User login
Alert System Could Warn of Impact of Severe Weather on Health
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
It’s Never Too Late to Convince Patients to Quit Smoking
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An estimated 450,000 US deaths are expected this year from conditions attributed to cigarette smoking. Although the percentage of adults who smoke declined from 21% in 2005 to 11% in 2022, the annual death toll has been stable since 2005 and isn’t expected to decline until 2030, owing to an aging population of current and former smokers.
In 2022, based on a national survey, two thirds of the 28.8 million US adult smokers wanted to quit, and more than half tried quitting on their own or with the help of clinicians, but less than 9% succeeded in kicking the habit. The health benefits of quitting, summarized in a patient education handout from the American Cancer Society, include a lower risk for cancer, diabetes, and cardiovascular disease. Furthermore, the handout states, “quitting smoking can add as much as 10 years to your life, compared to if you continued to smoke.”
For my patients older than age 50 who are lifelong smokers, the qualifier “as much as” can be a sticking point. Although most recognize that continuing to smoke exposes them to greater health risks and are willing to undergo lung cancer screening and receive pneumococcal vaccines, a kind of fatalism frequently sets in. I’ve heard more times than I can recall some version of the declaration, “It’s too late for quitting to make much difference for me.” Many smokers think that once they reach middle age, gains in life expectancy will be too small to be worth the intense effort and multiple failed attempts that are typically required to quit permanently. Until recently, there were few data I could call on to persuade them they were wrong.
In February 2024, Dr. Eo Rin Cho and colleagues pooled data from four national cohort studies (United States, United Kingdom, Norway, and Canada) to calculate mortality differences among current, former, and never smokers aged 20-79 years. Compared with never smokers, lifelong smokers died an average of 12-13 years earlier. However, quitting before age 50 nearly eliminated the excess mortality associated with smoking, and in the 50- to 59-year-old age group, cessation eventually reduced excess mortality by 92%-95%. Better yet, more than half of the benefits occurred within the first 3 years after cessation.
At first glance, these estimates may seem too good to be true. A few months later, though, a different research group, using data from a large cancer prevention study and 2018 US population census and mortality rates, largely confirmed their findings. Dr. Thuy Le and colleagues found that quitting at age 35, 45, 55, 65, or 75 years resulted in average life gains of 8, 5.6, 3.5, 1.7, and 0.7 years, respectively, relative to continuing to smoke. Because no patient is average, the analysis also presented some helpful probabilities. For example, a smoker who quits at age 65 has about a 1 in 4 chance of gaining at least 1 full year of life and a 1 in 6 chance of gaining at least 4 years. In other words, from a life expectancy perspective alone, it’s almost never too late to quit smoking.
Dr. Lin is a family physician and Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Whooping Cough Likely on Pace for a 5-Year High
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Could This COPD Treatment’s Cost Put It Out of Reach for Many?
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the US Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at a high annual cost, authors of a cost-effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second (FEV1) within 0-12 hours of administration compared with placebo. In addition, patients were found to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High Cost Barrier
But as authors of the analysis from the Boston-based Institute for Clinical and Economic Review (ICER) found, ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER, in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides,” he said.
As previously reported, as many as 1 in 6 persons with COPD in the United States miss or delay COPD medication doses owing to high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher volume sales, affording the drug maker a comparable profit to the higher cost but lower volume option.
Good Drug, High Price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added health care costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe, and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug Not Yet Available?
However, providers have not yet had experience to gauge the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of advance practice provider and clinical services for Colorado Springs Pulmonary Consultants and president and founder of the Association of Pulmonary Advanced Practice Providers. She learned that “they were going to release it to select specialty pharmacies in the third quarter of 2024. But all the ones we call do not have it, and no one knows who does. They haven’t sent any reps into the field in my area, so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no disclosures relevant to ensifentrine or Verona Pharma. Ms. Young is a member of the CHEST Physician Editorial Board.
A version of this article first appeared on Medscape.com.
Data Trends 2024: Respiratory Health
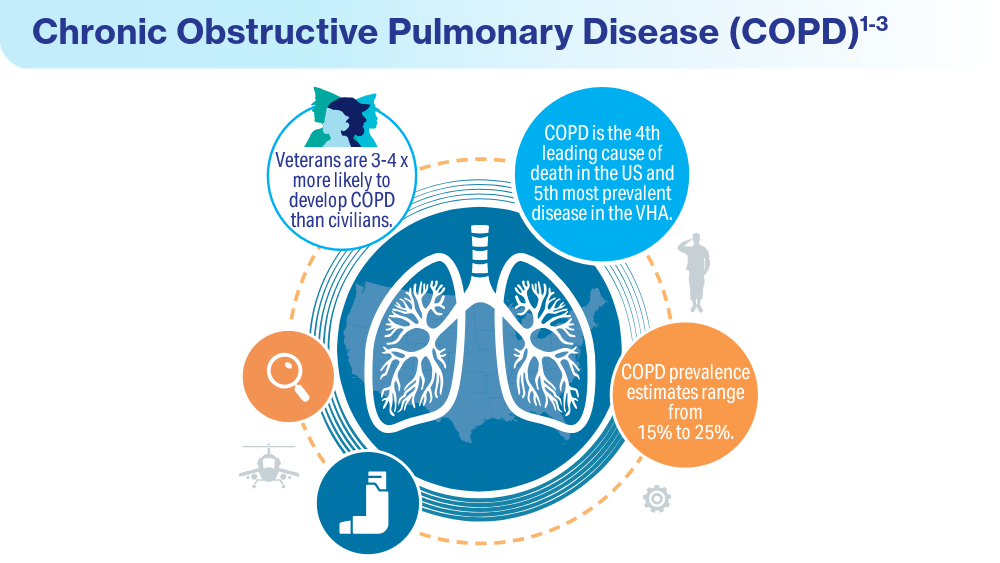
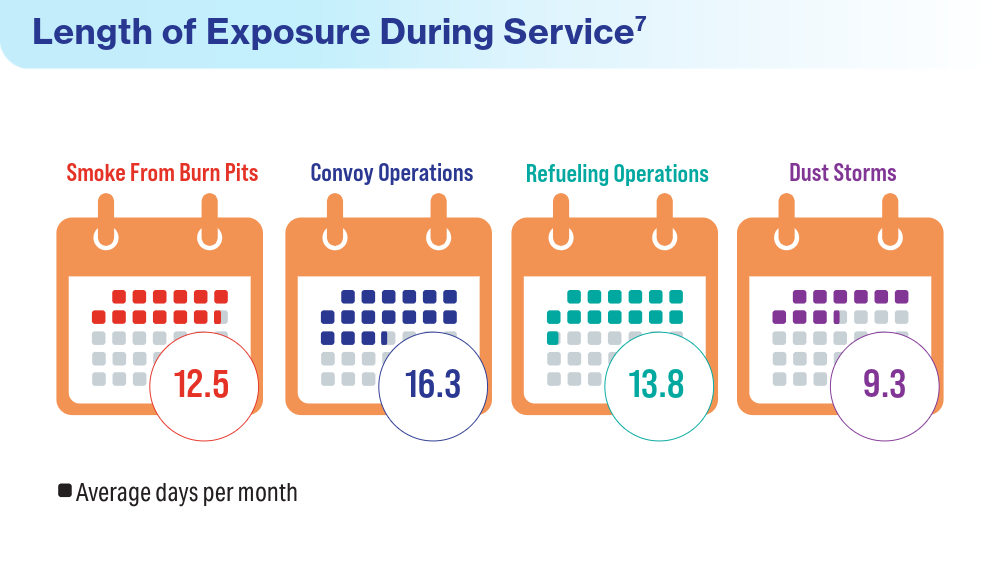
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
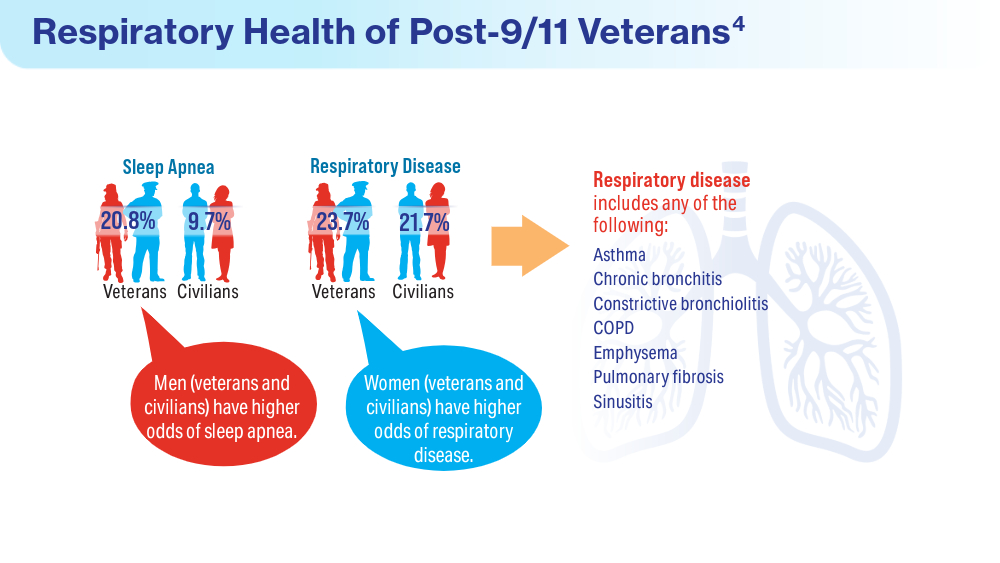
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
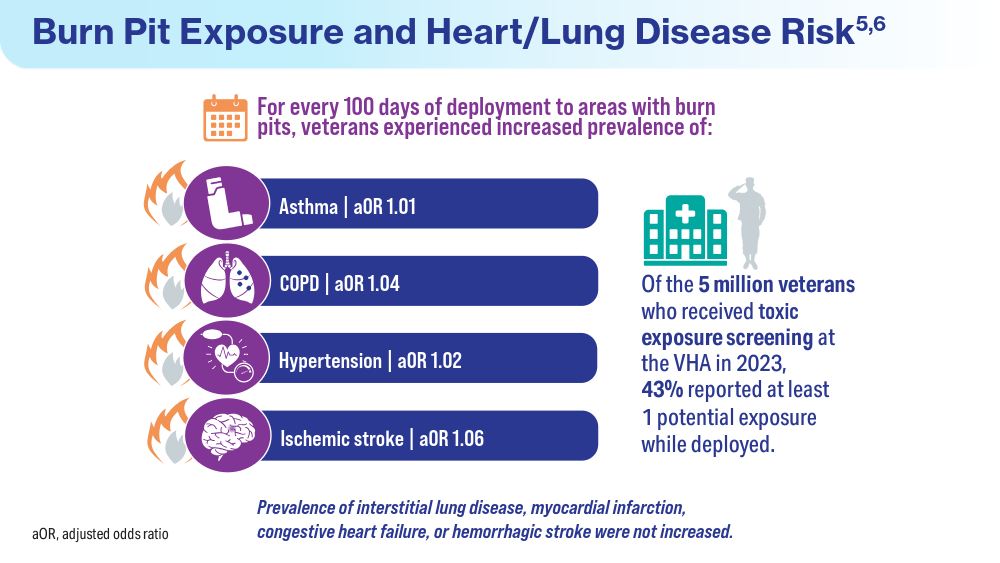
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
Federal Health Care Data Trends 2024
Could Mobile Tech Help to Minimize COPD Exacerbations?
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Viral Season 2024-2025: Try for An Ounce of Prevention
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
Rheumatoid Arthritis May Raise Lung Cancer Risk, Particularly in Those With ILD
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.