at the health system.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
In the Future, a Robot Intensivist May Save Your Life
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.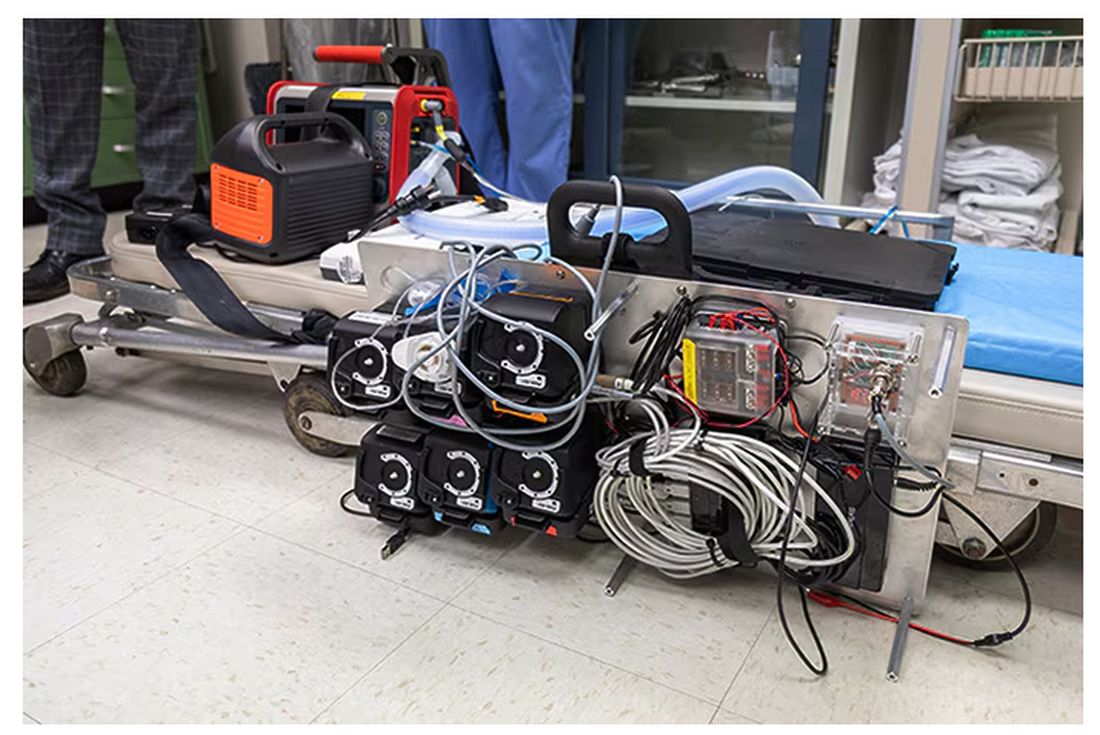
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.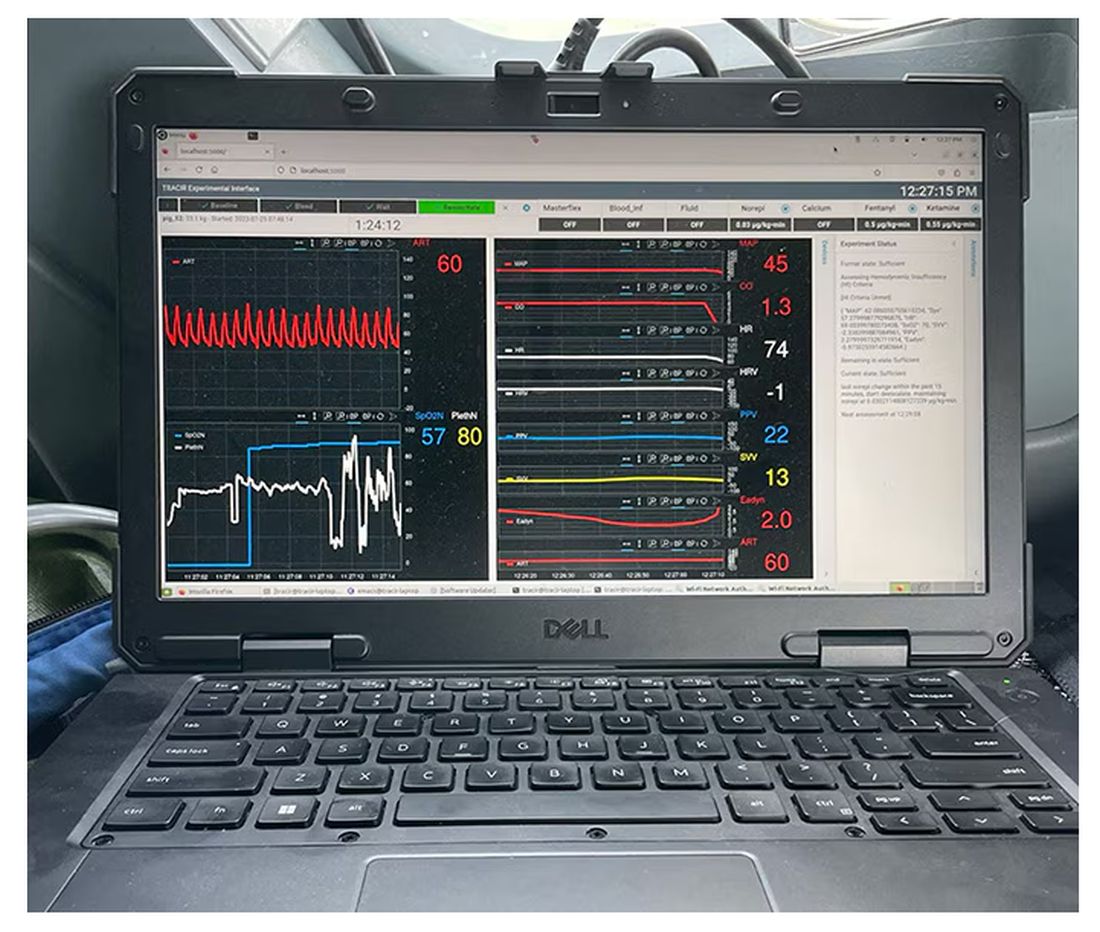
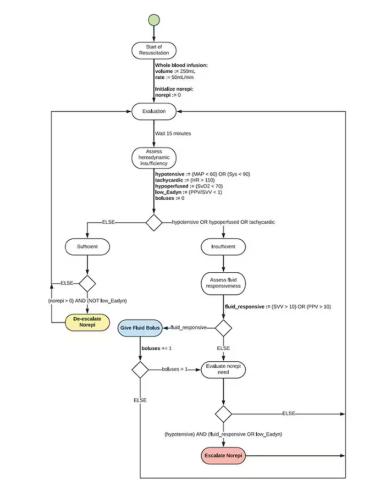
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.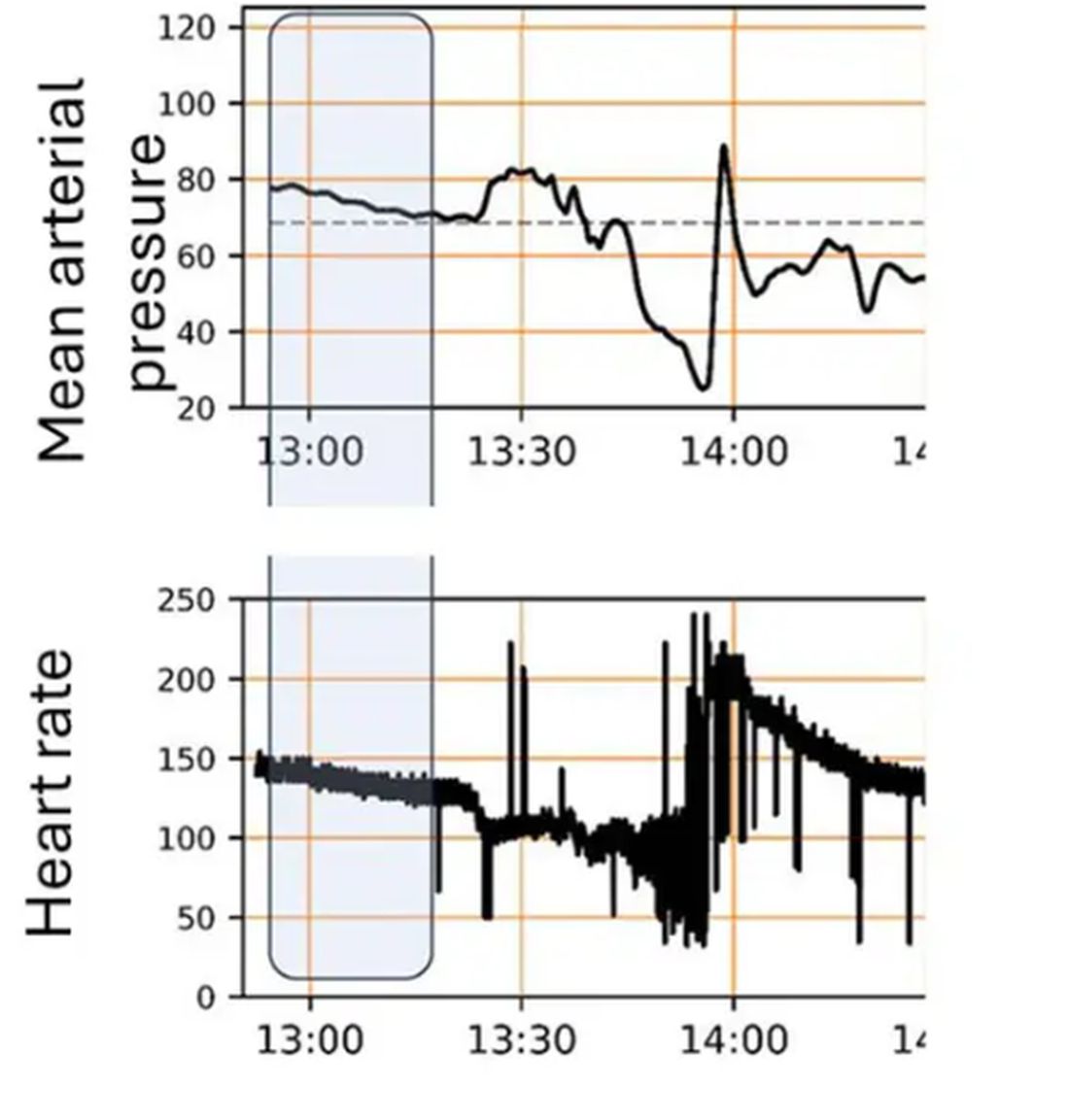
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 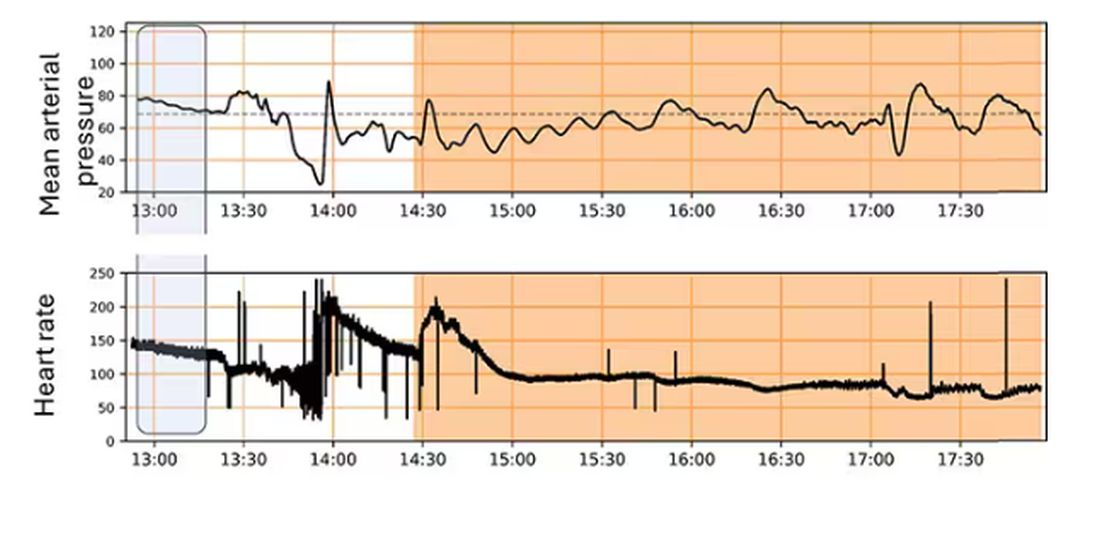
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New Administration Routes for Adrenaline in Anaphylaxis
PARIS — While anaphylaxis requires immediate adrenaline administration through autoinjection, the use of this treatment is not optimal. Therefore, the development of new adrenaline formulations (such as for intranasal, sublingual, and transcutaneous routes) aims to facilitate the drug’s use and reduce persistent delays in administration by patients and caregivers. An overview of the research was presented at the 19th French-speaking Congress of Allergology.
Anaphylaxis is a severe and potentially fatal immediate hypersensitivity reaction with highly variable and dynamic clinical presentations. It requires prompt recognition for immediate treatment with intramuscular (IM) adrenaline (at the anterolateral aspect of the mid-thigh).
One might think that this reflex is acquired, but in France, while the number of prescribed adrenaline autoinjection (AAI) devices has been increasing for a decade, reaching 965,944 units in 2022, this first-line treatment is underused. Anapen (150, 300, and 500 µg), EpiPen (150 and 300 µg), Jext (150 µg and 300 µg), and Emerade (150, 300, and 500 µg) are the four products marketed in France in 2024.
“Only 17.3% of individuals presenting to the emergency department in the Lorraine region used it in 2015,” said Catherine Neukirch, MD, a pneumologist at Hôpital Bichat–Claude Bernard in Paris, France, with rates of 11.3% for children and 20.3% for adults.
Anaphylaxis Incidence Increasing
Approximately 0.3% (95% CI, 0.1-0.5) of the population will experience an anaphylaxis episode in their lifetime. Incidence in Europe, across all causes, is estimated between 1.5 and 7.9 cases per 100,000 inhabitants per year. Although anaphylaxis is on the rise, its associated mortality remains low, ranging between 0.05 and 0.51 per million per year for drugs, between 0.03 and 0.32 per million per year for foods, and between 0.09 and 0.13 per million per year for hymenopteran venoms.
Data from the European Anaphylaxis Registry indicate that anaphylaxis manifests rapidly after allergen exposure: 55% of cases occur within 10 minutes and 80% within 30 minutes. In addition, a biphasic reaction, which can occur up to 72 hours after exposure, is observed in < 5% of cases.
While a delay in adrenaline use is associated with risk for increased morbidity and mortality, AAI significantly reduces error rates compared with manual treatments involving ampoules, needles, and syringes. It also reduces the associated panic risks. However, there are multiple barriers to adrenaline use. The clinical symptoms of anaphylaxis may be misleading, especially if it occurs without cutaneous and urticarial manifestations but with only acute bronchospasm. It may present as isolated laryngeal edema without digestive involvement, hypotension, or other respiratory problems.
Other limitations to adrenaline use include technical difficulties and the possibility of incorrect administration, the need for appropriate needle sizes for patients with obesity, needle phobia, potential adverse effects of adrenaline injections, failure to carry two autoinjectors, constraints related to storage and bulky transport, as well as the need for training and practice.
“These factors contribute to underuse of adrenaline by patients and caregivers,” said Dr. Neukirch, which results in delays in necessary administration.
Adrenaline Treatment Criteria?
An analysis published in 2023 based on pharmacovigilance data from 30 regional French centers from 1984 to 2022 included 42 reported cases (average age, 33 years; 26% children) of reactions to AAI, which probably is an underestimate. About 40% of AAI uses occurred during anaphylaxis. The remaining 60% were triggered outside of reactions. The main reasons were accidental injections, mainly in the fingers, and cases of not triggering the autoinjector, underlining the importance of patient education.
In 2015, the European Medicines Agency required pharmacological studies for injectable adrenaline on healthy volunteers. These studies include ultrasound measurements of bolus injection, pharmacokinetics (ie, absorption, distribution, metabolism, and excretion), and pharmacodynamics (ie, the effect of the drug and the mechanism of action in the body), with precise evaluation of cardiovascular effects (eg, systolic and diastolic blood pressures and heart rate).
Among the information collected with the different products, ultrasound studies have shown a different localization of the adrenaline bolus (ie, in muscle in patients with normal BMI and mostly in adipose tissue in patients with BMI indicating overweight and obesity). The consequences of this finding are still unknown.
In a study with 500 µg Anapen, women with overweight or obesity showed different pharmacokinetic or pharmacodynamic profiles from those in men with normal weight, with an increase in the area under the curve (0-240 min) and marked changes in the heart rate time curve.
IM administration of 0.5 mg produces rapid pharmacokinetic effects in patients with normal weight, overweight, or obesity, with a delay for the second peak in the latter case. This delay perhaps results from initial local vasoconstriction due to adrenaline.
The early peak plasma concentration occurs at 5-10 minutes for AAI, with a faster speed for Anapen and EpiPen.
Moreover, needle size is not the most important factor. Rather, it is the strength and speed of injection, which can vary depending on the AAI.
Also, the optimal plasma concentration of adrenaline to treat anaphylaxis is not known; studies cannot be conducted during anaphylaxis. In terms of pharmacokinetics, a small series discovered that increased skin or muscle thickness delays the absorption of EpiPen AAI.
Intranasal Adrenaline
To facilitate rapid adrenaline use and convince reluctant patients to carry and use adrenaline, intranasal, sublingual, or transcutaneous forms are under development.
Three intranasal forms of adrenaline are already well advanced, including Neffy from ARS Pharma, epinephrine sprays from Bryn Pharma and Hikma, and Oxero from Oragoo, which contains dry powder.
A comparison of intranasal adrenaline Neffy and AAI shows that the former has satisfactory pharmacokinetic and pharmacodynamic effects.
In a phase 1 randomized crossover study of 42 healthy adults comparing the pharmacokinetic effects of Neffy adrenaline (2 mg) and EpiPen (0.3 mg), as well as IM epinephrine 0.3 mg, several observations were made. For a single dose, the maximum concentration (Cmax) of Neffy was lower than that of EpiPen.
However, with repeated doses administered 10 minutes apart, the Cmax of Neffy was higher than that of EpiPen. At this stage, pharmacodynamic responses to intranasal products are at least comparable with those of approved injectable products.
A comparison of the pharmacodynamic effects, such as systolic and diastolic blood pressures and heart rate, of Neffy adrenaline and AAI concluded that the profile of Neffy is comparable with that of EpiPen and superior to that of IM epinephrine.
In patients with a history of allergic rhinitis, adrenaline Cmax appears to be increased, while time to peak plasma concentration (Tmax) is reduced. Low blood pressure does not prevent Neffy absorption. Neffy is currently under review by the American and European health authorities.
Intranasal absorption of dry powder adrenaline appears to be faster than that of EpiPen, thus offering a clinical advantage in the short therapeutic window for anaphylaxis treatment.
In an open-label trial conducted on 12 adults with seasonal allergic rhinitis without asthma, the pharmacokinetics, pharmacodynamics, and safety of adrenaline were compared between FMXIN002 (1.6 and 3.2 mg), which was administered intranasally with or without nasal allergen challenge, and IM EpiPen 0.3 mg. Pharmacokinetics varied by patient. Nevertheless, nasal FMXIN002 had a shorter Tmax, a doubled Cmax after the allergen challenge peak, and a higher area under the curve in the 8 hours following administration compared with EpiPen. Pharmacodynamic effects comparable with those of EpiPen were noted at 15 minutes to 4 hours after administration. The tolerance was good, with mild and local side effects. The powder seems to deposit slightly better in the nasal cavity. It remains stable for 6 months at a temperature of 40 °C and relative humidity of 75% and for 2 years at a temperature of 25 °C and relative humidity of 60%.
Sublingual Adrenaline Film
AQST-109 is a sublingual film that is intended to allow rapid administration of epinephrine 1, which is a prodrug of adrenaline. The product is the size of a postage stamp, weighs < 30 g, and dissolves on contact with the tongue.
The EPIPHAST II study was a phase 1, multiperiod, crossover study conducted on 24 healthy adults (age, 24-49 years) who were randomly assigned to receive either 12 or 0.3 mg of AQST-109 of manual IM adrenaline in the first two periods. All participants received 0.3 mg of EpiPen in the last period.
EpiPen 0.3 mg resulted in a higher Cmax than AQST-109 12 mg. AQST-109 12 mg had the fastest median Tmax of 12 minutes. The areas under the curve of AQST-109 12 mg fell between those of EpiPen 0.3 mg and manual IM adrenaline 0.3 mg.
Early increases in systolic blood pressure, diastolic blood pressure, and heart rate were observed with AQST-109 12 mg. Changes were more pronounced with AQST-109 12 mg despite a higher Cmax with EpiPen 0.3 mg.
Part 3 of the EPIPHAST study evaluated the impact of food exposure (ie, a peanut butter sandwich) on the pharmacokinetics of AQST-109 12 mg in 24 healthy adults. Oral food residues did not significantly affect pharmacodynamic parameters, and no treatment-related adverse events were reported.
Researchers concluded that AQST-109 12 mg absorption would not be altered by “real” situations if used during meals. “These results suggest that the sublingual adrenaline film could be promising in real situations,” said Dr. Neukirch, especially in cases of food allergy with recent ingestion of the allergenic food.
Transcutaneous Adrenaline
A transcutaneous form of adrenaline that uses the Zeneo device developed by Crossject, a company based in Dijon, France, comes in the form of an AAI that requires no needle. This project, funded by the European Union, uses a gas generator to propel the drug at very high speed through the skin in 50 milliseconds. This method allows for extended drug storage.
Dr. Neukirch reported financial relationships with Viatris, Stallergènes, ALK, Astrazeneca, Sanofi, GSK, and Novartis.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
PARIS — While anaphylaxis requires immediate adrenaline administration through autoinjection, the use of this treatment is not optimal. Therefore, the development of new adrenaline formulations (such as for intranasal, sublingual, and transcutaneous routes) aims to facilitate the drug’s use and reduce persistent delays in administration by patients and caregivers. An overview of the research was presented at the 19th French-speaking Congress of Allergology.
Anaphylaxis is a severe and potentially fatal immediate hypersensitivity reaction with highly variable and dynamic clinical presentations. It requires prompt recognition for immediate treatment with intramuscular (IM) adrenaline (at the anterolateral aspect of the mid-thigh).
One might think that this reflex is acquired, but in France, while the number of prescribed adrenaline autoinjection (AAI) devices has been increasing for a decade, reaching 965,944 units in 2022, this first-line treatment is underused. Anapen (150, 300, and 500 µg), EpiPen (150 and 300 µg), Jext (150 µg and 300 µg), and Emerade (150, 300, and 500 µg) are the four products marketed in France in 2024.
“Only 17.3% of individuals presenting to the emergency department in the Lorraine region used it in 2015,” said Catherine Neukirch, MD, a pneumologist at Hôpital Bichat–Claude Bernard in Paris, France, with rates of 11.3% for children and 20.3% for adults.
Anaphylaxis Incidence Increasing
Approximately 0.3% (95% CI, 0.1-0.5) of the population will experience an anaphylaxis episode in their lifetime. Incidence in Europe, across all causes, is estimated between 1.5 and 7.9 cases per 100,000 inhabitants per year. Although anaphylaxis is on the rise, its associated mortality remains low, ranging between 0.05 and 0.51 per million per year for drugs, between 0.03 and 0.32 per million per year for foods, and between 0.09 and 0.13 per million per year for hymenopteran venoms.
Data from the European Anaphylaxis Registry indicate that anaphylaxis manifests rapidly after allergen exposure: 55% of cases occur within 10 minutes and 80% within 30 minutes. In addition, a biphasic reaction, which can occur up to 72 hours after exposure, is observed in < 5% of cases.
While a delay in adrenaline use is associated with risk for increased morbidity and mortality, AAI significantly reduces error rates compared with manual treatments involving ampoules, needles, and syringes. It also reduces the associated panic risks. However, there are multiple barriers to adrenaline use. The clinical symptoms of anaphylaxis may be misleading, especially if it occurs without cutaneous and urticarial manifestations but with only acute bronchospasm. It may present as isolated laryngeal edema without digestive involvement, hypotension, or other respiratory problems.
Other limitations to adrenaline use include technical difficulties and the possibility of incorrect administration, the need for appropriate needle sizes for patients with obesity, needle phobia, potential adverse effects of adrenaline injections, failure to carry two autoinjectors, constraints related to storage and bulky transport, as well as the need for training and practice.
“These factors contribute to underuse of adrenaline by patients and caregivers,” said Dr. Neukirch, which results in delays in necessary administration.
Adrenaline Treatment Criteria?
An analysis published in 2023 based on pharmacovigilance data from 30 regional French centers from 1984 to 2022 included 42 reported cases (average age, 33 years; 26% children) of reactions to AAI, which probably is an underestimate. About 40% of AAI uses occurred during anaphylaxis. The remaining 60% were triggered outside of reactions. The main reasons were accidental injections, mainly in the fingers, and cases of not triggering the autoinjector, underlining the importance of patient education.
In 2015, the European Medicines Agency required pharmacological studies for injectable adrenaline on healthy volunteers. These studies include ultrasound measurements of bolus injection, pharmacokinetics (ie, absorption, distribution, metabolism, and excretion), and pharmacodynamics (ie, the effect of the drug and the mechanism of action in the body), with precise evaluation of cardiovascular effects (eg, systolic and diastolic blood pressures and heart rate).
Among the information collected with the different products, ultrasound studies have shown a different localization of the adrenaline bolus (ie, in muscle in patients with normal BMI and mostly in adipose tissue in patients with BMI indicating overweight and obesity). The consequences of this finding are still unknown.
In a study with 500 µg Anapen, women with overweight or obesity showed different pharmacokinetic or pharmacodynamic profiles from those in men with normal weight, with an increase in the area under the curve (0-240 min) and marked changes in the heart rate time curve.
IM administration of 0.5 mg produces rapid pharmacokinetic effects in patients with normal weight, overweight, or obesity, with a delay for the second peak in the latter case. This delay perhaps results from initial local vasoconstriction due to adrenaline.
The early peak plasma concentration occurs at 5-10 minutes for AAI, with a faster speed for Anapen and EpiPen.
Moreover, needle size is not the most important factor. Rather, it is the strength and speed of injection, which can vary depending on the AAI.
Also, the optimal plasma concentration of adrenaline to treat anaphylaxis is not known; studies cannot be conducted during anaphylaxis. In terms of pharmacokinetics, a small series discovered that increased skin or muscle thickness delays the absorption of EpiPen AAI.
Intranasal Adrenaline
To facilitate rapid adrenaline use and convince reluctant patients to carry and use adrenaline, intranasal, sublingual, or transcutaneous forms are under development.
Three intranasal forms of adrenaline are already well advanced, including Neffy from ARS Pharma, epinephrine sprays from Bryn Pharma and Hikma, and Oxero from Oragoo, which contains dry powder.
A comparison of intranasal adrenaline Neffy and AAI shows that the former has satisfactory pharmacokinetic and pharmacodynamic effects.
In a phase 1 randomized crossover study of 42 healthy adults comparing the pharmacokinetic effects of Neffy adrenaline (2 mg) and EpiPen (0.3 mg), as well as IM epinephrine 0.3 mg, several observations were made. For a single dose, the maximum concentration (Cmax) of Neffy was lower than that of EpiPen.
However, with repeated doses administered 10 minutes apart, the Cmax of Neffy was higher than that of EpiPen. At this stage, pharmacodynamic responses to intranasal products are at least comparable with those of approved injectable products.
A comparison of the pharmacodynamic effects, such as systolic and diastolic blood pressures and heart rate, of Neffy adrenaline and AAI concluded that the profile of Neffy is comparable with that of EpiPen and superior to that of IM epinephrine.
In patients with a history of allergic rhinitis, adrenaline Cmax appears to be increased, while time to peak plasma concentration (Tmax) is reduced. Low blood pressure does not prevent Neffy absorption. Neffy is currently under review by the American and European health authorities.
Intranasal absorption of dry powder adrenaline appears to be faster than that of EpiPen, thus offering a clinical advantage in the short therapeutic window for anaphylaxis treatment.
In an open-label trial conducted on 12 adults with seasonal allergic rhinitis without asthma, the pharmacokinetics, pharmacodynamics, and safety of adrenaline were compared between FMXIN002 (1.6 and 3.2 mg), which was administered intranasally with or without nasal allergen challenge, and IM EpiPen 0.3 mg. Pharmacokinetics varied by patient. Nevertheless, nasal FMXIN002 had a shorter Tmax, a doubled Cmax after the allergen challenge peak, and a higher area under the curve in the 8 hours following administration compared with EpiPen. Pharmacodynamic effects comparable with those of EpiPen were noted at 15 minutes to 4 hours after administration. The tolerance was good, with mild and local side effects. The powder seems to deposit slightly better in the nasal cavity. It remains stable for 6 months at a temperature of 40 °C and relative humidity of 75% and for 2 years at a temperature of 25 °C and relative humidity of 60%.
Sublingual Adrenaline Film
AQST-109 is a sublingual film that is intended to allow rapid administration of epinephrine 1, which is a prodrug of adrenaline. The product is the size of a postage stamp, weighs < 30 g, and dissolves on contact with the tongue.
The EPIPHAST II study was a phase 1, multiperiod, crossover study conducted on 24 healthy adults (age, 24-49 years) who were randomly assigned to receive either 12 or 0.3 mg of AQST-109 of manual IM adrenaline in the first two periods. All participants received 0.3 mg of EpiPen in the last period.
EpiPen 0.3 mg resulted in a higher Cmax than AQST-109 12 mg. AQST-109 12 mg had the fastest median Tmax of 12 minutes. The areas under the curve of AQST-109 12 mg fell between those of EpiPen 0.3 mg and manual IM adrenaline 0.3 mg.
Early increases in systolic blood pressure, diastolic blood pressure, and heart rate were observed with AQST-109 12 mg. Changes were more pronounced with AQST-109 12 mg despite a higher Cmax with EpiPen 0.3 mg.
Part 3 of the EPIPHAST study evaluated the impact of food exposure (ie, a peanut butter sandwich) on the pharmacokinetics of AQST-109 12 mg in 24 healthy adults. Oral food residues did not significantly affect pharmacodynamic parameters, and no treatment-related adverse events were reported.
Researchers concluded that AQST-109 12 mg absorption would not be altered by “real” situations if used during meals. “These results suggest that the sublingual adrenaline film could be promising in real situations,” said Dr. Neukirch, especially in cases of food allergy with recent ingestion of the allergenic food.
Transcutaneous Adrenaline
A transcutaneous form of adrenaline that uses the Zeneo device developed by Crossject, a company based in Dijon, France, comes in the form of an AAI that requires no needle. This project, funded by the European Union, uses a gas generator to propel the drug at very high speed through the skin in 50 milliseconds. This method allows for extended drug storage.
Dr. Neukirch reported financial relationships with Viatris, Stallergènes, ALK, Astrazeneca, Sanofi, GSK, and Novartis.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
PARIS — While anaphylaxis requires immediate adrenaline administration through autoinjection, the use of this treatment is not optimal. Therefore, the development of new adrenaline formulations (such as for intranasal, sublingual, and transcutaneous routes) aims to facilitate the drug’s use and reduce persistent delays in administration by patients and caregivers. An overview of the research was presented at the 19th French-speaking Congress of Allergology.
Anaphylaxis is a severe and potentially fatal immediate hypersensitivity reaction with highly variable and dynamic clinical presentations. It requires prompt recognition for immediate treatment with intramuscular (IM) adrenaline (at the anterolateral aspect of the mid-thigh).
One might think that this reflex is acquired, but in France, while the number of prescribed adrenaline autoinjection (AAI) devices has been increasing for a decade, reaching 965,944 units in 2022, this first-line treatment is underused. Anapen (150, 300, and 500 µg), EpiPen (150 and 300 µg), Jext (150 µg and 300 µg), and Emerade (150, 300, and 500 µg) are the four products marketed in France in 2024.
“Only 17.3% of individuals presenting to the emergency department in the Lorraine region used it in 2015,” said Catherine Neukirch, MD, a pneumologist at Hôpital Bichat–Claude Bernard in Paris, France, with rates of 11.3% for children and 20.3% for adults.
Anaphylaxis Incidence Increasing
Approximately 0.3% (95% CI, 0.1-0.5) of the population will experience an anaphylaxis episode in their lifetime. Incidence in Europe, across all causes, is estimated between 1.5 and 7.9 cases per 100,000 inhabitants per year. Although anaphylaxis is on the rise, its associated mortality remains low, ranging between 0.05 and 0.51 per million per year for drugs, between 0.03 and 0.32 per million per year for foods, and between 0.09 and 0.13 per million per year for hymenopteran venoms.
Data from the European Anaphylaxis Registry indicate that anaphylaxis manifests rapidly after allergen exposure: 55% of cases occur within 10 minutes and 80% within 30 minutes. In addition, a biphasic reaction, which can occur up to 72 hours after exposure, is observed in < 5% of cases.
While a delay in adrenaline use is associated with risk for increased morbidity and mortality, AAI significantly reduces error rates compared with manual treatments involving ampoules, needles, and syringes. It also reduces the associated panic risks. However, there are multiple barriers to adrenaline use. The clinical symptoms of anaphylaxis may be misleading, especially if it occurs without cutaneous and urticarial manifestations but with only acute bronchospasm. It may present as isolated laryngeal edema without digestive involvement, hypotension, or other respiratory problems.
Other limitations to adrenaline use include technical difficulties and the possibility of incorrect administration, the need for appropriate needle sizes for patients with obesity, needle phobia, potential adverse effects of adrenaline injections, failure to carry two autoinjectors, constraints related to storage and bulky transport, as well as the need for training and practice.
“These factors contribute to underuse of adrenaline by patients and caregivers,” said Dr. Neukirch, which results in delays in necessary administration.
Adrenaline Treatment Criteria?
An analysis published in 2023 based on pharmacovigilance data from 30 regional French centers from 1984 to 2022 included 42 reported cases (average age, 33 years; 26% children) of reactions to AAI, which probably is an underestimate. About 40% of AAI uses occurred during anaphylaxis. The remaining 60% were triggered outside of reactions. The main reasons were accidental injections, mainly in the fingers, and cases of not triggering the autoinjector, underlining the importance of patient education.
In 2015, the European Medicines Agency required pharmacological studies for injectable adrenaline on healthy volunteers. These studies include ultrasound measurements of bolus injection, pharmacokinetics (ie, absorption, distribution, metabolism, and excretion), and pharmacodynamics (ie, the effect of the drug and the mechanism of action in the body), with precise evaluation of cardiovascular effects (eg, systolic and diastolic blood pressures and heart rate).
Among the information collected with the different products, ultrasound studies have shown a different localization of the adrenaline bolus (ie, in muscle in patients with normal BMI and mostly in adipose tissue in patients with BMI indicating overweight and obesity). The consequences of this finding are still unknown.
In a study with 500 µg Anapen, women with overweight or obesity showed different pharmacokinetic or pharmacodynamic profiles from those in men with normal weight, with an increase in the area under the curve (0-240 min) and marked changes in the heart rate time curve.
IM administration of 0.5 mg produces rapid pharmacokinetic effects in patients with normal weight, overweight, or obesity, with a delay for the second peak in the latter case. This delay perhaps results from initial local vasoconstriction due to adrenaline.
The early peak plasma concentration occurs at 5-10 minutes for AAI, with a faster speed for Anapen and EpiPen.
Moreover, needle size is not the most important factor. Rather, it is the strength and speed of injection, which can vary depending on the AAI.
Also, the optimal plasma concentration of adrenaline to treat anaphylaxis is not known; studies cannot be conducted during anaphylaxis. In terms of pharmacokinetics, a small series discovered that increased skin or muscle thickness delays the absorption of EpiPen AAI.
Intranasal Adrenaline
To facilitate rapid adrenaline use and convince reluctant patients to carry and use adrenaline, intranasal, sublingual, or transcutaneous forms are under development.
Three intranasal forms of adrenaline are already well advanced, including Neffy from ARS Pharma, epinephrine sprays from Bryn Pharma and Hikma, and Oxero from Oragoo, which contains dry powder.
A comparison of intranasal adrenaline Neffy and AAI shows that the former has satisfactory pharmacokinetic and pharmacodynamic effects.
In a phase 1 randomized crossover study of 42 healthy adults comparing the pharmacokinetic effects of Neffy adrenaline (2 mg) and EpiPen (0.3 mg), as well as IM epinephrine 0.3 mg, several observations were made. For a single dose, the maximum concentration (Cmax) of Neffy was lower than that of EpiPen.
However, with repeated doses administered 10 minutes apart, the Cmax of Neffy was higher than that of EpiPen. At this stage, pharmacodynamic responses to intranasal products are at least comparable with those of approved injectable products.
A comparison of the pharmacodynamic effects, such as systolic and diastolic blood pressures and heart rate, of Neffy adrenaline and AAI concluded that the profile of Neffy is comparable with that of EpiPen and superior to that of IM epinephrine.
In patients with a history of allergic rhinitis, adrenaline Cmax appears to be increased, while time to peak plasma concentration (Tmax) is reduced. Low blood pressure does not prevent Neffy absorption. Neffy is currently under review by the American and European health authorities.
Intranasal absorption of dry powder adrenaline appears to be faster than that of EpiPen, thus offering a clinical advantage in the short therapeutic window for anaphylaxis treatment.
In an open-label trial conducted on 12 adults with seasonal allergic rhinitis without asthma, the pharmacokinetics, pharmacodynamics, and safety of adrenaline were compared between FMXIN002 (1.6 and 3.2 mg), which was administered intranasally with or without nasal allergen challenge, and IM EpiPen 0.3 mg. Pharmacokinetics varied by patient. Nevertheless, nasal FMXIN002 had a shorter Tmax, a doubled Cmax after the allergen challenge peak, and a higher area under the curve in the 8 hours following administration compared with EpiPen. Pharmacodynamic effects comparable with those of EpiPen were noted at 15 minutes to 4 hours after administration. The tolerance was good, with mild and local side effects. The powder seems to deposit slightly better in the nasal cavity. It remains stable for 6 months at a temperature of 40 °C and relative humidity of 75% and for 2 years at a temperature of 25 °C and relative humidity of 60%.
Sublingual Adrenaline Film
AQST-109 is a sublingual film that is intended to allow rapid administration of epinephrine 1, which is a prodrug of adrenaline. The product is the size of a postage stamp, weighs < 30 g, and dissolves on contact with the tongue.
The EPIPHAST II study was a phase 1, multiperiod, crossover study conducted on 24 healthy adults (age, 24-49 years) who were randomly assigned to receive either 12 or 0.3 mg of AQST-109 of manual IM adrenaline in the first two periods. All participants received 0.3 mg of EpiPen in the last period.
EpiPen 0.3 mg resulted in a higher Cmax than AQST-109 12 mg. AQST-109 12 mg had the fastest median Tmax of 12 minutes. The areas under the curve of AQST-109 12 mg fell between those of EpiPen 0.3 mg and manual IM adrenaline 0.3 mg.
Early increases in systolic blood pressure, diastolic blood pressure, and heart rate were observed with AQST-109 12 mg. Changes were more pronounced with AQST-109 12 mg despite a higher Cmax with EpiPen 0.3 mg.
Part 3 of the EPIPHAST study evaluated the impact of food exposure (ie, a peanut butter sandwich) on the pharmacokinetics of AQST-109 12 mg in 24 healthy adults. Oral food residues did not significantly affect pharmacodynamic parameters, and no treatment-related adverse events were reported.
Researchers concluded that AQST-109 12 mg absorption would not be altered by “real” situations if used during meals. “These results suggest that the sublingual adrenaline film could be promising in real situations,” said Dr. Neukirch, especially in cases of food allergy with recent ingestion of the allergenic food.
Transcutaneous Adrenaline
A transcutaneous form of adrenaline that uses the Zeneo device developed by Crossject, a company based in Dijon, France, comes in the form of an AAI that requires no needle. This project, funded by the European Union, uses a gas generator to propel the drug at very high speed through the skin in 50 milliseconds. This method allows for extended drug storage.
Dr. Neukirch reported financial relationships with Viatris, Stallergènes, ALK, Astrazeneca, Sanofi, GSK, and Novartis.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Vacationing Doctors Fight to Revive a Drowned Child
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
FDA Approves AI Diagnostic Tool for Early Sepsis Detection
The US Food and Drug Administration (FDA) has approved a medical device named the Sepsis ImmunoScore, which is an artificial intelligence/machine learning software, to guide rapid diagnosis and prediction of sepsis. The authorization was granted through the FDA’s De Novo pathway.
Sepsis is a complex condition, so diagnosing it early is difficult and has been a decades-long challenge for the US healthcare system.
Using both biomarkers and clinical data with the assistance of AI, the Sepsis ImmunoScore helps assess the risk for the presence of or progression to sepsis within 24 hours of patient evaluation in the emergency department or hospital. By considering 22 diverse parameters, the AI-powered tool provides a comprehensive evaluation of the patient’s biological condition, resulting in a risk score and categorization into four distinct risk levels.
It’s important to note that this system is not an alert mechanism. These risk categories are correlated with the risk for patient deterioration, including length of hospital stay, in-hospital mortality, and the need for escalated care within 24 hours (such as intensive care unit admission, mechanical ventilation, or vasopressor use). The diagnostic software is integrated directly into hospital electronic medical records.
This is the first AI diagnostic tool for sepsis to receive marketing authorization from the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a medical device named the Sepsis ImmunoScore, which is an artificial intelligence/machine learning software, to guide rapid diagnosis and prediction of sepsis. The authorization was granted through the FDA’s De Novo pathway.
Sepsis is a complex condition, so diagnosing it early is difficult and has been a decades-long challenge for the US healthcare system.
Using both biomarkers and clinical data with the assistance of AI, the Sepsis ImmunoScore helps assess the risk for the presence of or progression to sepsis within 24 hours of patient evaluation in the emergency department or hospital. By considering 22 diverse parameters, the AI-powered tool provides a comprehensive evaluation of the patient’s biological condition, resulting in a risk score and categorization into four distinct risk levels.
It’s important to note that this system is not an alert mechanism. These risk categories are correlated with the risk for patient deterioration, including length of hospital stay, in-hospital mortality, and the need for escalated care within 24 hours (such as intensive care unit admission, mechanical ventilation, or vasopressor use). The diagnostic software is integrated directly into hospital electronic medical records.
This is the first AI diagnostic tool for sepsis to receive marketing authorization from the FDA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a medical device named the Sepsis ImmunoScore, which is an artificial intelligence/machine learning software, to guide rapid diagnosis and prediction of sepsis. The authorization was granted through the FDA’s De Novo pathway.
Sepsis is a complex condition, so diagnosing it early is difficult and has been a decades-long challenge for the US healthcare system.
Using both biomarkers and clinical data with the assistance of AI, the Sepsis ImmunoScore helps assess the risk for the presence of or progression to sepsis within 24 hours of patient evaluation in the emergency department or hospital. By considering 22 diverse parameters, the AI-powered tool provides a comprehensive evaluation of the patient’s biological condition, resulting in a risk score and categorization into four distinct risk levels.
It’s important to note that this system is not an alert mechanism. These risk categories are correlated with the risk for patient deterioration, including length of hospital stay, in-hospital mortality, and the need for escalated care within 24 hours (such as intensive care unit admission, mechanical ventilation, or vasopressor use). The diagnostic software is integrated directly into hospital electronic medical records.
This is the first AI diagnostic tool for sepsis to receive marketing authorization from the FDA.
A version of this article appeared on Medscape.com.
Near-Death Experiences During CPR: An Impetus for Better Care
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.